Concentration, Distribution and Biomagnification of Novel Brominated Flame Retardant in Grassland Food Chain and Sheep from Inner Mongolia, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical Analysis
2.3. Quality Control and Assurance
3. Results and Discussion
3.1. NBFRs Concentrations in the Xilingol Grassland Samples
3.2. NBFRs Congener Patterns in the Different Xilingol Grassland Samples
3.3. NBFRs Biomagnification in the Grassland Food Chain in Inner Mongolia
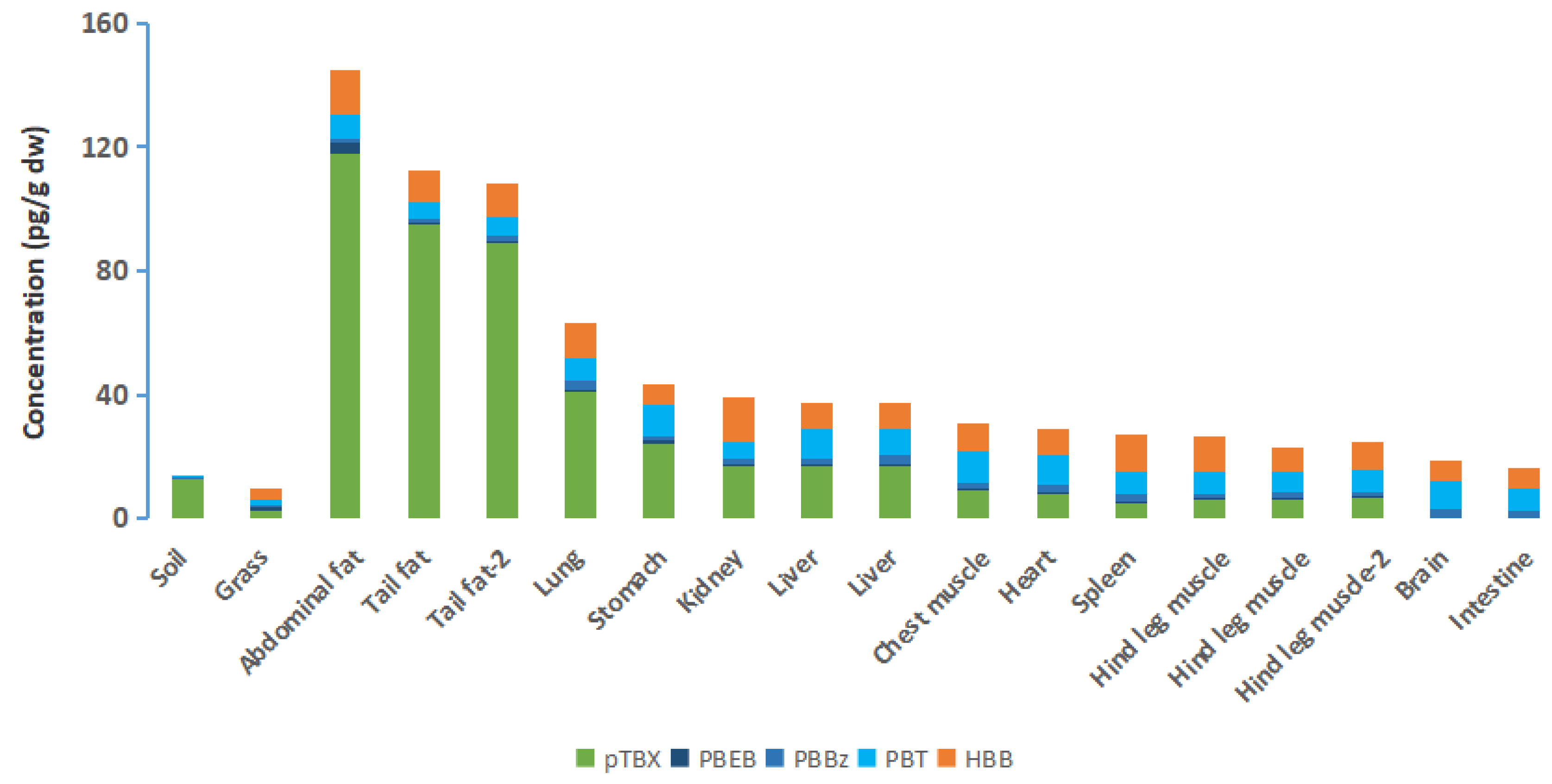
3.4. NBFRs Bioaccumulation in the Organs and Tissues of Sheep
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venier, M.; Salamova, A.; Hites, R.A. Halogenated Flame Retardants in the Great Lakes Environment. Acc. Chem. Res. 2015, 48, 1853–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Sjodin, A.; Kurzon, M.; Lederman, S.A.; Perera, F. Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect. 2010, 118, 712–719. [Google Scholar] [CrossRef] [PubMed]
- UNEP/POPS/COP.8/32. Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the Work of Its Eighth Meeting, Geneva, Switzerland, 24 April–5 May 2017; UNEP: Nairobi, Kenya, 2017.
- Geens, T.; Ali, N.; Roosens, L.; Neels, H.; Covaci, A. Analytical characteristics of several new brominated flame retardants. Talanta 2010, 81, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Å.; Rydén, A.; Law, R.J.; de Boer, J.; Covaci, A.; Alaee, M.; Birnbaum, L.; Petreas, M.; Rose, M.; Sakai, S.; et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012, 49, 57–82. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Dong, S.; Wang, R.; Wang, P.; Ruan, Z.; Sun, X.; Rao, Q.; Liu, Z.; Su, X. Novel brominated flame retardant (NBFR) concentrations and spatial distributions in global fishmeal. Ecotoxicol. Environ. Saf. 2018, 170, 306–313. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, R.; Zhang, Q.; Zhang, H.; Xiao, K.; Zhang, H.; Wang, Y.; Lam, P.K.; Jiang, G. Current Levels and Composition Profiles of Emerging Halogenated Flame Retardants and Dehalogenated Products in Sewage Sludge from Municipal Wastewater Treatment Plants in China. Environ. Sci. Technol. 2014, 48, 12586–12594. [Google Scholar] [CrossRef]
- Poma, G.; Roscioli, C.; Guzzella, L. PBDE, HBCD, and novel brominated flame retardant contamination in sediments from Lake Maggiore (Northern Italy). Environ. Monit. Assess. 2014, 186, 7683–7692. [Google Scholar] [CrossRef]
- Ali, N.; Harrad, S.; Goosey, E.; Neels, H.; Covaci, A. “Novel” brominated flame retardants in Belgian and UK indoor dust: Implications for human exposure. Chemosphere 2011, 83, 1360–1365. [Google Scholar] [CrossRef]
- Cruz, R.; Cunha, S.C.; Casal, S. Brominated flame retardants and seafood safety: A review. Environ. Int. 2015, 77, 116–131. [Google Scholar] [CrossRef]
- Vorkamp, K.; Bossi, R.; Rigét, F.F.; Skov, H.; Sonne, C.; Dietz, R. Novel brominated flame retardants and dechlorane plus in Greenland air and biota. Environ. Pollut. 2014, 196, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, P.; Jin, J.; Wang, Y.; Wang, Q. Current halogenated flame retardant concentrations in serum from residents of Shandong Province, China, and temporal changes in the concentrations. Environ. Res. 2017, 155, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.M.; Higley, E.B.; Hecker, M.; Mankidy, R.; Giesy, J.P. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol. Lett. 2013, 223, 252–259. [Google Scholar]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Morin, A.E.; Gobas, F.A.P.C. Food Web–Specific Biomagnification of Persistent Organic Pollutants. Science 2007, 317, 236–239. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, Q.; Wang, P.; Yang, H.; Jiang, G.; Wei, F. Evaluation of atmospheric sources of PCDD/Fs, PCBs and PBDEs around a steel industrial complex in northeast China using passive air samplers. Chemosphere 2011, 84, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Nie, M.; Huang, Y.M. The impacts of lizards on the community diversity and aboveground biomass of Inner Mongolia Steppe. Acta Agrestia Sin. 2015, 23, 246–251. (In Chinese) [Google Scholar]
- Guo, L. Analysis of the feeding habits of sparrows in Shazhen, Zhungeer banner, Inner Mongolia. Chin. J. Zool. 1975, 1, 37–38. (In Chinese) [Google Scholar]
- Yin, X.C.; Li, D.H. Preliminary investigation on the feeding habits of grassland birds in Yushu area, Qinghai. Chin. J. Zool. 1966, 3, 56–57. (In Chinese) [Google Scholar]
- Jin, Z.M.; Yang, C.W.; Liu, Z.; Qiao, Z.L.; Li, D.W. Study on the diet of Bufo gargarizans. Chin. J. Anhui Agric. 2010, 38, 3510–3511. (In Chinese) [Google Scholar]
- Wang, G.M.; Zhou, Q.Q. Food habits of brandt’s vole. Acta Theriol. Sin. 1992, 12, 57–64. (In Chinese) [Google Scholar]
- Zhao, K.T. Study on ecology of red–cheeked souslik in desert steppe from Inner Mongolia. Acta Sci. Nat. Univ. Intramongolicae 1981, 12, 67–78. (In Chinese) [Google Scholar]
- Wang, W.; Ma, J.Z.; Zou, H.F. Food habits of Siberian Ferrets in Badarhu Region, Inner Mongolia. J. Northeast. For. Univ. 2006, 34, 33–35. (In Chinese) [Google Scholar]
- Guo, Y.H.; Zhang, Q.L. Snake feeding habits and its impact on agroforestry ecosystem. Chin. Biol. Teach. 2018, 43, 2–4. (In Chinese) [Google Scholar]
- Chen, W.; Li, J.; Dong, Z.; Bao, J.; Zhang, A.; Shen, G.; Wang, Y.; Hu, J.; Jin, J. Correlations between dechlorane plus concentrations in paired hair and indoor dust samples and differences between dechlorane plus isomer concentrations in hair from males and females. Chemosphere 2019, 231, 378–384. [Google Scholar] [CrossRef]
- Wang, Q.H.; Yuan, H.D.; Jin, J.; Li, P.; Ma, Y.L.; Wang, Y. Concentration and Distribution of Novel Brominated Flame Retardants in Human Serum from Three Chinese Cities. Environ. Sci. 2019, 39, 1438–1444. (In Chinese) [Google Scholar]
- Wu, H.; Jin, J.; Wang, Y. Comparative Study of the Level and Distribution of Polybrominated Diphenyl Ethers and New Brominated Flame Retardants in the Atmosphere of Typical Urban. Environ. Sci. 2014, 35, 1230–1237. (In Chinese) [Google Scholar]
- Carlsson, P.; Vrana, B.; Sobotka, J.; Borgå, K.; Nizzetto, P.B.; Varpe, Ø. New brominated flame retardants and dechlorane plus in the Arctic: Local sources and bioaccumulation potential in marine benthos. Chemosphere 2018, 211, 1193–1202. [Google Scholar] [CrossRef]
- Wu, J.-P.; Guan, Y.-T.; Zhang, Y.; Luo, X.-J.; Zhi, H.; Chen, S.-J.; Mai, B.-X. Trophodynamics of Hexabromocyclododecanes and Several Other Non-PBDE Brominated Flame Retardants in a Freshwater Food Web. Environ. Sci. Technol. 2010, 44, 5490–5495. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Zhou, H.; Zhang, A.; Qi, H. Levels, occurrence and human exposure to novel brominated flame retardants (NBFRs) and Dechlorane Plus (DP) in dust from different indoor environments in Hangzhou, China. Sci. Total Environ. 2018, 631–632, 1212–1220. [Google Scholar] [CrossRef]
- Mcgrath, T.J.; Morrison, P.D.; Ball, A.S.; Clarke, B.O. Spatial Distribution of Novel and Legacy Brominated Flame Retardants in Soils Surrounding Two Australian Electronic Waste Recycling Facilities. Environ. Sci. Technol. 2018, 52, 8194–8204. [Google Scholar] [CrossRef]
- He, C.; Jin, J.; Wu, H.; Wang, Y.; Li, G.Y.; Tian, Y. Determination of Halogenated Flame Retardants in Willow Bark by Solid Phase Extraction-Gas Chromatography-Mass Spectrometry. Chin. J. Anal. Chem. 2013, 11, 64–70. (In Chinese) [Google Scholar] [CrossRef]
- Vénisseau, A.; Bichon, E.; Brosseaud, A.; Vaccher, V.; Lesquin, E.; Larvor, F.; Durand, S.; Dervilly-Pinel, G.; Marchand, P.; Le Bizec, B. Occurence of legacy and novel brominated flame retardants in food and feed in France for the period 2014 to 2016. Chemosphere 2018, 207, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; De Silva, A.O.; Small, J.; Williamson, M.; Wang, X.W.; Morris, A.; Katz, S.; Gamberg, M.; Muir, D.C.G. Biomagnification of Perfluorinated Compounds in a Remote Terrestrial Food Chain: Lichen–Caribou–Wolf. Environ. Sci. Technol. 2011, 45, 8665–8673. [Google Scholar] [CrossRef] [PubMed]
- Gobas, F.A.; Burkhard, L.P.; Doucette, W.J.; Sappington, K.G.; Verbruggen, E.M.; Hope, B.K.; Bonnell, M.A.; Arnot, J.A.; Tarazona, J.V. Review of existing terrestrial bioaccumulation models and terrestrial bioaccumulation modeling needs for organic chemicals. Integr. Environ. Assess. Manag. 2016, 12, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Zeng, Y.; Deng, M.; Tu, W.; Wu, Y.; Mai, B. Bioaccumulation and biomagnification of hexabromocyclododecane (HBCDD) in insect-dominated food webs from a former e-waste recycling site in South China. Chemosphere 2020, 240, 124813. [Google Scholar] [CrossRef]
- Kurt-Karakus, P.B.; Muir, D.C.; De Jourdan, B.; Teixeira, C.; Martindale, J.E.; Embers, H.; Wang, X.; Keir, M.; Backus, S. Bioaccumulation of Selected Halogenated Organic Flame Retardants in Lake Ontario. Environ. Toxicol. Chem. 2019, 38, 1198–1210. [Google Scholar] [CrossRef]
- Wu, J.-P.; Wu, S.-K.; Tao, L.; She, Y.-Z.; Chen, X.-Y.; Feng, W.-L.; Zeng, Y.-H.; Luo, X.-J.; Mai, B.-X. Bioaccumulation characteristics of PBDEs and alternative brominated flame retardants in a wild frog-eating snake. Environ. Pollut. 2019, 258, 113661. [Google Scholar] [CrossRef]

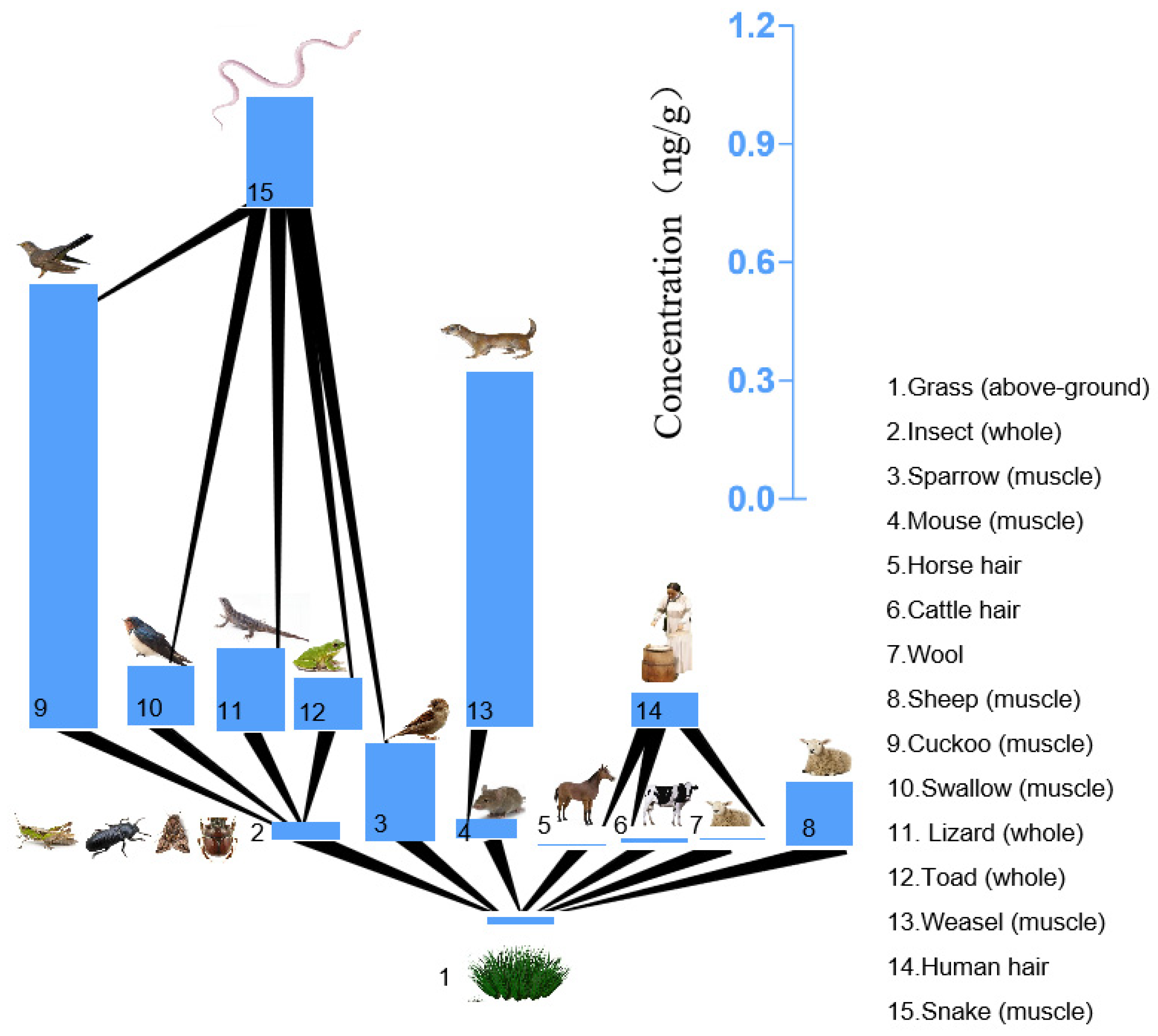
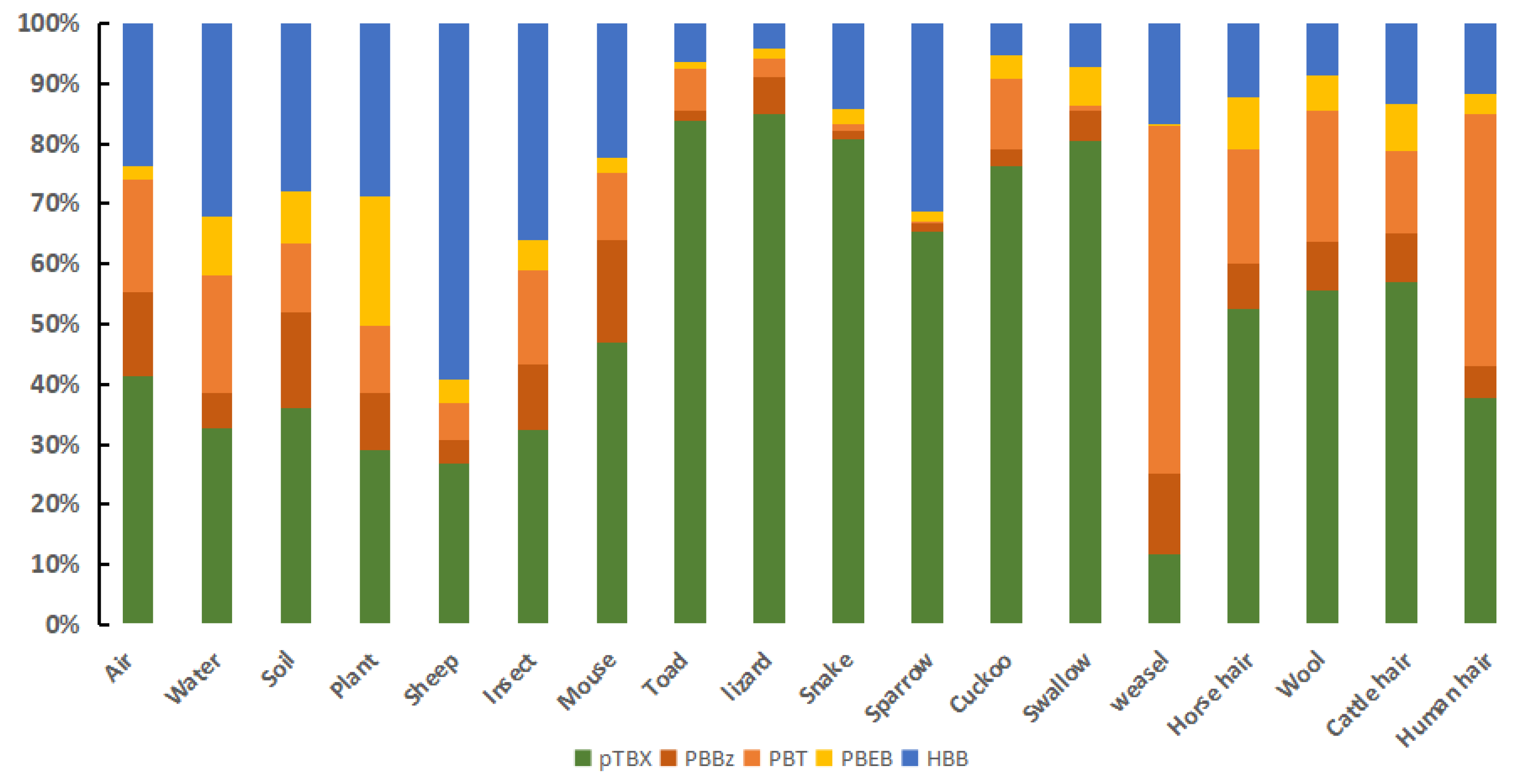

| Type | Consumer (Part) | Food (Part) | pTBX | PBBz | PBT | PBEB | HBB |
|---|---|---|---|---|---|---|---|
| Insect | Insect (whole) | Grass | 0.654 | 0.640 | 0.823 | 0.132 | 0.719 |
| Mammals | Mouse (muscle) | Grass | 1.05 | 1.09 | 0.551 | 0.0963 | 0.436 |
| Sheep (muscle) | Grass | 2.49 | 1.14 | 1.47 | 0.500 | 5.57 | |
| weasel (muscle) | Mouse (muscle) | 1.31 | 4.08 | 27.9 | 0.663 | 4.23 | |
| Wool | Grass | 0.680 | 0.310 | 0.693 | 0.0951 | 0.109 | |
| Cattle hair | Grass | 0.978 | 0.436 | 0.609 | 0.188 | 0.233 | |
| Horse hair | Grass | 0.452 | 0.200 | 0.426 | 0.0991 | 0.108 | |
| Human hair | Wool | 7.85 | 7.55 | 22.3 | 6.89 | 15.6 | |
| Human hair | Cattle hair | 5.46 | 5.37 | 25.4 | 3.49 | 7.32 | |
| Human hair | Horse hair | 11.8 | 11.7 | 36.4 | 6.60 | 15.7 | |
| Birds | Sparrow (muscle) | Grass | 5.98 | 0.349 | 0.0611 | 0.211 | 2.91 |
| Swallow (muscle) | Insect (whole) | 9.54 | 1.94 | 0.192 | 5.12 | 0.793 | |
| Cuckoo (muscle) | Insect (whole) | 34.3 | 3.93 | 11.0 | 11.1 | 2.27 | |
| Amphibians and reptiles | Toad (whole) | Insect (whole) | 7.61 | 0.467 | 0.357 | 0.756 | 0.530 |
| lizard (whole) | Insect (whole) | 5.23 | 1.41 | 0.408 | 0.734 | 0.501 | |
| Snake (muscle) | Mouse (muscle) | 2.55 | 0.221 | 0.157 | 1.14 | 1.07 | |
| Snake (muscle) | Sparrow (muscle) | 0.450 | 0.694 | 1.41 | 0.519 | 0.161 | |
| Snake (muscle) | Swallow (muscle) | 0.432 | 0.195 | 0.546 | 0.162 | 0.820 | |
| Snake (muscle) | Cuckoo (muscle) | 0.120 | 0.0963 | 0.00951 | 0.0749 | 0.286 | |
| Snake (muscle) | Toad (whole) | 0.541 | 0.811 | 0.294 | 1.10 | 1.23 | |
| Snake (muscle) | Lizard (whole) | 0.787 | 0.268 | 0.257 | 1.13 | 1.30 |
| Tissues and Organs | pTBX | PBT | PBBz | HBB | PBEB |
|---|---|---|---|---|---|
| Abdominal fat | 49.8 | 3.71 | 1.86 | 4.61 | 2.67 |
| Tail fat | 40.2 | 2.64 | 1.39 | 3.23 | 0.562 |
| Tail fat-2 | 37.6 | 3.07 | 1.42 | 3.42 | 0.597 |
| Lung | 17.3 | 3.52 | 2.98 | 3.73 | 0.669 |
| Stomach | 10.3 | 5.05 | 1.49 | 2.12 | 0.676 |
| Kidney | 7.22 | 2.79 | 1.99 | 4.52 | 0.362 |
| Liver | 7.22 | 4.63 | 2.40 | 2.54 | 0.433 |
| Liver | 7.14 | 4.21 | 3.25 | 2.62 | 0.532 |
| Chest muscle | 3.98 | 4.78 | 2.31 | 2.97 | 0.410 |
| Heart | 3.41 | 4.56 | 3.15 | 2.75 | 0.290 |
| Hind leg muscle | 2.59 | 3.31 | 1.85 | 3.59 | 0.382 |
| Hind leg muscle | 2.70 | 3.35 | 2.01 | 2.35 | 0.382 |
| Hind leg muscle-2 | 2.78 | 3.35 | 2.03 | 2.82 | 0.456 |
| Spleen | 2.24 | 3.77 | 2.61 | 3.67 | 0.231 |
| Brain | 0.00 | 4.48 | 3.03 | 2.16 | 0.302 |
| Intestine | 0.00 | 3.57 | 2.80 | 1.95 | 0.324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Bu, T.; Li, T.; Bao, J.; Wang, Y.; Hu, J.; Jin, J. Concentration, Distribution and Biomagnification of Novel Brominated Flame Retardant in Grassland Food Chain and Sheep from Inner Mongolia, China. Int. J. Environ. Res. Public Health 2022, 19, 12785. https://doi.org/10.3390/ijerph191912785
Chen W, Bu T, Li T, Bao J, Wang Y, Hu J, Jin J. Concentration, Distribution and Biomagnification of Novel Brominated Flame Retardant in Grassland Food Chain and Sheep from Inner Mongolia, China. International Journal of Environmental Research and Public Health. 2022; 19(19):12785. https://doi.org/10.3390/ijerph191912785
Chicago/Turabian StyleChen, Wenming, Te Bu, Tianwei Li, Junsong Bao, Ying Wang, Jicheng Hu, and Jun Jin. 2022. "Concentration, Distribution and Biomagnification of Novel Brominated Flame Retardant in Grassland Food Chain and Sheep from Inner Mongolia, China" International Journal of Environmental Research and Public Health 19, no. 19: 12785. https://doi.org/10.3390/ijerph191912785





