Biological, Psychological, and Physical Performance Variations in Football Players during the COVID-19 Lockdown: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Training Program
2.4. Anthropometric Evaluation
2.5. Blood Parameters
2.6. Physical Performances
2.7. Statistical Analysis
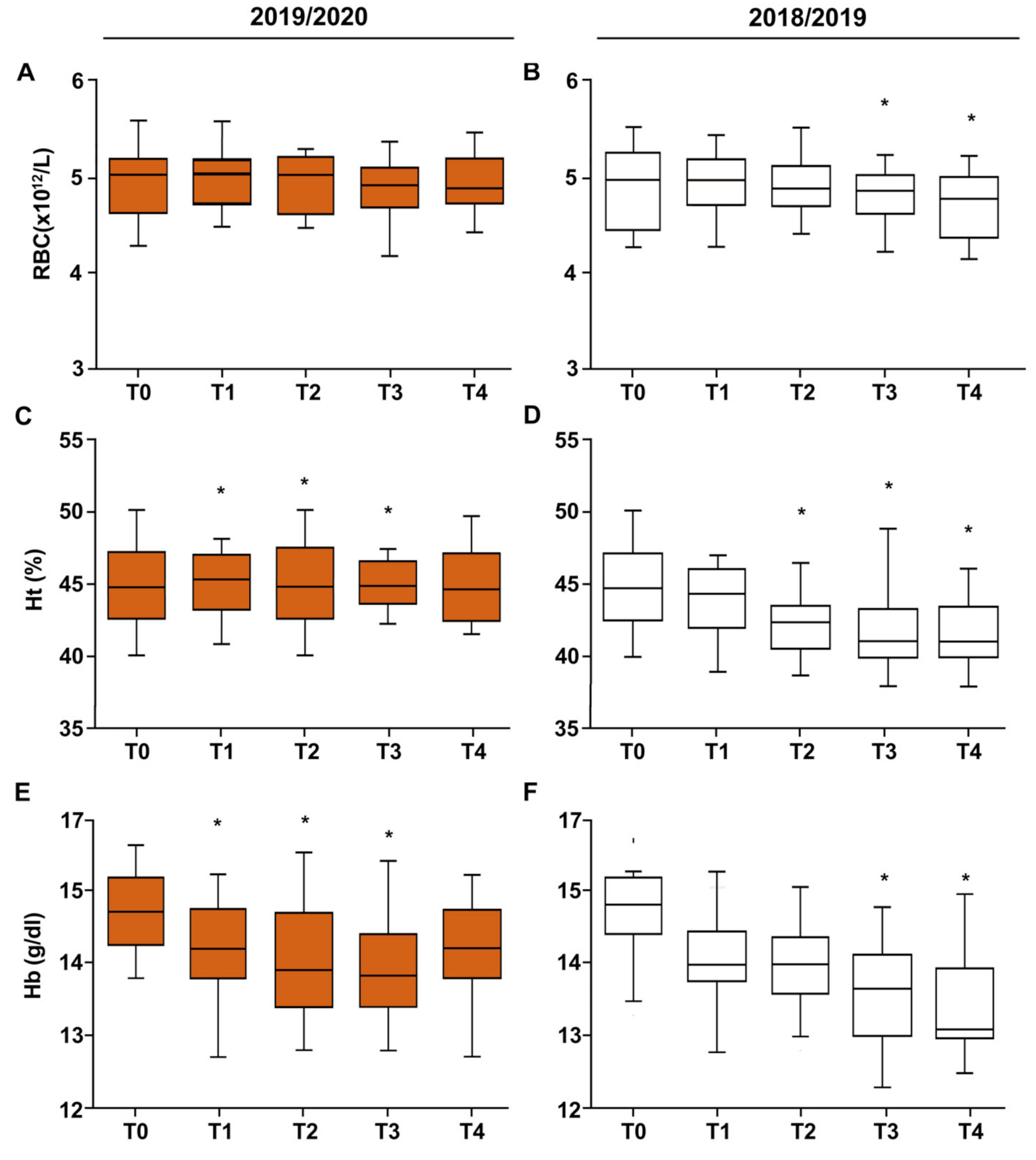
3. Results
3.1. Anthropometric Characteristics of Football Players
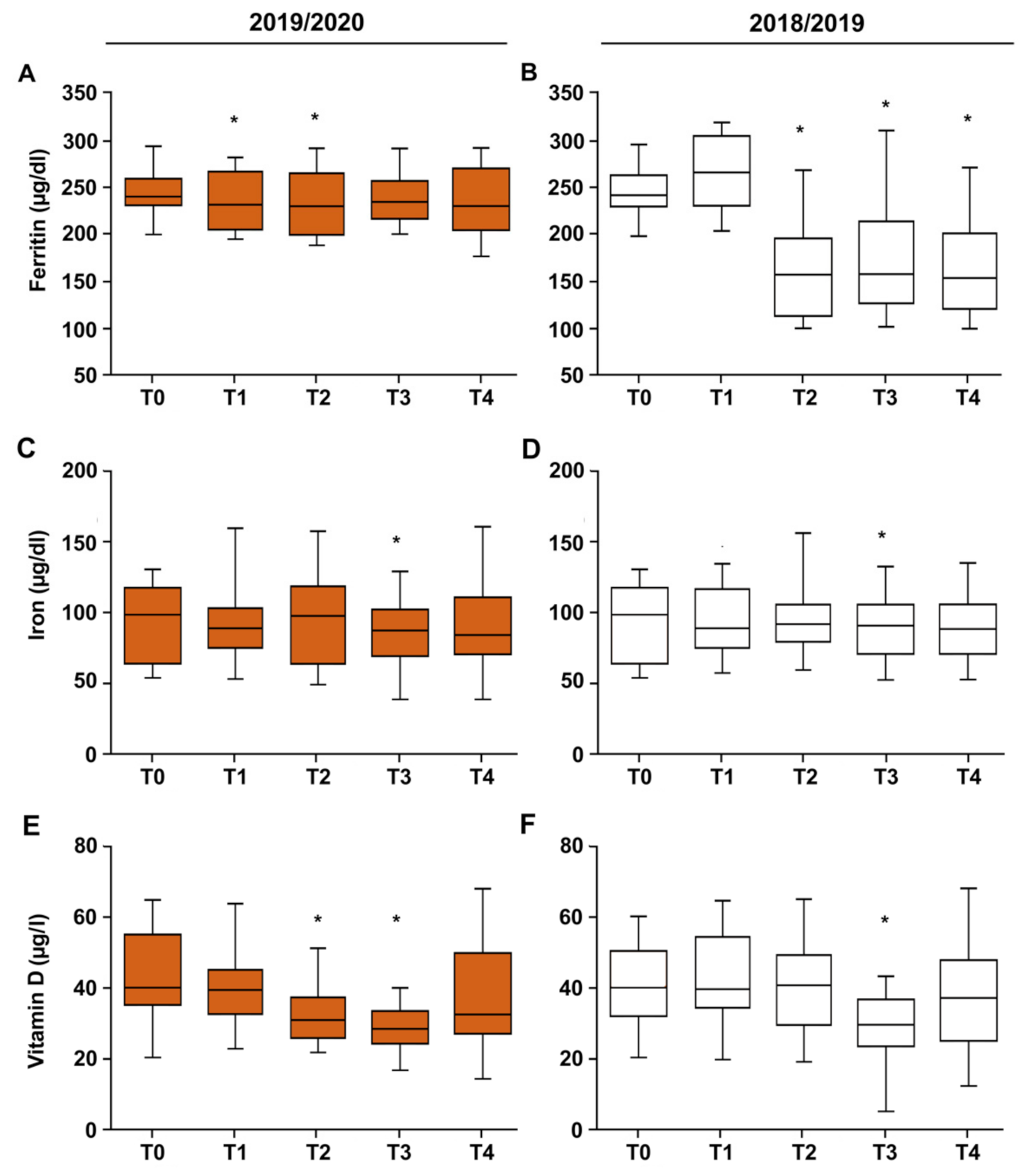
3.2. Vitamin D–Iron–Ferritin
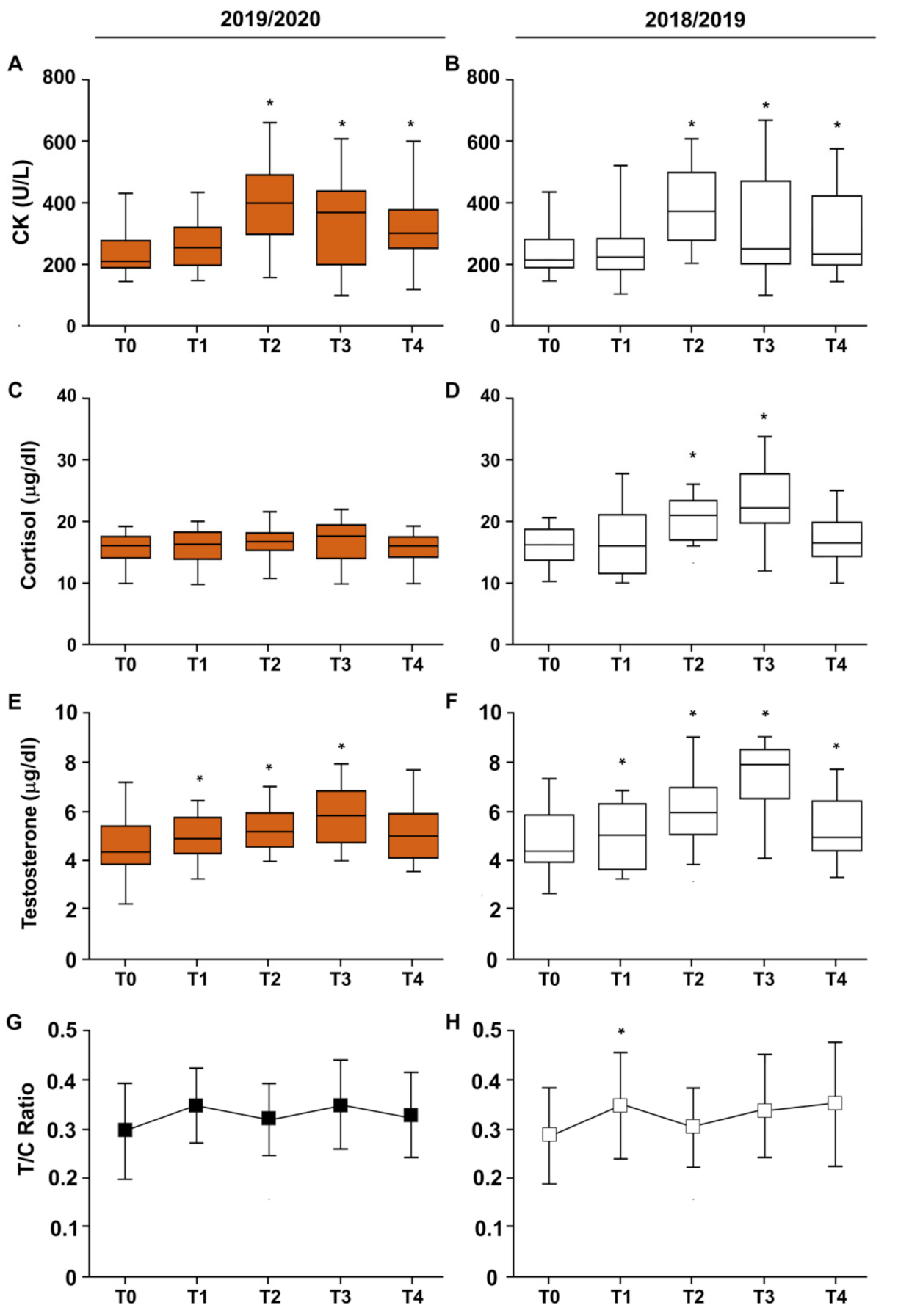
3.3. CK–Cortisol–Testosterone–T/C Ratio
3.4. Electrolytes
3.5. Physical Performances
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, M.; Sax, P.E. Challenges of “return to work” in an ongoing pandemic. N. Engl. J. Med. 2020, 383, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Mehrsafar, A.H.; Gazerani, P.; Zadeh, A.M.; Sánchez, J.C.J. Addressing potential impact of COVID-19 pandemic on physical and mental health of elite athletes. Brain Behav. Immun. 2020, 87, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.E.; Insigne, K.D.; Jones, E.M.; Hastings, Q.A.; Boldridge, W.C.; Kosuri, S. Dissection of c-AMP Response Element Architecture by Using Genomic and Episomal Massively Parallel Reporter Assays. Cell Syst. 2020, 11, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A.; Jönsson, C.; Kenttä, G. Psychological distress and problem gambling in elite athletes during COVID-19 restrictions—A web survey in top leagues of three sports during the pandemic. Int. J. Environ. Res. Public Health 2020, 17, 6693. [Google Scholar] [CrossRef]
- Marotta, N.; de Sire, A.; Gimigliano, A.; Demeco, A.; Moggio, L.; Vescio, A.; Iona, T.; Ammendolia, A. Impact of COVID-19 lockdown on the epidemiology of soccer muscle injuries in Italian Serie A professional football players. J. Sports Med. Phys. Fit. 2022, 62, 356–360. [Google Scholar] [CrossRef]
- De Souza, D.B.; Gonzalez-Garca, J.; Campo, R.L.D. Players’ physical performance in LaLiga across the season: Insights for competition continuation after COVID-19. Biol. Sport 2021, 38, 3–7. [Google Scholar] [CrossRef]
- Leguizamo, F.; Olmedilla, A.; Nez, A. Personality, Coping Strategies, and Mental Health in High- Performance Athletes during Confinement Derived From the COVID-19 Pandemic. Front. Public Health 2021, 8, 561198. [Google Scholar] [CrossRef]
- Nevill, A.M.; Metsios, G.S.; Jackson, A.S.; Wang, J.; Thornton, J.; Gallagher, D. Can we use the Jackson and Pollock equations to predict body density/fat of obese individuals in the 21st century? Int. J. Body Compos. Res. 2008, 6, 114–121. [Google Scholar]
- Saidi, K.; Abderrahman, A.B.; Boullosa, D.; Dupont, G.; Hackney, A.C.; Bideau, B.; Pavillon, T.; Granacher, U.; Zouhal, H. The Interplay between plasma hormonal concentrations, physical fitness, workload and mood state changes to periods of congested match play in professional soccer players. Front. Physiol. 2020, 11, 835. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Spedicato, M.; Stefàno, E.; Marsigliante, S. The effects of training on hormonal concentrations in young soccer players. J. Cell. Physiol. 2019, 234, 20685–20693. [Google Scholar] [CrossRef]
- Impellizzeri, F.M.; Rampinini, E.; Marcora, S.M. Physiological assessment of aerobic training in soccer. J. Sports Sci. 2005, 23, 583–592. [Google Scholar] [CrossRef]
- Neumann, G.; Schmidt, H.; Fröhner, G. Development of functional stability of the musculoskeletal system in athletes in childhood and adolescence. Arztl. Jugendkd. 1989, 80, 73–79. [Google Scholar]
- Eichner, E.R. Runner’s macrocytosis: A clue to footstrike hemolysis. Runner’s anemia as a benefit versus runner’s hemolysis as a detriment. Am. J. Med. 1985, 78, 321–325. [Google Scholar] [CrossRef]
- Hunding, A.; Jordal, R.; Paulev, P.E. Runner’s anemia and iron deficiency. Acta Med. Scand. 1981, 209, 315–318. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liu, L.; Liu, S.; Li, X.; Zhou, T.; Zhang, J. Vitamin D deficiency and the risk of anemia: A meta-analysis of observational studies. Ren. Fail. 2015, 37, 929–934. [Google Scholar] [CrossRef]
- Silva, J.R.; Rebelo, A.; Marques, F.; Pereira, L.; Seabra, A.; Ascensão, A. Biochemical impact of soccer: An analysis of hormonal, muscle damage, and redox markers during the season. Appl. Physiol. Nutr. Metlab. 2014, 39, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Meister, S.; Faude, O.; Ammann, T.; Schnittker, R.; Meyer, T. Indicators for high physical strain and overload in elite football players. Scand. J. Med. Sci. Sports 2013, 23, 156–163. [Google Scholar] [CrossRef]
- Requena, B.; García, I.; Suárez-Arrones, L.; Sáez de Villarreal, E.; Naranjo Orellana, J.; Santalla, A. Off-Season Effects on Functional Performance, Body Composition, and Blood Parameters in Top-Level Professional Soccer Players. J. Strength Cond. Res. 2017, 31, 939–946. [Google Scholar] [CrossRef]
- Jaspers, A.; De Beéck, T.O.; Brink, M.S.; Frencken, W.G.P.; Staes, F.; Davis, J.J.; Helsen, W.F. Relationships Between the External and Internal Training Load in Professional Soccer: What Can We Learn from Machine Learning? Int. J. Sports Physiol. Perform. 2018, 13, 625–630. [Google Scholar] [CrossRef]
- Demarie, S.; Galvani, C.; Billat, V.L. Horse-Riding Competitions Pre and Post COVID-19: Effect of Anxiety, sRPE and HR on Performance in Eventing. Int. J. Environ. Res. Public Health 2020, 17, 8648. [Google Scholar] [CrossRef]
- Milsom, J.; Naughton, R.; O’Boyle, A.; Iqbal, Z.; Morgans, R.; Drust, B. Body composition assessment of English Premier League soccer players: A comparative DXA analysis of first team, U21 and U18 squads. J. Sports Sci. 2015, 33, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Suhr, F.; Porten, S.; Hertrich, T.; Brixius, K.; Schmidt, A.; Platen, P. Intensive exercise induces changes of endothelial nitric oxide synthase pattern in human erythrocytes. Nitric Oxide 2009, 20, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Klausen, K. Development of aerobic power in pubescent male soccer players related to hematocrit, hemoglobin and maturation: A longitudinal study. J. Sports Med. Phys. Fit. 2004, 44, 219. [Google Scholar]
- Hu, M.; Finni, T.; Sedliak, M.; Zhou, W.; Alen, M.; Cheng, S. Seasonal variation of red blood cell variables in physically inactive men: Effects of strength training. Int. J. Sports Med. 2008, 29, 564–568. [Google Scholar] [CrossRef]
- Bekris, E.; Gioldasis, A.; Gissis, I.; Anagnostakos, K.; Eleftherios, M. From Preparation to Competitive Period in Soccer: Hematological Changes. Sport Sci. Rev. 2015, 24, 103–114. [Google Scholar] [CrossRef]
- Banfi, G.; Mauri, C.; Morelli, B.; Di Gaetano, N.; Malgeri, U.; Melegati, G. Reticulocyte count, mean reticulocyte volume, immature reticulocyte fraction, and mean sphered cell volume in elite athletes: Reference values and comparison with the general population. Clin. Chem. Lab. Med. 2006, 44, 616–622. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381. [Google Scholar] [CrossRef]
- Convertino, V.A.; Brock, P.J.; Keil, L.C.; Bernauer, E.M.; Greenleaf, J.E. Exercise training-induced hypervolemia: Role of plasma albumin, renin, and vasopressin. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 665–669. [Google Scholar] [CrossRef]
- Rago, V.; Leo, I.; Marrocco, A.; Izzo, R.; Filetti, C. Blood and performance adaptations to individual training load in professional soccer: A team study. J. Sports Med. Phys. Fit. 2021. [Google Scholar] [CrossRef]
- Silva, A.S.; Santhiago, V.; Papoti, M.; Gobatto, C.A. Hematological parameters and anaerobic threshold in Brazilian soccer players throughout a training program. Int. J. Lab. Hematol. 2008, 30, 158–166. [Google Scholar] [CrossRef]
- Heisterberg, M.F.; Fahrenkrug, J.; Krustrup, P.; Storskov, A.; Kjær, M.; Andersen, J.L. Extensive monitoring through multiple blood samples in professional soccer players. J. Strength Cond. Res. 2013, 27, 1260–1271. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ahmetovic, Z. Indicators of iron status in elite soccer players during the sports season. Int. J. Lab. Hematol. 2009, 31, 447–452. [Google Scholar] [CrossRef]
- Maria, T.S.; de Arruda, M.; Portella, D.; Vitoria, R.V.; Campos, R.G.; Martinez, C. Hematological parameters of elite soccer players during the competitive period. J. Exerc. Physiol. Online 2013, 16, 68–77. [Google Scholar]
- Thirup, P. Haematocrit: Within-subjected and seasonal variation. Sports Med. 2003, 33, 231–243. [Google Scholar] [CrossRef]
- Sawka, M.N.; Convertino, V.A.; Schneider, E.R.; Schnieder, S.M.; Young, A.J. Blood Volume: Importance and Adaptations to Exercise Training, Environmental Stresses and Trauma Sickness; U.S. Army Institute of Surgical Research: Fort Sam Houston, TX, USA, 2000; Volume 32, pp. 332–348. [Google Scholar]
- Escanero, J.F.; Villanueva, J.; Rojo, A.; Herrera, A.; Del Diego, C.; Guerra, M. Iron stores in professional athletes throughout the sports season. Physiol. Behav. 1997, 62, 811–814. [Google Scholar] [CrossRef]
- Banfi, G.; del Fabbro, M.; Mauri, C.; Corsi, M.M.; Melegati, G. Haematological parameters in elite rugby players during a competitive season. Clin. Lab. Haematol. 2006, 28, 183–188. [Google Scholar] [CrossRef]
- Schumacher, Y.O.; Schmid, A.; Grathwohl, D.; Bultermann, D.; Berg, A. Hematological indices and iron status in athletes of various sports and performances. Med. Sci. Sports Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef]
- Malcovati, L.; Pascutto, C.; Cazzola, M. Hematologic passport for athletes competing in endurance sports: A feasibility study. Haematologica 2003, 88, 570–581. [Google Scholar]
- Meneghini, R. Iron homeostasis, oxidative stress and DNA damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Quan, M.; Cao, Z.B. Effect of Vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.W.; Song, J.K.; Jung, H.C.; Kim, S.W.; Kim, J.H.; Lee, J.M. The associations of vitamin D status with athletic performance and blood-borne markers in adolescent athletes: A cross-sectional study. Int. J. Environ. Res. Public Health 2019, 16, 3422. [Google Scholar] [CrossRef] [Green Version]
- Ksiazek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes—A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.P.; Iqbal, Z.; Drust, B.; Burgess, D.; Close, G.L.; Brukner, P.D. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl. Physiol. Nutr. Metab. 2012, 37, 798–802. [Google Scholar] [CrossRef] [Green Version]
- Galan, F.; Ribas, J.; Sánchez-Martinez, P.M.; Calero, T.; Sánchez, A.B.; Muñoz, A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin. Nutr. 2012, 31, 132–136. [Google Scholar] [CrossRef]
- Książek, A.; Zagrodna, A.; Bohdanowicz-Pawlak, A.; Lwow, F.; Słowińska-Lisowska, M. Relationships between Vitamin D and Selected Cytokines and Hemogram Parameters in Professional Football Players-Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7124. [Google Scholar] [CrossRef]
- Qu, C.; Wu, Z.; Xu, M.; Qin, F.; Dong, Y.; Wang, Z.; Zhao, J. Cryotherapy Models and Timing-Sequence Recovery of Exercise-Induced Muscle Damage in Middle- and Long-Distance Runners. J. Athl. Train. 2020, 55, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Reichel, T.; Boßlau, T.K.; Palmowski, J.; Eder, K.; Ringseis, R.; Mooren, F.C.; Walscheid, R.; Bothur, E.; Samel, S.; Frech, T.; et al. Reliability and suitability of physiological exercise response and recovery markers. Sci. Rep. 2020, 10, 11924. [Google Scholar] [CrossRef]
- Silva, J.R.; Rumpf, M.C.; Hertzog, M.; Castagna, C.; Farooq, A.; Girard, O.; Hader, K. Acute and Residual Soccer Match-Related Fatigue: A Systematic Review and Meta-analysis. Sports Med. 2018, 48, 539–583. [Google Scholar] [CrossRef]
- Souglis, A.; Bogdanis, G.C.; Chryssanthopoulos, C.; Apostolidis, N.; Geladas, N.D. Time Course of Oxidative Stress, Inflammation, and Muscle Damage Markers for 5 Days After a Soccer Match: Effects of Sex and Playing Position. J. Strength Cond. Res. 2018, 32, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Jukić, I. Muscle Damage during a Soccer World Cup Preparatory and Competition Period. Int. J. Sports Physiol. 2019, 2, 496–502. [Google Scholar] [CrossRef] [PubMed]
- García-Romero-Pérez, Á.; Ordonez, F.J.; Reyes-Gil, F.; Rodríguez-López, E.S.; Oliva-Pascual-Vaca, Á. Muscle Damage Biomarkers in Congestion Weeks in English Premier League Soccer Players: A Prospective Study for Two Consecutive Seasons. Int. J. Environ. Res. Public Health 2021, 18, 7960. [Google Scholar] [CrossRef] [PubMed]
- Mougios, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 2007, 41, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Bangsbo, J.; Mohr, M.; Krustrup, P. Physical and metabolic demands of training and match-play in the elite football player. J. Sports Sci. 2006, 24, 665–674. [Google Scholar] [CrossRef]
- Fry, A.C.; Kraemer, W.J.; Stone, M.H.; Warren, B.J.; Kearney, J.T.; Maresh, C.M.; Weseman, C.A.; Fleck, S.J. Endocrine and performance responses to high volume training and amino acid supplementation in elite junior weightlifters. Int. J. Sport Nutr. Exerc. Metab. 1993, 3, 306–322. [Google Scholar] [CrossRef]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef]
- Andrzejewski, M.; Podgórski, T.; Kryściak, J.; Chmura, P.; Konefał, M.; Chmura, J.; Marynowicz, J.; Adrian, J.; Pluta, B. Anabolic–catabolic hormonal responses in youth soccer players during a half-season. Res. Sports Med. 2020, 29, 141–154. [Google Scholar] [CrossRef]
- Kraemer, W.J. Endocrine responses to resistance exercise. In Essentials of Strength Training and Conditioning; Human Kinetic Press: Champaign, IL, USA, 2000; pp. 91–114. [Google Scholar]
- Michailidis, Y. Stress hormonal analysis in elite soccer players during a season. J. Sports Health Sci. 2014, 3, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Kamran, A.; Verma, S.; Ahmad, I.; Singla, D.; Saleem, M.; Hussein, M.E. Comparison of complex versus contrast training on steroid hormones and sports performance in male soccer players. J. Chiropr. Med. 2019, 18, 131–138. [Google Scholar]
- Cooke, J.E.; Eirich, R.; Racine, N.; Madigan, S. Prevalence of posttraumatic and general psychological stress during COVID-19: A rapid review and meta-analysis. Psychiatry Res. 2020, 292, 113347. [Google Scholar] [CrossRef]
- Cay, M.; Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. North. Clin. Istanb. 2018, 5, 295–301. [Google Scholar]
- Madsen, E.E.; Krustrup, P.; Larsen, C.H.; Elbe, A.M.; Wikman, J.M.; Ivarsson, A.; Lautenbach, F. Resilience as a protective factor for well-being and emotional stability in elite-level football players during the first wave of the COVID-19 pandemic. Sci. Med. Footb. 2021, 5 (Suppl. S1), 62–69. [Google Scholar] [CrossRef]
- De Souza, G.; dos Santos, W.; Paes, P.; Fidelix, Y.; de Paula Oliveira, L.; da Costa, M.; dos Santos, W. Consequences of COVID-19 preventive measures on anxiety, stress and depression in football athletes. Soccer Soc. 2021, 1–9. [Google Scholar] [CrossRef]
- Demarie, S.; Chirico, E.; Galvani, C. Prediction and Analysis of Tokyo Olympic Games Swimming Results: Impact of the COVID-19 Pandemic on Swimmers’ Performance. Int. J. Environ. Res. Public Health 2022, 19, 2110. [Google Scholar] [CrossRef]
- Saidi, K.; Abderrahman, A.B.; Hackney, A.C.; Bideau, B.; Zouita, S.; Granacher, U.; Zouhal, H. Hematology, Hormones, Inflammation, and Muscle Damage in Elite and Professional Soccer Players: A Systematic Review with Implications for Exercise. Sports Med. 2021, 51, 2607–2627. [Google Scholar] [CrossRef]
- Hammami, M.A.; Ben Abderrahman, A.; Hackney, A.C.; Kebsi, W.; Owen, A.L.; Nebigh, A.; Racil, G.; Tabka, Z.; Zouhal, H. Hormonal (Cortical-Gonadotropic Axis) and Physical Changes with Two Years Intense Exercise Training in Elite Young Soccer Players. J. Strength Cond. Res. 2017, 31, 2388–2397. [Google Scholar] [CrossRef]
- Filaire, E.; Bernain, X.; Sagnol, M.; Lac, G. Preliminary results on mood state, salivary testosterone: Cortisol ratio and team performance in a professional soccer team. Eur. J. Appl. Physiol. 2001, 86, 179–184. [Google Scholar]
- Opaszowski, B.H.; Tyc, Z.; Obmiński, Z.; Danielik, T.; Korkuć, M.; Długołȩcka, B. The influence of a 7-week preparatory period on hormonal and metabolic responses in soccer players/metabolic and hormonal response to training. Pol. J. Sport Tour. 2012, 19, 178–183. [Google Scholar] [CrossRef] [Green Version]

| PERIOD | Weeks (n) | Weekly Volume Mean of Training + Matches (min) | Official Matches/Week (n) | Training Sessions/Week (n) | Training Distribution (%) | Training Week Activities Description | ||
|---|---|---|---|---|---|---|---|---|
| Aerobic | Anaerobic | Other | ||||||
| 2018/2019 SEASON | 50 | 571 (min 526–max 616) | ½ | 5 | 35 | 20 | 45 | ~3 high-intensity technical tacticalsessions including simulated soccer matches + 2 or 3 low-intensity technical-tactical sessions + 1 or 2 speed training sessions + 1 or 2 strength training sessions in the gym |
| 2019/2020 SEASON (From week 1 to week 34 and from week 51 to week 56) | 40 | 571 (min 526–max 616) | ½ | 5 | 35 | 20 | 45 | ~3 high-intensity technical tacticalsessions including simulated soccer matches + 2 or 3 low-intensity technical-tactical sessions + 1 or 2 speed training sessions + 1 or 2 strength training sessions in the gym |
| LOCKDOWN (From week 35 to week 50) | 16 | 0 | 5 | 60 | 30 | 10 | 4 or 5 aerobic sessions performed at home with fixed devices (treadmill or bike) or with bodyweight + 2 or 3 strength training sessions using body weight and small weights + 1 or 2 running sessions, (close to home and individually) | |
| Parameters | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| COVID season | |||||
| WEIGHT (kg) | 86.3 ± 6.0 | 86.9 ± 6.5 | 86.3 ± 6.5 | 87.7 ± 7.4 § | 86.8 ± 7.0 *,° |
| HEIGHT (m) | 1.78 ± 4.9 | 1.78 ± 4.9 | 1.78 ± 4.9 | 1.78 ± 4.9 | 1.78 ± 4.9 |
| BMI (kg/m2) | 24.7 ± 0.6 | 24.6 ± 0.9 | 24.4 ± 0,7 | 24.8 ± 1.0 § | 24.6 ± 0.9 |
| BFP (%) | 9.4 ± 1.8 | 9.1 ± 2.1 | 8.1 ± 1.2 *,# | 7.9 ± 1.4 *,# | 7.9 ± 1.4 *,# |
| FFM (kg) | 78.5 ± 4.6 | 79.5± 5.3 | 80.5 ± 5.9 | 80.3 ± 6.6 *,A | 79.8 ± 6.21 *,A |
| 2018/2019 season | |||||
| WEIGHT (kg) | 85.6 ± 2.5 | 85.0 ± 3.5 | 85.1 ± 3.2 | 84.5 ± 3.0 *,# | 84.0 ± 2.7 *,# |
| HEIGHT (m) | 1.77 ± 5.6 | 1.77 ± 5.6 | 1.77 ± 5.6 | 1.77 ± 5.6 | 1.77 ± 5.6 |
| BMI (kg/m2) | 24.7 ± 1.0 | 24.5 ± 0.4 | 24.6 ± 0.9 | 24.4 ± 1.0 | 24.3 ± 1.1 |
| BFP (%) | 8.9 ± 0.7 | 8.6 ± 1.1 | 8.5 ± 0.9 | 7.9 ± 1.0 *,#,§ | 7.6 ± 1.1 *,# |
| FFM (kg) | 78.2 ± 2.3 | 79.0 ± 3.2 | 80.3 ± 2.2 | 81.6 ± 2.1 *,# | 82.3 ± 2.0 *,# |
| COVID Δ | 2018/2019 Δ | p | |
|---|---|---|---|
| Erythrocytes (M/mm3) | |||
| T0–T1 | 0.02 ± 0.07 | −0.2 ± 0.10 | 0.05 |
| T1–T2 | −0.04 ± 0.35 | −0.04 ± 0.43 | 0.48 |
| T2–T3 | −0.03 ± 0.34 | −0.07 ± 0.16 | 0.26 |
| T3–T4 | 0.05 ± 0.39 | −0.12 ± 0.27 | 0.03 |
| T0–T4 | 0.01 ± 0.37 | −0.25 ± 0.45 | 0.02 |
| Hematocrit (%) | |||
| T0–T1 | 0.26 ± 2.77 | −0.75 ± 3.42 | 0.06 |
| T1–T2 | −0.15 ± 2.79 | −1.74 ± 2.43 | 0.03 |
| T2–T3 | 0.13 ± 2.96 | −0.57 ± 2.81 | 0.22 |
| T3–T4 | 0.03 ± 2.62 | −0.23 ± 0.75 | 0.32 |
| T0–T4 | 0.28 ± 3.35 | −3.28 ± 3.59 | 0.0001 |
| Hemoglobin (g/dL) | |||
| T0–T1 | −0.54 ± 0.77 | −0.57 ± 0.69 | 0.44 |
| T1–T2 | −0.14 ± 1.00 | −0.15 ± 0.72 | 0.49 |
| T2–T3 | −0.12 ± 0.43 | −0.34 ± 0.82 | 0.10 |
| T3–T4 | 0.29 ± 0.83 | −0.32 ± 0.81 | 0.009 |
| T0–T4 | −0.51 ± 0.71 | −1.37 ± 0.84 | 0.001 |
| Δ PV (%) | |||
| T0–T1 | −11.07 ± 9.28 | 5.41 ± 10.19 | 0.000004 |
| T1–T2 | 1.77 ± 9.66 | 4.73 ± 8.79 | 0.05 |
| T2–T3 | 0.87 ± 7.38 | 2.78 ± 8.10 | 0.15 |
| T3–T4 | −1.76 ± 9.31 | 2.58 ± 6.89 | 0.06 |
| T0–T4 | −11.22 ± 9.99 | 15.27 ± 10.16 | 0.0000002 |
| COVID Δ | 2018/2019 Δ | p | |
|---|---|---|---|
| Ferritin | |||
| T0–T1 | −8.72 ± 3.66 | 12.17 ± 3.84 | 0.02 |
| T1–T2 | −3.35 ± 3.98 | −98.91 ± 6.57 | 0.000006 |
| T2–T3 | 5.97 ± 4.00 | 17.35 ± 6.07 | 0.26 |
| T3–T4 | −1.55 ± 0.47 | −14.60 ± 6.41 | 0.23 |
| T0–T4 | −7.65 ± 4.14 | −3.89 ± 5.16 | 0.000001 |
| Iron | |||
| T0–T1 | −3.94 ± 2.98 | −1.54 ± 3.6 | 0.31 |
| T1–T2 | 4.94 ± 3.16 | 1.50 ± 2.98 | 0.31 |
| T2–T3 | −10.20 ± 3.44 | −3.96 ± 2.74 | 0.24 |
| T3–T4 | 7.65 ± 3.79 | −1.00 ± 4.90 | 0.14 |
| T0–T4 | −1.55 ± 3.46 | −5.00 ± 3.22 | 0.33 |
| Vitamin D | |||
| T0–T1 | −4.09 ± 1.53 | −0.37 ± 1.12 | 0.13 |
| T1–T2 | −7.14 ± 1,12 | −1.92 ± 1.83 | 0.10 |
| T2–T3 | −3.29 ± 9.09 | −10.70 ± 1.24 | 0.01 |
| T3–T4 | 8.10 ± 1.57 | 5.39 ± 1.51 | 0.16 |
| T0–T4 | −6.43 ± 1.57 | −7.60 ± 1.65 | 0.16 |
| COVID Δ | 2018/2019 Δ | p | |
|---|---|---|---|
| CPK (U/L) | |||
| T0–T1 | 35.3 ± 8.6 | 3.9 ± 4.2 | 0.05 |
| T1–T2 | 135.5 ± 1.6 | 141.8 ± 1.6 | 0.43 |
| T2–T3 | −64.8 ± 2.13 | −64.8 ± 1.7 | 0.50 |
| T3–T4 | −11.3 ± 2.19 | −8.2 ± 5.7 | 0.47 |
| T0–T4 | 94.7 ± 1.43 | 72.7 ± 1.29 | 0.30 |
| Cortisol (µg/dL) | |||
| T0–T1 | 0.1 ± 3.48 | 1.1 ± 6.01 | 0.19 |
| T1–T2 | 0.7 ± 3.46 | 3.4 ± 6.83 | 0.03 |
| T2–T3 | 0.2 ± 4.26 | 2.9 ± 6.84 | 0.03 |
| T3–T4 | −0.9 ± 3.69 | −6.8 ± 6.21 | 0.0001 |
| T0–T4 | 0.1 ± 3.35 | 0.6 ± 4.02 | 0.23 |
| Testosterone (µg/dL) | |||
| T0–T1 | 0.9 ± 1.81 | 1.0 ± 1.45 | 0.40 |
| T1–T2 | −0.2 ± 1.26 | 0.6 ± 1.05 | 0.002 |
| T2–T3 | 0.5 ± 1.69 | 1.4 ± 1.66 | 0.05 |
| T3–T4 | −0.6 ± 1.66 | −2.0 ± 1.89 | 0.007 |
| T0–T4 | 0.6 ± 1.54 | 1.0 ± 1.45 | 0.14 |
| T/C ratio | |||
| T0–T1 | 5.1 ± 13.67 | 6.0 ± 15.00 | 0.39 |
| T1–T2 | −2.5 ± 10.84 | −4.3 ± 13.54 | 0.30 |
| T2–T3 | 2.9 ± 13.29 | 2.9 ± 11.70 | 0.49 |
| T3–T4 | −2.2 ± 10.58 | 1.8 ± 15.80 | 0.01 |
| T0–T4 | 3.3 ± 10.41 | 6.4 ± 13.05 | 0.20 |
| Parameters | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| 2018/2019 season | |||||
| Magnesium (mg/dL) | 2.25 ± 0.16 | 1.86 ± 0.14 # | 2.04 ± 0.13 #,* | 1.90 ± 0.13 # | 2.15 ± 0.12 *,$ |
| Sodium (mmol/L) | 142.25 ± 3.60 | 142.29 ± 3.59 | 140.17 ± 4.91 | 144.29 ± 3.51 § | 141.25 ± 3.84 $ |
| Potassium (mmol/L) | 4.37 ± 0.30 | 4.38 ± 0.29 | 4.57 ± 0.28 | 4.44 ± 0.23 | 4.41 ± 0.27 |
| 2019/2020 season | |||||
| Magnesium (mg/dL) | 2.25 ± 0.12 | 1.94 ± 0.12 # | 2.03 ± 0.13 # | 2.05 ± 0.25 #,*,A | 2.21 ± 0.17 *,§ |
| Sodium (mmol/L) | 142.25 ± 3.84 | 142.58 ± 1.53 | 140.92 ± 1.32 * | 143.33 ± 1.17 §,A | 141.96 ± 1.52 |
| Potassium (mmol/L) | 4.37 ± 0.27 | 4.36 ± 0.29 | 4.71 ± 0.33 #,* | 4.60 ± 0.36 | 4.46 ± 0.38 |
| COVID Δ | 2018/2019 Δ | p | |
|---|---|---|---|
| Lactate—mmol × L−1 | |||
| T0–T1 | −0.81 ± 0.06 | −0.90 ± 0.10 | 0.34 |
| T1–T2 | −0.86 ± 0.15 | −0.92 ± 0.34 | 0.08 |
| T2–T3 | −0.45 ± 0.34 | −0.60 ± 0.15 | 0.26 |
| T3–T4 | 0.90 ± 0.39 | −0.50 ± 0.30 | 0.01 |
| T0–T4 | −0.80 ± 0.37 | −1.60 ± 0.50 | 0.03 |
| CMJ—height jump (cm) | |||
| T0–T1 | 1.17 ± 0.77 | 1.10 ± 0.69 | 0.44 |
| T1–T2 | 1.76 ± 1.00 | 1.80 ± 0.22 | 0.49 |
| T2–T3 | 0.84 ± 0.43 | 0.90 ± 0.82 | 0.10 |
| T3–T4 | 0.10 ± 0.83 | 1.52 ± 0.81 | 0.02 |
| T0–T4 | −0.51 ± 0.71 | 3.61 ± 0.84 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
My, G.; Marsigliante, S.; Bianco, A.; Zangla, D.; Silva, C.M.d.; Muscella, A. Biological, Psychological, and Physical Performance Variations in Football Players during the COVID-19 Lockdown: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 2739. https://doi.org/10.3390/ijerph19052739
My G, Marsigliante S, Bianco A, Zangla D, Silva CMd, Muscella A. Biological, Psychological, and Physical Performance Variations in Football Players during the COVID-19 Lockdown: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(5):2739. https://doi.org/10.3390/ijerph19052739
Chicago/Turabian StyleMy, Giulia, Santo Marsigliante, Antonino Bianco, Daniele Zangla, Carlos Marques da Silva, and Antonella Muscella. 2022. "Biological, Psychological, and Physical Performance Variations in Football Players during the COVID-19 Lockdown: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 5: 2739. https://doi.org/10.3390/ijerph19052739







