Ozone Eliminates SARS-CoV-2 from Difficult-to-Clean Office Supplies and Clinical Equipment
Abstract
:1. Introduction
2. Methods
2.1. Samples, Study Design, and Outcome Assessment
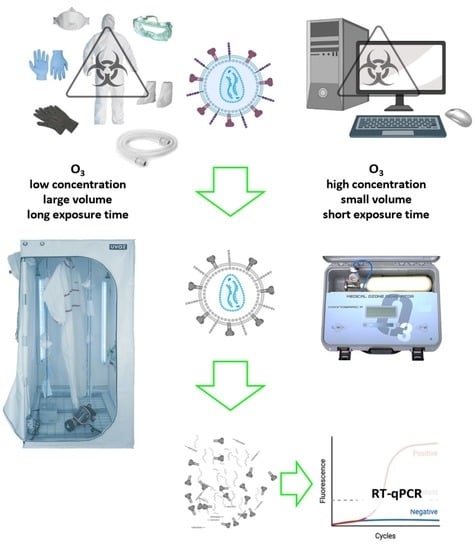
2.2. Ozone Exposure Conditions
2.3. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
3. Results
3.1. Low Ozone Concentrations
3.2. High Ozone Concentrations
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| CPAP | Continuous positive airway pressure tube |
| DNA | Deoxyribonucleic acid |
| O2 | Molecular oxygen |
| O3 | Ozone |
| PPE | Personal protective equipment |
| RNA | Ribonucleic acid |
| RT-qPCR | Real-time polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| UV | Ultraviolet |
| VERO | African green monkey kidney epithelial cells |
| WHO | World Health Organization |
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 10 September 2020).
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Carraturo, F.; Del Giudice, C.; Morelli, M.; Cerullo, V.; Libralato, G.; Galdiero, E.; Guida, M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020, 265, 115010. [Google Scholar] [CrossRef] [PubMed]
- Cordoba-Lanus, E.; Garcia-Perez, O.; Cazorla-Rivero, S.; Rodriguez-Esparragon, F.; Pinero, J.E.; Clavo, B.; Lorenzo-Morales, J. Persistence of SARS-CoV-2 infection on personal protective equipment (PPE). BMC Infect. Dis. 2021, 21, 1169. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, A.; Jeddi, F.; Ghobadi, H.; Vosoughi, M.; Karami, C.; Sarailoo, M.; Hadisi, A.; Mokhtari, S.A.; Haghighi, S.B.; Sadeghi, H.; et al. Evaluation of masks’ internal and external surfaces used by health care workers and patients in coronavirus-2 (SARS-CoV-2) wards. Environ. Res. 2021, 196, 110948. [Google Scholar] [CrossRef] [PubMed]
- Percivalle, E.; Clerici, M.; Cassaniti, I.; Vecchio Nepita, E.; Marchese, P.; Olivati, D.; Catelli, C.; Berri, A.; Baldanti, F.; Marone, P.; et al. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: A preliminary evaluation. J. Hosp. Infect. 2021, 110, 33–36. [Google Scholar] [CrossRef]
- World Health Organization. Infection Prevention and Control. Available online: https://www.who.int/health-topics/infection-prevention-and-control#tab=tab_1 (accessed on 28 October 2021).
- Cristiano, L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020, 61, E301–E303. [Google Scholar]
- Clavo, B.; Cordoba-Lanus, E.; Rodriguez-Esparragon, F.; Cazorla-Rivero, S.E.; Garcia-Perez, O.; Pinero, J.E.; Villar, J.; Blanco, A.; Torres-Ascension, C.; Martin-Barrasa, J.L.; et al. Effects of Ozone Treatment on Personal Protective Equipment Contaminated with SARS-CoV-2. Antioxidants 2020, 9, 1222. [Google Scholar] [CrossRef]
- Blanco, A.; Ojembarrena, F.B.; Clavo, B.; Negro, C. Ozone potential to fight against SAR-COV-2 pandemic: Facts and research needs. Environ. Sci. Pollut. Res. Int. 2021, 28, 16517–16531. [Google Scholar] [CrossRef]
- Habibi Najafi, M.B.; Haddad Khodaparast, M.H. Efficacy of ozone to reduce microbial populations in date fruits. Food Control 2009, 20, 27–30. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Manjunath, S.N.; Sakar, M.; Katapadi, M.; Geetha Balakrishna, R. Recent case studies on the use of ozone to combat coronavirus: Problems and perspectives. Environ. Technol. Innov. 2021, 21, 101313. [Google Scholar] [CrossRef]
- Martinez De Alba, A.E.; Rubio, M.B.; Moran-Diez, M.E.; Bernabeu, C.; Hermosa, R.; Monte, E. Microbiological Evaluation of the Disinfecting Potential of UV-C and UV-C Plus Ozone Generating Robots. Microorganisms 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Farooq, S.; Hashmi, I. Ozone Disinfection Efficiency for Indicator Microorganisms at Different pH Values and Temperatures. Ozone Sci. Eng. 2017, 39, 407–416. [Google Scholar] [CrossRef]
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Criscuolo, E.; Diotti, R.A.; Ferrarese, R.; Alippi, C.; Viscardi, G.; Signorelli, C.; Mancini, N.; Clementi, M.; Clementi, N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021, 10, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.K.; Ohmine, S.; Tomer, D.P.; Jensen, K.J.; Johnson, F.B.; Kirsi, J.J.; Robison, R.A.; O’Neill, K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods 2008, 153, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Tizaoui, C. Ozone: A Potential Oxidant for COVID-19 Virus (SARSCoV-2). Ozone Sci. Eng. 2020, 42, 378–385. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Anachkov, M.; Rakovsky, S.; Zaikov, G.E. Ozone decomposition. Interdiscip. Toxicol. 2014, 7, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Thill, S.A.; Spaltenstein, M. Toward Efficient Low-Temperature Ozone Gas Sterilization of Medical Devices. Ozone Sci. Eng. 2020, 42, 386–398. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Amarilla, A.A.; Trollope, B.; Sng, J.D.J.; Setoh, Y.X.; Deering, N.; Modhiran, N.; Weng, S.H.; Melo, M.C.; Hutley, N.; et al. Assessing the potential of unmanned aerial vehicle spraying of aqueous ozone as an outdoor disinfectant for SARS-CoV-2. Environ. Res. 2021, 196, 110944. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bong, C.; Lim, W.; Bae, P.K.; Abafogi, A.T.; Baek, S.H.; Shin, Y.-B.; Bak, M.S.; Park, S.B. Fast and easy disinfection of coronavirus-contaminated face masks using ozone gas produced by a dielectric barrier discharge plasma generator. Environ. Sci. Technol. Lett. 2021, 8, 339–344. [Google Scholar] [CrossRef]
- Uppal, T.; Khazaieli, A.; Snijders, A.M.; Verma, S.C. Inactivation of Human Coronavirus by FATHHOME’s Dry Sanitizer Device: Rapid and Eco-Friendly Ozone-Based Disinfection of SARS-CoV-2. Pathogens 2021, 10, 339. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Makita, Y.; Kaneko, A.; Matsuda, S.; Ogawa, H.; Oh, H. Inactivation of SARS-CoV-2 by Ozonated Glycerol. Food Environ. Virol. 2021, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Franke, G.; Knobling, B.; Brill, F.H.; Becker, B.; Klupp, E.M.; Belmar Campos, C.; Pfefferle, S.; Lutgehetmann, M.; Knobloch, J.K. An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. J. Hosp. Infect. 2021, 112, 108–113. [Google Scholar] [CrossRef]
- Dennis, R.; Cashion, A.; Emanuel, S.; Hubbard, D. Ozone Gas: Scientific Justification and Practical Guidelines for Improvised Disinfection using Consumer-Grade Ozone Generators and Plastic Storage Boxes. J. Sci. Med. 2020, 2. [Google Scholar] [CrossRef]
- Tizaoui, C.; Stanton, R.; Statkute, E.; Rubina, A.; Lester-Card, E.; Lewis, A.; Holliman, P.; Worsley, D. Ozone for SARS-CoV-2 inactivation on surfaces and in liquid cell culture media. J. Hazard. Mater. 2022, 428, 128251. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidance on Implementing Regulatory Requirements for Biosafety and Biosecurity in Biomedical Laboratories: A Stepwise Approach. 2020. Available online: https://apps.who.int/iris/handle/10665/332244 (accessed on 29 November 2021).
- Bayarri, B.; Cruz-Alcalde, A.; Lopez-Vinent, N.; Mico, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658. [Google Scholar] [CrossRef]
- Ataei-Pirkooh, A.; Alavi, A.; Kianirad, M.; Bagherzadeh, K.; Ghasempour, A.; Pourdakan, O.; Adl, R.; Kiani, S.J.; Mirzaei, M.; Mehravi, B. Destruction mechanisms of ozone over SARS-CoV-2. Sci. Rep. 2021, 11, 18851. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Loibner, M.; Puff, M.; Melischnig, A.; Zatloukal, K. SARS-CoV2 neutralizing activity of ozone on porous and non-porous materials. New Biotechnol. 2021, 66, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Behzadinasab, S.; Benmamoun, Z.; Ducker, W.A. The viability of SARS-CoV-2 on solid surfaces. Curr. Opin. Colloid Interface Sci. 2021, 55, 101481. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Medronho, B.; Alves, L.; Norgren, M.; Nordenskiold, L. Hydrophobic interactions control the self-assembly of DNA and cellulose. Q. Rev. Biophys. 2021, 54, e3. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Comeau, A.M. Cellulose as a matrix for nucleic acid purification. Anal. Biochem. 1999, 267, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Baiersdorfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Kariko, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comission, E. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en#thematicareas (accessed on 3 November 2021).
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of Natural Rubber. Polym. Degrad. Stab. 2021, 186, 109514. [Google Scholar] [CrossRef]



| Supply Type (by Duplicate) | Ozone Concentration (ppm) | Time of Treatment (Minutes) | Relative Humidity (%) | RT-qPCR | |
|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | ||||
| Face masks | 19 | 30 | 80–90 | ✓✓✓ | ✓✓ |
| 19 | 60 | 85–90 | ✓✓✓ | ✓ | |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| 2000 | 5 | 60–75 | ✓✓✓ | X | |
| 2000 | 10 | 60–75 | ✓✓✓ | X | |
| 4000 | 5 | 60–75 | ✓✓✓ | X | |
| Vinyl lab glove | 19 | 30 | 80–90 | ✓✓✓ | X |
| Nitrile lab glove | 90 | 120 | 65–70 | ✓✓✓ | X |
| Cover needle | 90 | 120 | 65–70 | ✓✓✓ | X |
| Cover syringe | 90 | 120 | 65–70 | X | n.v. |
| CPAP tube | 70 a | 120 | 60–75 | ✓✓✓ | ✓ |
| 90 a | 120 | 65–70 | ✓✓✓ | X | |
| 4000 b | 10 | 60–75 | ✓✓✓ | ✓ | |
| 10,000 b | 10 | 60–75 | ✓✓✓ | X | |
| Lab grid | 70 | 120 | 60–75 | ✓✓✓ | X |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| Reactant flask | 70 | 120 | 60–75 | ✓✓✓ | X |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| Reactant flask tag | 70 | 120 | 60–75 | X | n.v. |
| 90 | 120 | 65–70 | X | n.v. | |
| Test tube | 70 | 120 | 60–75 | ✓✓✓ | X |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| Between keys of mouse | 70 | 120 | 60–75 | X | n.v. |
| 90 | 120 | 65–70 | X | n.v. | |
| Computer mouse | 33 | 120 | 60–75 | ✓✓✓ | ✓ |
| 70 | 120 | 60–75 | ✓✓✓ | ✓✓✓ | |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| 4000 | 10 | 60–75 | ✓ | X | |
| 10,000 | 10 | 60–75 | ✓ | X | |
| Computer screen | 33 | 120 | 60–75 | ✓✓✓ | ✓✓✓ |
| 70 | 120 | 60–75 | ✓✓✓ | X | |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| 4000 | 10 | 60–75 | ✓✓✓ | X | |
| 10,000 | 10 | 60–75 | ✓✓✓ | X | |
| Keyboard key | 33 | 120 | 60–75 | ✓✓✓ | ✓✓✓ |
| 70 | 120 | 60–75 | ✓✓✓ | X | |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
| 4000 | 10 | 60–75 | X | n.v. | |
| 10,000 | 10 | 60–75 | X | n.v. | |
| Between keys of keyboard | 33 | 120 | 60–75 | ✓✓✓ | ✓✓✓ |
| 70 | 120 | 60–75 | ✓✓✓ | ✓✓✓ | |
| 90 | 120 | 65–70 | X | n.v. | |
| 4000 | 10 | 60–75 | ✓✓✓ | X | |
| 10,000 | 10 | 60–75 | ✓✓✓ | X | |
| Cellphone | 70 | 120 | 60–75 | ✓✓✓ | X |
| 90 | 120 | 65–70 | ✓✓✓ | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Mata, L.B.; García-Pérez, O.; Rodríguez-Esparragón, F.; Blanco, A.; Villar, J.; Ruiz-Apodaca, F.; Martín-Barrasa, J.L.; González-Martín, J.M.; Serrano-Aguilar, P.; Piñero, J.E.; et al. Ozone Eliminates SARS-CoV-2 from Difficult-to-Clean Office Supplies and Clinical Equipment. Int. J. Environ. Res. Public Health 2022, 19, 8672. https://doi.org/10.3390/ijerph19148672
Torres-Mata LB, García-Pérez O, Rodríguez-Esparragón F, Blanco A, Villar J, Ruiz-Apodaca F, Martín-Barrasa JL, González-Martín JM, Serrano-Aguilar P, Piñero JE, et al. Ozone Eliminates SARS-CoV-2 from Difficult-to-Clean Office Supplies and Clinical Equipment. International Journal of Environmental Research and Public Health. 2022; 19(14):8672. https://doi.org/10.3390/ijerph19148672
Chicago/Turabian StyleTorres-Mata, Laura B., Omar García-Pérez, Francisco Rodríguez-Esparragón, Angeles Blanco, Jesús Villar, Fernando Ruiz-Apodaca, José L. Martín-Barrasa, Jesús M. González-Martín, Pedro Serrano-Aguilar, José E. Piñero, and et al. 2022. "Ozone Eliminates SARS-CoV-2 from Difficult-to-Clean Office Supplies and Clinical Equipment" International Journal of Environmental Research and Public Health 19, no. 14: 8672. https://doi.org/10.3390/ijerph19148672