Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017
Abstract
:1. Introduction
2. Methods
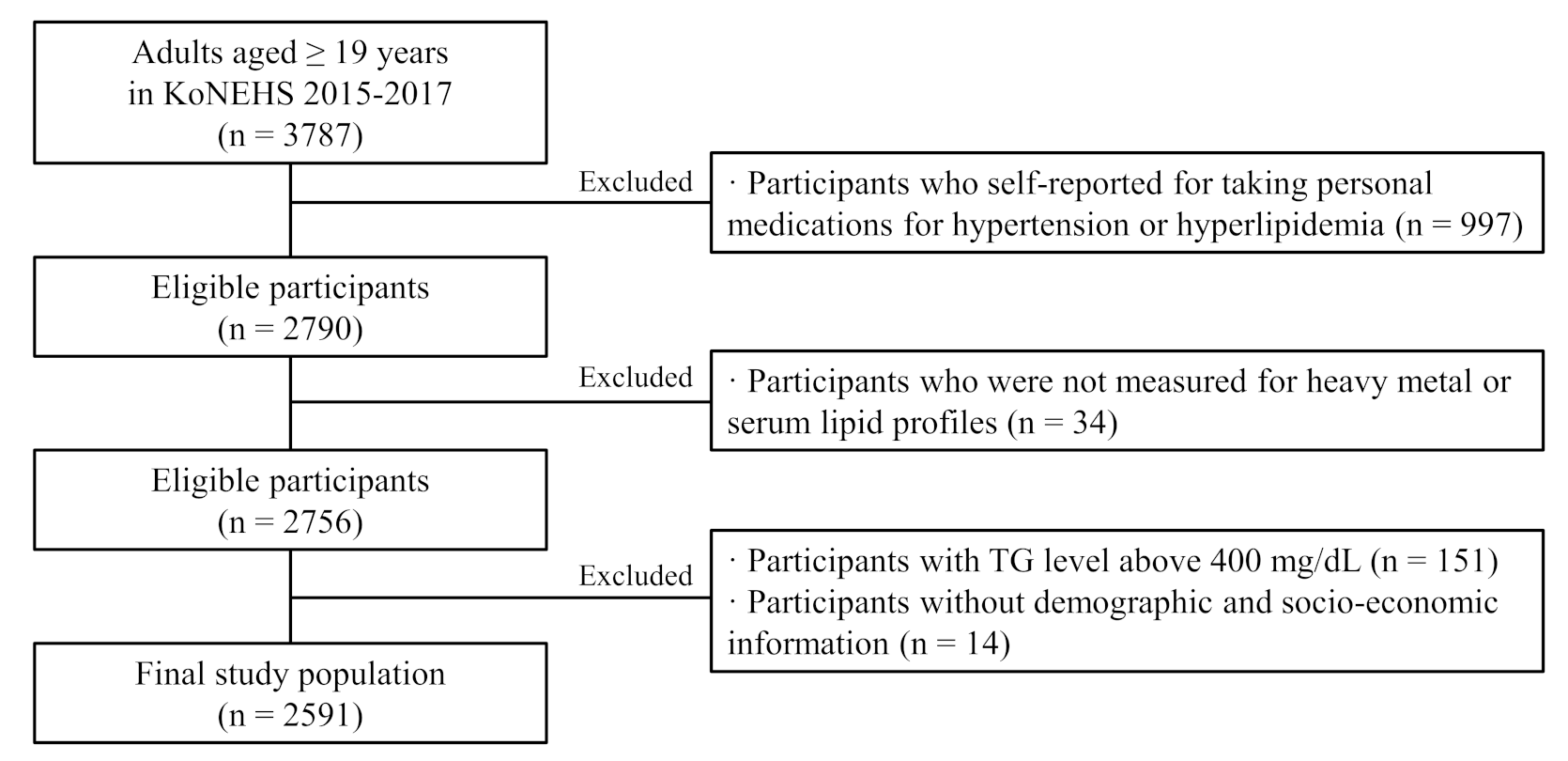
2.1. Study Population
2.2. Measurement of Heavy Metals
2.3. Measurement of Serum Lipid Profile
2.4. Evaluation of Serum Lipid Profile
2.5. Covariates
2.6. Statistical Analysis
2.7. Sensitivity Analysis
3. Results
Demographic Characteristics and Serum Lipid Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitsavos, C.; Panagiotakos, D.; Weinem, M.; Stefanadis, C. Diet, exercise and the metabolic syndrome. Rev. Diabet. Stud. 2006, 3, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, P.P.; Potter, D.; Ming, E.E. Prevalence of lipid abnormalities in the united states: The national health and nutrition examination survey 2003–2006. J. Clin. Lipidol. 2012, 6, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Deedwania, P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr. Hypertens. Rep. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Pires, A.; Sena, C.; Seiça, R. Dyslipidemia and cardiovascular changes in children. Curr. Opin. Cardiol. 2016, 31, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Hirano, T. Dyslipidemia and atherosclerosis. Nihon Rinsho. Jpn. J. Clin. Med. 2011, 69, 138–143. [Google Scholar]
- Asghari, S.; Aref-Eshghi, E.; Godwin, M.; Duke, P.; Williamson, T.; Mahdavian, M. Single and mixed dyslipidaemia in Canadian primary care settings: Findings from the Canadian primary care sentinel surveillance network database. BMJ Open 2015, 5, e007954. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Lee, J. Prevalence and management of dyslipidemia among Korean adults: KNHANES 2010–2012. J. Korea Acad.-Ind. Coop. Soc. 2015, 16, 7978–7989. [Google Scholar]
- Jeon, M.Y.; Choi, W.H.; Seo, Y.M. Risk factors of dyslipidemia and related factors of medication adherence in Korea adults: KNHANES 2013–2015. J. Korean Biol. Nurs. Sci. 2017, 19, 131–140. [Google Scholar]
- Knopp, R.H.; LaRosa, J.C.; Burkman, R.T., Jr. Contraception and dyslipidemia. Am. J. Obstet. Gynecol. 1993, 168, 1994–2005. [Google Scholar] [CrossRef]
- Carmena, R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am. Heart J. 2005, 150, 859–870. [Google Scholar] [CrossRef]
- Packard, C.J.; Boren, J.; Taskinen, M.-R. Causes and consequences of hypertriglyceridemia. Front. Endocrinol. 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)—The Program. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Feng, H.; Ha, F.; Hu, G.; Wu, Y.; Yu, S.; Ji, Z.; Feng, W.; Wang, T.; Jia, G. Concentration of chromium in whole blood and erythrocytes showed different relationships with serum apolipoprotein levels in Cr (VI) exposed subjects. J. Trace Elem. Med. Biol. 2018, 50, 384–392. [Google Scholar] [CrossRef]
- Mendrick, D.L.; Diehl, A.M.; Topor, L.S.; Dietert, R.R.; Will, Y.; La Merrill, M.A.; Bouret, S.; Varma, V.; Hastings, K.L.; Schug, T.T. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol. Sci. 2018, 162, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef]
- Cho, H.W.; Kim, S.-H.; Park, M.J. An association of blood mercury levels and hypercholesterolemia among Korean adolescents. Sci. Total Environ. 2020, 709, 135965. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Gao, W.; Sun, X.; Xu, S. Comprehensive analysis of heavy metals in soils from Baoshan District, Shanghai: A heavily industrialized area in China. Environ. Earth Sci. 2012, 67, 2331–2343. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res.-Granthaalayah 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Shen, M.; Zeng, G. Heavy metals in soils and sediments from Dongting Lake in China: Occurrence, sources, and spatial distribution by multivariate statistical analysis. Environ. Sci. Pollut. Res. 2018, 25, 13687–13696. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, S.; Kaur, H. Heavy metals toxicity and the environment. J. Pharmacogn. Phytochem. SP1 2019, 247–249. [Google Scholar] [CrossRef]
- Fatema, K.; Shoily, S.S.; Ahsan, T.; Haidar, Z.; Sumit, A.F.; Sajib, A.A. Effects of arsenic and heavy metals on metabolic pathways in cells of human origin: Similarities and differences. Toxicol. Rep. 2021, 8, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Poręba, R.; Gać, P.; Poręba, M.; Andrzejak, R. Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environ. Toxicol. Pharmacol. 2011, 31, 267–277. [Google Scholar] [CrossRef]
- Xu, H.; Mao, Y.; Xu, B.; Hu, Y. Low-level environmental lead and cadmium exposures and dyslipidemia in adults: Findings from the NHANES 2005–2016. J. Trace Elem. Med. Biol. 2021, 63, 126651. [Google Scholar] [CrossRef]
- Kim, K. Blood cadmium concentration and lipid profile in Korean adults. Environ. Res. 2012, 112, 225–229. [Google Scholar] [CrossRef]
- Park, K.; Seo, E. Toenail mercury and dyslipidemia: Interaction with selenium. J. Trace Elem. Med. Biol. 2017, 39, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Kim, D.-W.; Ock, J.; Moon, K.-W.; Park, C.-H. Association between Pb, Cd, and Hg exposure and liver injury among Korean adults. Int. J. Environ. Res. Public Health 2021, 18, 6783. [Google Scholar] [CrossRef] [PubMed]
- NIER. Guidebook of the National Institute of Environmental Research; NIER: Incheon, Korea, 2019. [Google Scholar]
- NIER. Analysis Manual of the National Institute of Environmental Research (Heavy Metals); NIER: Incheon, Korea, 2018. [Google Scholar]
- NIER. Clinical Analysis Manual of the National Institute of Environmental Research; NIER: Incheon, Korea, 2019. [Google Scholar]
- Ma, C.; Schupp, C.; Armstrong, E.; Armstrong, A. Psoriasis and dyslipidemia: A population-based study analyzing the National Health and Nutrition Examination Survey (NHANES). J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1109–1112. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Choi, H.; Shim, J.-S.; Lee, M.H.; Yoon, Y.M.; Choi, D.P.; Kim, H.C. Comparison of formulas for calculating low-density lipoprotein cholesterol in general population and high-risk patients with cardiovascular disease. Korean Circ. J. 2016, 46, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.H.; Heo, H.C.; Jo, S.; Park, C.; Sakong, J. The association between mercury concentrations and lipid profiles in the Korean National Environmental Health Survey (KoNEHS) cycle 3. Ann. Occup. Environ. Med. 2020, 32, e19. [Google Scholar] [CrossRef]
- Virani, S.S. Non-HDL cholesterol as a metric of good quality of care: Opportunities and challenges. Tex. Heart Inst. J. 2011, 38, 160. [Google Scholar]
- Havel, R.J.; Rapaport, E. Management of primary hyperlipidemia. N. Engl. J. Med. 1995, 332, 1491–1498. [Google Scholar] [CrossRef]
- Rana, J.S.; Boekholdt, S.M.; Kastelein, J.J.; Shah, P.K. The role of non-HDL cholesterol in risk stratification for coronary artery disease. Curr. Atheroscler. Rep. 2012, 14, 130–134. [Google Scholar] [CrossRef]
- Ni, W.-Q.; Liu, X.-L.; Zhuo, Z.-P.; Yuan, X.-L.; Song, J.-P.; Chi, H.-S.; Xu, J. Serum lipids and associated factors of dyslipidemia in the adult population in Shenzhen. Lipids Health Dis. 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-E.; Kim, G.B.; Hwang, S.H.; Ha, M.; Lee, K.-M. Longitudinal trends of blood lead levels before and after leaded gasoline regulation in Korea. Environ. Health Toxicol. 2017, 32, e2017019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-González, M.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Usman, A.R.; Lee, S.S.; Abd El-Azeem, S.A.; Choi, B.; Hashimoto, Y.; Yang, J.E. Effects of rapeseed residue on lead and cadmium availability and uptake by rice plants in heavy metal contaminated paddy soil. Chemosphere 2011, 85, 677–682. [Google Scholar] [CrossRef]
- Park, S.; Lee, B.-K. Strong positive association of traditional Asian-style diets with blood cadmium and lead levels in the Korean adult population. Int. J. Environ. Health Res. 2013, 23, 531–543. [Google Scholar] [CrossRef]
- Ademuyiwa, O.; Ugbaja, R.N.; Idumebor, F.; Adebawo, O. Plasma lipid profiles and risk of cardiovascular disease in occupational lead exposure in Abeokuta, Nigeria. Lipids Health Dis. 2005, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Cho, S.-R.; Jeong, I.; Park, J.B.; Shin, M.-Y.; Kim, S.; Kim, J.H. Mercury exposure and associations with hyperlipidemia and elevated liver enzymes: A nationwide cross-sectional survey. Toxics 2020, 8, 47. [Google Scholar] [CrossRef]
- Cho, Y.M. Fish consumption, mercury exposure, and the risk of cholesterol profiles: Findings from the Korea National Health and Nutrition Examination Survey 2010–2011. Environ. Health Toxicol. 2017, 32, e2017014. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M.; Igisu, H. Effects of heavy metals on mitogen-activated protein kinase pathways. Environ. Health Prev. Med. 2002, 6, 210–217. [Google Scholar] [CrossRef]
- Newsholme, P.; Keane, K.N.; Carlessi, R.; Cruzat, V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol.-Cell Physiol. 2019, 317, C420–C433. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; King, G.L. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Baeeri, M.; Bahadar, H.; Soltany-Rezaee-Rad, M.; Gholami, M.; Abdollahi, M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ. Toxicol. Pharmacol. 2015, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.B.; Hafida, S.; Stemmer, P.; Adhami, A.; Leff, T. Lead (Pb) exposure promotes diabetes in obese rodents. J. Trace Elem. Med. Biol. 2017, 39, 221–226. [Google Scholar] [CrossRef]
- Chen, Y.W.; Huang, C.F.; Yang, C.Y.; Yen, C.C.; Tsai, K.S.; Liu, S.H. Inorganic mercury causes pancreatic β-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol. Appl. Pharmacol. 2010, 243, 323–331. [Google Scholar] [CrossRef]
- Schumacher, L.; Abbott, L.C. Effects of methyl mercury exposure on pancreatic beta cell development and function. J. Appl. Toxicol. 2017, 37, 4–12. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Y.-h.; Pi, H.-f.; Gao, P.; Li, M.; Zhang, L.; Pei, L.-p.; Mei, X.; Liu, L.; Zhao, Q. Cadmium exposure is associated with the prevalence of dyslipidemia. Cell. Physiol. Biochem. 2016, 40, 633–643. [Google Scholar] [CrossRef]
- Lee, B.-K.; Kim, Y. Association of blood cadmium level with metabolic syndrome after adjustment for confounding by serum ferritin and other factors: 2008–2012 Korean National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2016, 171, 6–16. [Google Scholar] [CrossRef]
- Noor, N.; Zong, G.; Seely, E.W.; Weisskopf, M.; James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in US adults: The National Health and Nutrition Examination Survey 2001–2014. Environ. Int. 2018, 121, 349–356. [Google Scholar] [CrossRef]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, F.; Lei, Y. Dietary intake and urinary level of cadmium and breast cancer risk: A meta-analysis. Cancer Epidemiol. 2016, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
| n (%) | TC (mg/dL (SE)) | TG (mg/dL (SE)) | LDL-C (mg/dL (SE)) | Non-HDL-C (mg/dL (SE)) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 2591 | (100) | 185.70 | (0.97) | 145.42 | (2.17) | 99.10 | (0.87) | 185.70 | (0.97) |
| Sex | ||||||||||
| Male | 1111 | (49.4) | 182.52 | (1.67) | 145.45 | (3.08) | 95.00 | (1.51) | 128.48 | (1.63) |
| Female | 1480 | (50.6) | 182.58 | (1.11) | 110.71 | (1.98) | 93.86 | (0.97) | 119.03 | (1.12) |
| p-value | <0.001 | <0.001 | 0.249 | <0.001 | ||||||
| Age group (years) | ||||||||||
| 19–39 | 766 | (29.56) | 175.58 | (1.54) | 116.95 | (3.11) | 88.32 | (1.41) | 115.55 | (1.51) |
| 40–59 | 1152 | (44.46) | 190.12 | (1.20) | 134.35 | (2.34) | 100.57 | (0.99) | 131.07 | (1.14) |
| ≥60 | 673 | (25.97) | 183.13 | (2.06) | 137.16 | (3.53) | 96.81 | (1.83) | 128.63 | (2.07) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| BMI (kg/m2) | ||||||||||
| <18.5 | 82 | (3.92) | 174.05 | (5.25) | 95.39 | (6.33) | 89.15 | (3.62) | 108.23 | (4.01) |
| 18.5–22.9 | 944 | (39.0) | 177.41 | (1.33) | 115.01 | (2.82) | 91.84 | (1.11) | 114.84 | (1.25) |
| 23.0–25.0 | 677 | (25.9) | 191.55 | (1.95) | 154.45 | (3.47) | 104.59 | (1.92) | 135.48 | (2.03) |
| >25 | 888 | (31.8) | 192.36 | (1.75) | 180.91 | (4.00) | 104.61 | (1.56) | 140.80 | (1.64) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Household income (US$/month) | ||||||||||
| Low (840) | 332 | (12.81) | 180.64 | (3.08) | 139.78 | (5.73) | 94.08 | (2.75) | 126.74 | (2.97) |
| Middle (840–4220) | 1777 | (68.58) | 181.64 | (1.09) | 124.58 | (2.23) | 94.05 | (1.01) | 122.59 | (1.08) |
| High (≥4220) | 482 | (18.60) | 185.86 | (2.02) | 129.04 | (4.42) | 95.64 | (1.78) | 125.65 | (1.98) |
| p-value | 0.063 | 0.006 | 0.729 | 0.103 | ||||||
| Education levels | ||||||||||
| ≤Middle school | 641 | (24.74) | 187.29 | (1.69) | 135.53 | (2.99) | 99.84 | (1.60) | 131.36 | (1.60) |
| High school | 847 | (32.69) | 184.23 | (1.47) | 130.27 | (3.16) | 96.52 | (1.19) | 126.35 | (1.40) |
| ≥College | 1103 | (42.57) | 180.52 | (1.37) | 122.76 | (2.66) | 92.05 | (1.31) | 120.35 | (1.32) |
| p-value | 0.001 | 0.025 | <0.001 | <0.001 | ||||||
| Smoking status | ||||||||||
| Never | 1694 | (65.38) | 181.05 | (1.05) | 116.05 | (2.03) | 93.44 | (0.92) | 120.01 | (1.06) |
| Former | 455 | (17.56) | 187.55 | (2.11) | 149.54 | (4.36) | 99.08 | (1.81) | 133.42 | (1.93) |
| Current | 442 | (17.06) | 183.27 | (2.15) | 145.87 | (5.08) | 93.81 | (1.88) | 127.41 | (2.21) |
| p-value | 0.002 | <0.001 | 0.001 | <0.001 | ||||||
| Drinking status | ||||||||||
| Never | 783 | (30.22) | 182.08 | (1.57) | 123.99 | (4.23) | 95.32 | (1.44) | 123.86 | (1.69) |
| Light | 889 | (34.31) | 180.61 | (1.78) | 118.92 | (3.07) | 95.37 | (1.48) | 122.64 | (1.77) |
| Heavy | 919 | (35.47) | 184.57 | (1.42) | 135.69 | (3.51) | 93.06 | (1.40) | 124.31 | (1.40) |
| p-value | 0.118 | <0.001 | 0.099 | 0.760 | ||||||
| Physical activity | ||||||||||
| None | 1458 | (56.27) | 182.55 | (1.21) | 126.51 | (2.32) | 94.40 | (1.11) | 123.51 | (1.27) |
| Moderate | 204 | (7.87) | 184.92 | (3.00) | 138.31 | (7.15) | 93.85 | (2.86) | 125.82 | (3.57) |
| Hardly | 929 | (35.85) | 182.08 | (1.70) | 124.70 | (3.00) | 94.58 | (1.48) | 123.31 | (1.65) |
| p-value | 0.631 | 0.014 | 0.856 | 0.575 | ||||||
| n | BPb (μg/dL (95% CIs)) | BHg (μg/L (95% CIs)) a | UHg (μg/L (95% CIs)) a | UCd (μg/L (95% CIs)) | |||||
|---|---|---|---|---|---|---|---|---|---|
| TC (mg/dL) | |||||||||
| <200 | 1703 | 1.50 | (1.46, 1.55) | 2.56 | (2.41, 2.71) | 0.38 | (0.36, 0.41) | 0.39 | (0.36, 0.42) |
| ≥200 | 888 | 1.64 | (1.57, 1.71) * | 3.06 | (2.89, 3.24) * | 0.42 | (0.39, 0.45) | 0.43 | (0.39, 0.47) |
| TG (mg/dL) | |||||||||
| <150 | 2254 | 1.45 | (1.40, 1.50) | 2.53 | (2.38, 2.68) | 0.39 | (0.36, 0.41) | 0.38 | (0.35, 0.42) |
| ≥150 | 337 | 1.72 | (1.65, 1.79) * | 3.02 | (2.85, 3.20) * | 0.40 | (0.38, 0.43) | 0.43 | (0.39, 0.47) |
| LDL-C (mg/dL) | |||||||||
| <160 | 2168 | 1.52 | (1.47, 1.56) | 2.62 | (2.48, 2.76) | 0.38 | (0.36, 0.40) | 0.39 | (0.36, 0.42) |
| ≥160 | 423 | 1.72 | (1.63, 1.83) * | 3.32 | (3.10, 3.56) * | 0.46 | (0.41, 0.51) * | 0.48 | (0.43, 0.53) * |
| Non-HDL-C (mg/dL) | |||||||||
| <130 | 2091 | 1.51 | (1.46, 1.55) | 2.61 | (2.48, 2.76) | 0.38 | (0.36, 0.40) | 0.39 | (0.36, 0.42) |
| ≥130 | 500 | 1.76 | (1.67, 1.86) * | 3.20 | (2.97, 3.45) * | 0.45 | (0.41, 0.50) * | 0.45 | (0.40, 0.50) * |
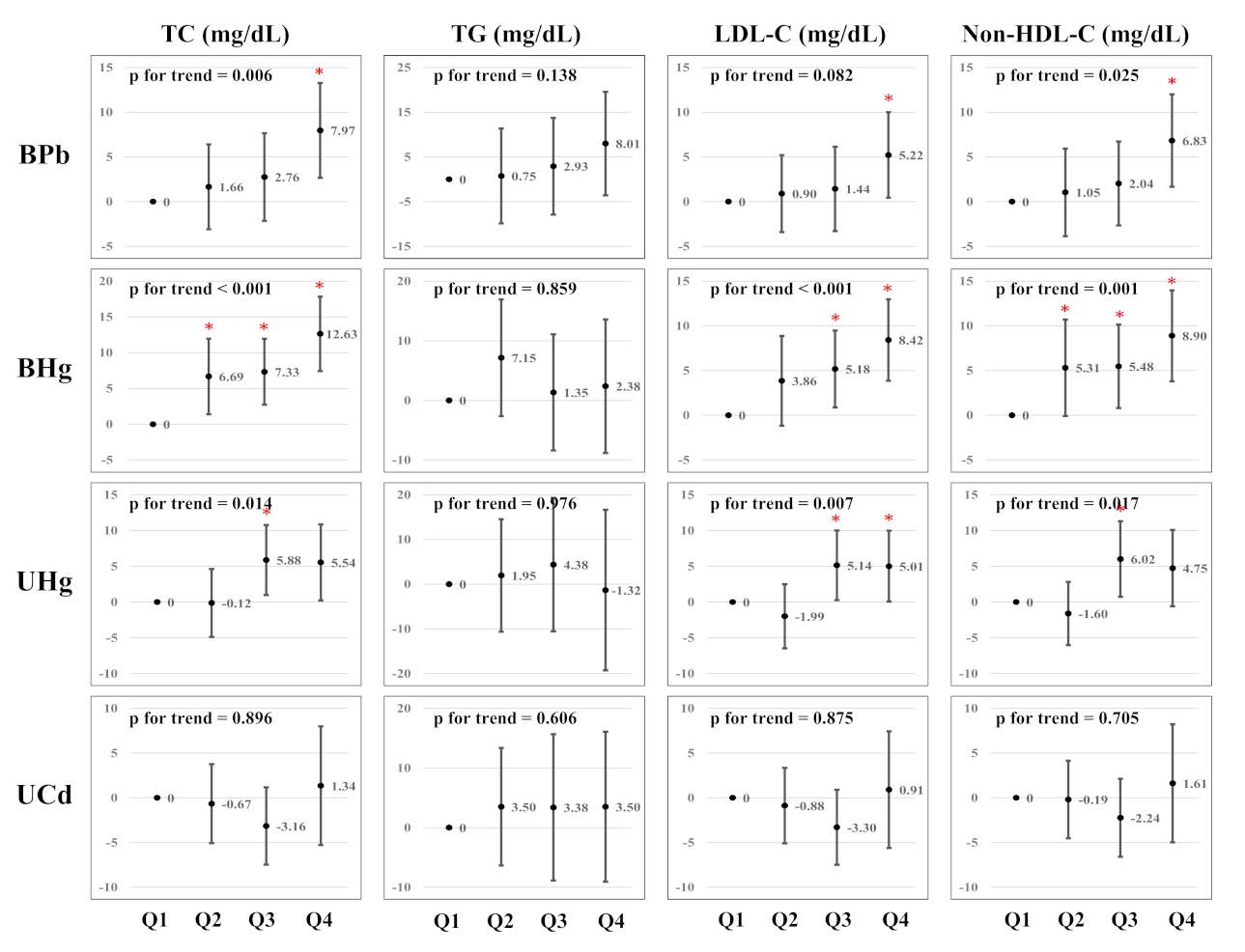
| β (95% CI) of Serum Lipid Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Elevated TC (≥200 mg/dL) | Elevated TG (≥150 mg/dL) | Elevated LDL-C (≥130 mg/dL) | Elevated Non-HDL-C (≥160 mg/dL) | ||||||
| BPb (μg/dL) | ||||||||||
| Q1 | (0.37–1.21) | 651 | Reference | Reference | Reference | Reference | ||||
| Q2 | (1.21–1.65) | 644 | 1.05 | (0.76, 1.45) | 1.11 | (0.77, 1.62) | 0.96 | (0.59, 1.53) | 1.03 | (0.66, 1.60) |
| Q3 | (1.65–2.23) | 647 | 1.14 | (0.87, 1.50) | 1.13 | (0.78, 1.65) | 1.24 | (0.84, 1.84) | 1.27 | (0.84, 1.92) |
| Q4 | (2.23–20.58) | 649 | 1.49 | (1.07, 2.08) * | 1.43 | (0.98, 2.08) | 1.57 | (1.02, 2.40) * | 1.71 | (1.09, 2.68) * |
| p for trend | 0.084 | 0.249 | 0.041 | 0.049 | ||||||
| BHg (μg/L) a | ||||||||||
| Q1 | (0.33–1.85) | 647 | Reference | Reference | Reference | Reference | ||||
| Q2 | (1.86–2.77) | 648 | 1.17 | (0.86, 1.59) | 1.23 | (0.81, 1.56) | 1.63 | (1.08, 2.45) * | 1.20 | (0.80, 1.79) |
| Q3 | (2.77–4.30) | 649 | 1.21 | (0.87, 1.68) | 1.00 | (0.73, 1.37) | 1.77 | (1.17, 2.68) * | 1.02 | (0.64, 1.64) |
| Q4 | (4.30–60.60) | 647 | 1.72 | (1.21, 2.44) * | 0.86 | (0.59, 1.24) | 2.21 | (1.49, 3.28) * | 1.52 | (1.01, 2.28) * |
| p for trend | 0.016 | 0.478 | 0.012 | 0.011 | ||||||
| UHg (μg/L) a | ||||||||||
| Q1 | (0.10–0.23) | 655 | Reference | Reference | Reference | Reference | ||||
| Q2 | (0.24–0.35) | 641 | 0.87 | (0.64, 1.18) | 0.95 | (0.66, 1.36) | 0.89 | (0.55, 1.44) | 0.79 | (0.51, 1.20) |
| Q3 | (0.36–0.64) | 648 | 1.19 | (0.85, 1.66) | 1.10 | (0.74, 1.62) | 1.36 | (0.88, 2.11) | 1.32 | (0.86, 2.02) |
| Q4 | (0.65–8.70) | 647 | 1.32 | (0.88, 1.96) | 0.77 | (0.52, 1.15) | 1.55 | (0.98, 2.45) | 1.37 | (0.89, 2.10) |
| p for trend | 0.154 | 0.185 | 0.055 | 0.037 | ||||||
| UCd (μg/L) | ||||||||||
| Q1 | (0.05–0.22) | 688 | Reference | Reference | Reference | Reference | ||||
| Q2 | (0.22–0.45) | 607 | 1.00 | (0.70, 1.43) | 1.18 | (0.88, 1.58) | 0.84 | (0.58, 1.22) | 1.16 | (0.65, 2.06) |
| Q3 | (0.45–0.87) | 648 | 0.78 | (0.56, 1.09) | 1.06 | (0.74, 1.52) | 0.61 | (0.39, 0.94) | 1.72 | (0.95, 3.11) |
| Q4 | (0.87–16.82) | 648 | 0.86 | (0.59, 1.24) | 1.14 | (0.83, 1.56) | 0.85 | (0.56, 1.29) | 1.58 | (0.83, 2.99) |
| p for trend | 0.396 | 0.657 | 0.661 | 0.172 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-w.; Ock, J.; Moon, K.-W.; Park, C.-H. Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017. Int. J. Environ. Res. Public Health 2022, 19, 3181. https://doi.org/10.3390/ijerph19063181
Kim D-w, Ock J, Moon K-W, Park C-H. Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017. International Journal of Environmental Research and Public Health. 2022; 19(6):3181. https://doi.org/10.3390/ijerph19063181
Chicago/Turabian StyleKim, Do-won, Jeongwon Ock, Kyong-Whan Moon, and Choong-Hee Park. 2022. "Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017" International Journal of Environmental Research and Public Health 19, no. 6: 3181. https://doi.org/10.3390/ijerph19063181






