Impact of Endocrine-Disrupting Chemicals in Breast Milk on Postpartum Depression in Korean Mothers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Postpartum Depression Scale
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Levels of Chemicals in the Breast Milk
3.3. Association of Chemicals with Postpartum Depression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal Depression: A Systematic Review of Prevalence and Incidence. Obstet. Gynecol. 2005, 106, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Pinsonneault, J.K.; Sullivan, D.; Sadee, W.; Soares, C.N.; Hampson, E.; Steiner, M. Association Study of the Estrogen Receptor Gene ESR1 with Postpartum Depression—A Pilot Study. Arch. Womens Ment. Health 2013, 16, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, C.E.; Meltzer-Brody, S.; Rubinow, D.R. The Role of Reproductive Hormones in Postpartum Depression. CNS Spectr. 2015, 20, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Dunn, E.; Born, L. Hormones and Mood: From Menarche to Menopause and Beyond. J. Affect. Disord. 2003, 74, 67–83. [Google Scholar] [CrossRef]
- Green, A.D.; Barr, A.M.; Galea, L.A. Role of Estradiolwithdrawal in ‘Anhedonic’ Sucrose Consumption: A Model of Postpartum Depression. Physiol. Behav. 2009, 97, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Gomez, P.; Perez, M.; Avila, J.; Garcia-Segura, L.M.; Wandosell, F. Estradiol Inhibits GSK3 and Regulates Interaction of Estrogen Receptors, GSK3, and Beta-Catenin in the Hippocampus. Mol. Cell. Neurosci. 2004, 25, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Finocchi, C.; Ferrari, M. Female Reproductive Steroids and Neuronal Excitability. Neurol. Sci. 2011, 32, S31–S35. [Google Scholar] [CrossRef]
- Marín-Morales, D.; Toro-Molina, S.; Peñacoba-Puente, C.; Losa-Iglesias, M.; Carmona-Monge, F.J. Relationship Between Postpartum Depression and Psychological and Biological Variables in the Initial Postpartum Period. Matern. Child Health J. 2018, 22, 866–873. [Google Scholar] [CrossRef]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D. A Human Exposure to Endocrine Disrupting Chemicals: Effects on the Male and Female Reproductive System. Environ. Toxiocol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.; Moon, S.M.; Yang, E.J. Associations of Lifestyle Factors with Phthalate Metabolites, Bisphenol A, Parabens, and Triclosan Concentrations in Breast Milk of Korean Mothers. Chemosphere 2020, 249, 126149. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben Esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. A Review and Update of Mechanisms of Estrogen in the Hippocampus and Amygdala for Anxiety and Depression Behavior. Neuropsychopharmacology 2006, 31, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hong, X.; Xie, L.; Li, T.; Yang, Y.; Zhang, Q.; Zhang, G.; Liu, X. Gestational and Lactational Exposure to Bisphenol-A Affects Anxiety- And Depression-Like Behaviors in Mice. Horm. Behav. 2012, 62, 480–490. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, R.; Wang, Y.; Ruan, Q.; Lu, Y. Perinatal Exposure to Di-(2-Ethylhexyl) Phthalate Affects Anxiety- And Depression-Like Behaviors in Mice. Chemosphere 2015, 124, 22–31. [Google Scholar] [CrossRef]
- Kim, K.N.; Choi, Y.H.; Lim, Y.H.; Hong, Y.C. Urinary Phthalate Metabolites and Depression in an Elderly Population: National Health and Nutrition Examination Survey 2005–2012. Environ. Res. 2016, 145, 61–67. [Google Scholar] [CrossRef]
- Lee, K.S.; Lim, Y.H.; Kim, K.N.; Choi, Y.H.; Hong, Y.C.; Lee, N. Urinary Phthalate Metabolites Concentrations and Symptoms of Depression in an Elderly Population. Sci. Total Environ. 2018, 625, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A Exposure and Symptoms of Anxiety and Depression Among Inner City Children at 10–12 Years of Age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.K.; Nash, M.; Barr, D.B.; Starr, J.M.; Scott Clifton, M.; Sobus, J.R. Distribution, Variability, and Predictors of Urinary Bisphenol A Levels in 50 North Carolina Adults over a Six-Week Monitoring Period. Environ. Int. 2018, 112, 85–99. [Google Scholar] [CrossRef]
- Bever, C.S.; Rand, A.A.; Nording, M.; Taft, D.; Kalanetra, K.M.; Mills, D.A.; Breck, M.A.; Smilowitz, J.T.; German, J.B.; Hammock, B.D. Effects of Triclosan in Breast Milk on the Infant Fecal Microbiome. Chemosphere 2018, 203, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Toms, L.M.; Allmyr, M.; Mueller, J.F.; Adolfsson-Erici, M.; McLachlan, M.; Murby, J.; Harden, F.A. Triclosan in Individual Human Milk Samples from Australia. Chemosphere 2011, 85, 1682–1686. [Google Scholar] [CrossRef]
- Shiue, I. Urinary Heavy Metals, Phthalates and Polyaromatic Hydrocarbons Independent of Health Events Are Associated with Adult Depression: USA NHANES, 2011–2012. Environ. Sci. Pollut. Res. 2015, 22, 17095–17103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, D.R.; Kwak, J.M.; Lee, J.K. Concentration and Variability of Urinary Phthalate Metabolites, Bisphenol A, Triclosan, and Parabens in Korean Mother–Infant Pairs. Sustainability 2020, 12, 8516. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Di Angerer, J. Di (2- Ethylhexyl) Phthalate (DEHP) Metabolites in Human Urine and Serum after a Single Oral Dose of Deuterium-Labelled DEHP. Arch. Toxicol. 2004, 78, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New Metabolites of di (2- Ethylhexyl) Phthalate (DEHP) in Human Urine and Serum After Single Oral Doses of Deuterium-Labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A Distribution in Serum, Urine, Placenta, Breast Milk, and Umbilical Cord Serum in a Birth Panel of Mother–Neonate Pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef]
- Nakao, T.; Akiyama, E.; Kakutani, H.; Mizuno, A.; Aozasa, O.; Akai, Y.; Ohta, S. Levels Oftetrabromobisphenol A, Tribromobisphenol A, Dibromobisphenol A, Monobromobisphenol A, and Bisphenol A in Japanese Breast Milk. Chem. Res. Toxicol. 2015, 28, 722–728. [Google Scholar] [CrossRef]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of Environmental Phenols and Parabens in Milk, Urine and Serum of Lactating North Carolina Women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Adenuga, A.A.; Ayinuola, O.; Adejuyigbe, E.A.; Ogunfowokan, A.O. Biomornitoring of phthalate esters in breast milk and urine samples as biomarkers for neonates’ exposure, using modified quechers method with agricultural biochar as dispersive solid-phase extraction absorbent. Microchem. J. 2020, 152, 104277. [Google Scholar] [CrossRef]
- Lakind, J.S.; Idri, F.; Naiman, D.Q.; Verner, M.A. Biomonitoring and Nonpersistent Chemicals—Understanding and Addressing Variability and Exposure Misclassification. Curr. Environ. Health Rep. 2019, 6, 16–21. [Google Scholar] [CrossRef]
- Koch, H.M.; Aylward, L.L.; Hays, S.M.; Smolders, R.; Moos, R.K.; Cocker, J.; Jones, K.; Warren, N.; Levy, L.; Bevan, R. Inter- and Intra-Individual Variation in Urinary Biomarker Concentrations over a 6-Day Sampling Period. Part 2: Personal Care Product Ingredients. Toxicol. Lett. 2014, 231, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, R.; Santonicola, S. Investigation on Bisphenol A Levels in Human Milk and Dairy Supply Chain: A Review. Food Chem. Toxicol. 2018, 114, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, B.; Yang, R.; Wu, Y.; Zhao, Y.; Li, C.; Zhang, J.; Xing, Y.; Shao, B. Bisphenol Analogues and Their Chlorinated Derivatives in Breast Milk in China: Occurrence and Exposure Assessment. J. Agric. Food Chem. 2021, 69, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Gatewood, J.D.; Howeth, C.; Rissman, E.F. Gestational Exposure to Bisphenol A and Cross-Fostering Affect Behaviors in Juvenile Mice. Haematol. Behav. 2010, 58, 754–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, X.-H.; Wang, Y.-M.; Luo, Q.-Q.; Ye, Y.-P. Perinatal Exposure to BPA Affects Sexual Differnentiation of Behaviors in Offspring Mice. Acta Psychol. Sin. 2009, 41, 832–841. [Google Scholar]
- Fujimoto, T.; Kubo, K.; Aou, S. Environmental Impact on Brain Functions: Low Dose Effects of Bisphenol A During Perinatal Critical Period. Int. Congr. Ser. 2007, 1301, 226–229. [Google Scholar] [CrossRef]
- Tian, Y.-H.; Baek, J.-H.; Lee, S.-Y.; Jang, C.-G. Prenatal and Postnatal Exposure to BPA Induces Anxiolytic Behaviors and Cognitive Deficits in Mice. Synapse 2006, 64, 432–439. [Google Scholar] [CrossRef]
- Guerranti, C.; Sbordoni, I.; Fanello, E.L.; Borghini, F.; Corsi, I.; Focardi, S.E. Levels of Phthalates in Human Milk Samples from Central Italy. Microchem. J. 2013, 107, 178–181. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Fisher, M.; MacPherson, S.; Lang, C.; Provencher, G.; LeBlanc, A.; Hauser, R.; Feeley, M.; Ayotte, P.; Neisa, A.; et al. Maternal and Early Life Exposure to Phthalates: The Plastics and Personal-Care Products Use in Pregnancy (P4) Study. Sci. Tot. Environ. 2016, 551–552, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression. Development of the 10-Item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Eberhard-Gran, M.; Eskild, A.; Tambs, K.; Opjordsmoen, S.; Samuelsen, S.O. Review of Validation Studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr. Scand. 2001, 104, 243–249. [Google Scholar] [CrossRef]
- Calafat, A.M.; Slakman, A.R.; Silva, M.J.; Herbert, A.R.; Needham, L.L. Automated Solid Phase Extraction and Quantitative Analysis of Human Milk for 13 Phthalate Metabolites. J. Chromatogr. B 2004, 805, 49–56. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Needham, L.L.; Calafat, A.M. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for Measuring Parabens, Triclosan, and Other Environmental Phenols in Human Milk. Anal. Chim. Acta 2008, 622, 150–156. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Kim, S.; Eom, S.; Kim, H.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D.; et al. Association Between Maternal Exposure to Major Phthalates, Heavy Metals, and Persistent Organic Pollutants, and the Neurodevelopmental Performances of Their Children at 1 to 2 Years of Age- CHECK Cohort Study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Stein, C.R.; Liu, M.; Ackerman, M.G.; Blakemore, J.K.; Long, S.E.; Pinna, G.; Romay-Tallon, R.; Kannan, K.; Zhu, H.; et al. Prenatal exposure to bisphenols and phthalates and postpartum depression: The role of neurosteroid hormone disruption. J. Clin. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.H.; Mukherjee, B.; Meeker, J.D. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in Pregnant Women: A Repeated Measures Analysis. Environ. Health Perspect. 2015, 123, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Zuo, H.X.; Li, J.Q.; Han, B.; Ke, C.J.; Liu, X.D.; Zhang, Y.C.; Li, L.; Yang, X. Di-(n-Butyl)-Phthalate-Induced Oxidative Stress and Depression-Like Behavior in Mice with or Without Ovalbumin Immunization. Biomed. Environ. Sci. 2014, 27, 268–280. [Google Scholar]
- Xu, Y.; Agrawal, S.; Cook, T.J.; Knipp, G.T. Di-(2-Ethylhexyl)-Phthalate Affects Lipid Profiling in Fetal Rat Brain upon Maternal Exposure. Arch. Toxicol. 2007, 81, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, W.; Li, Y.; Chen, X.; Xu, S. Mono-(2-Ethylhexyl) Phthalate Impairs Neurodevelopment: Inhibition of Proliferation and Promotion of Differentiation in PC12 Cells. Toxicol. Lett. 2011, 201, 34–41. [Google Scholar] [CrossRef]
- Wang, R.; Xu, X.; Zhu, Q. Pubertal Exposure to Di-(2-Ethylhexyl) Phthalate Influences Social Behavior and Dopamine Receptor D2 of Adult Female Mice. Chemosphere 2016, 144, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Wei, L.; Li, L. DEHP Reduces Thyroid Hormones via Interacting with Hormone Synthesis-Related Proteins, Deiodinases, Transthyretin, Receptors, and Hepatic Enzymes in Rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 12711–12719. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Gudsnuk, K.; Herbstman, J.B.; Tang, D.; Perera, F.P.; Champagne, F.A. DNA Methylation of BDNF as a Biomarker of Early-Life Adversity. Proc. Natl. Acad. Sci. USA 2015, 112, 6807–6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajaszan; Leranth. BPA Interferes with Synaptic Remodeling. Front. Neuroendocrinol. 2010, 31, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From Environmental Studies to Human Health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to BPA and Parabens During Pregnancy and Relations to Steroid Changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human Exposure to Endocrine Disrupting Compounds: Their Role in Reproductive Systems, Metabolic Syndrome and Breast Cancer. A Review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef]
- Jo, A.; Kim, S.; Ji, K.; Kho, Y.; Choi, K. Influence of Vegetarian Dietary Intervention on Urinary Paraben Concentrations: A Pilot Study with ‘Temple Stay’ Participants. Toxics 2020, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Kyung, M.S.; Ko, A.; Park, J.H.; Hwang, M.S.; Kwon, J.E.; Suh, J.H.; Lee, H.S.; Moon, G.I.; Hong, J.H.; et al. Urinary Concentrations of Parabens and Their Association with Demographic Factors: A Population-Based Cross-Sectional Study. Environ. Res. 2016, 146, 245–251. [Google Scholar] [CrossRef]
- Olaniyan, L.W.; Mkwetshana, N.; Okoh, A.I. Triclosan in Water, Implications for Human and Environmental Health. Springerplus 2016, 5, 1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yueh, M.; Tukey, R.H. Triclosan: A Widespread Environmental Toxicants with Many Biological Effects. Rec. 20.447-469. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weatherly, L.M.; Gosse, J.A. Triclosan Exposure, Transformation, and Human Health Effects. J. Toxicol. Environ. Health B 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R.; et al. Maternal Prenatal Exposures to Environmental Chemicals and Psychosocial/Stressors in the ECHO Program-Implications for Research on Perinatal Outcomes. J. Perinatal. 2020, 40, 10–24. [Google Scholar] [CrossRef]
| Characteristics | Categories | Total | Non-PPD Group (n = 126) | PPD Group (n = 95) | t/x2 | p |
|---|---|---|---|---|---|---|
| n (%) or M (SD) | ||||||
| Maternal age (years) | 31.3 (3.4) | 31.7 (3.6) | 30.7 (4.1) | 1.93 | 0.045 | |
| Pre-pregnancy BMI (kg/m2) | 21.3 (3.1) | 21.3 (3.3) | 21.4 (2.9) | −0.35 | 0.726 | |
| weight (kg) | 61.8 (9.4) | 62.0 (9.3) | 61.5 (9.6) | 0.45 | 0.652 | |
| Education | <College | 24 (10.6) | 6 (4.8) | 18 (18.9) | 1.94 | 0.160 |
| ≥College | 197 (89.4) | 120 (95.2) | 77 (81.1) | |||
| Household income (USD/month) | <5000 | 124 (56.1) | 66 (52.4) | 58 (61.1) | 0.63 | 0.030 |
| ≥5000 | 97 (43.9) | 60 (47.6) | 37 (38.9) | |||
| Employment status | Working | 167 (75.6) | 97 (77.0) | 70 (73.7) | 0.26 | 0.613 |
| Not working | 54 (24.4) | 29 (23.0) | 25 (26.3) | |||
| Age of first menarche | 13.1 (1.4) | 13.2 (1.3) | 13.0 (1.3) | 1.18 | 0.241 | |
| Menstruation cycle | Regular | 77 (34.8) | 45 (35.7) | 32 (33.7) | 0.09 | 0.764 |
| Irregular | 144 (65.2) | 81 (64.3) | 63 (66.3) | |||
| Menstrual pain | Yes | 185 (83.7) | 104 (82.5) | 81 (85.3) | 0.29 | 0.593 |
| No | 36 (16.3) | 22 (17.5) | 14 (14.7) | |||
| Neonatal gender | Male | 108 (48.9) | 57 (45.2) | 51 (53.7) | 1.24 | 0.266 |
| Female | 113 (51.1) | 69 (54.8) | 44 (46.3) | |||
| Neonatal age (day) | 33.7 (25.9) | 32.7 (25.5) | 35.0 (26.6) | −0.64 | 0.123 | |
| Neonatal birth weight (kg) | 3.2 (0.4) | 3.3 (0.4) | 3.20 (0.5) | 1.12 | 0.262 | |
| Neonatal birth height (cm) | 50.4 (2.3) | 50.3 (2.3) | 50.6 (2.3) | −1.08 | 0.283 | |
| Neonatal weight (kg) | 4.4 (1.2) | 4.40(1.16) | 4.36 (1.2) | 0.25 | 0.802 | |
| Neonatal height (cm) | 55.3 (4.8) | 55.5 (4.9) | 54.9 (4.6) | 0.63 | 0.530 | |
| EPDS score | 9.1 (4.3) | 4.0 (2.7) | 12.8 (3.4) | −20.94 | <0.001 | |
| Analyte | Percentile (μg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| LOD | n (% > LOD) | GM (SD) | 5th | 25th | 50th | 75th | 95th | |
| MEP | 0.131 | 102 (46.2) | 0.17 (2.37) | <LOD | <LOD | <LOD | 0.27 | 1.96 |
| MnBP | 0.282 | 161 (72.9) | 0.83 (3.16) | <LOD | <LOD | 0.89 | 1.86 | 8.46 |
| MiBP | 0.188 | 153 (69.2) | 0.47 (3.19) | <LOD | <LOD | 0.49 | 0.93 | 7.44 |
| MBzP | 0.082 | 12 (5.4) | 0.06 (1.37) | <LOD | <LOD | <LOD | <LOD | 0.10 |
| MEHP | 0.139 | 184 (83.3) | 1.44 (6.00) | <LOD | 0.35 | 1.72 | 4.88 | 24.38 |
| MiNP | 0.043 | 158 (71.5) | 0.10 (2.84) | <LOD | <LOD | 0.08 | 0.22 | 0.61 |
| BPA | 0.076 | 107 (48.4) | 0.12 (2.91) | <LOD | <LOD | <LOD | 0.23 | 0.88 |
| TCS | 0.035 | 57 (25.8) | 0.04 (2.62) | <LOD | <LOD | <LOD | 0.04 | 0.29 |
| MP | 0.101 | 130 (58.8) | 0.33 (5.61) | <LOD | <LOD | 0.18 | 1.12 | 10.34 |
| EP | 0.035 | 195 (88.2) | 0.46 (5.23) | <LOD | 0.14 | 0.62 | 1.62 | 4.60 |
| PP | 0.134 | 93 (42.1) | 0.21 (3.30) | <LOD | <LOD | <LOD | 0.39 | 2.17 |
| Characteristics | Total | Non-PPD Group | PPD Group | t/x2 | p |
|---|---|---|---|---|---|
| Mean (SD) | |||||
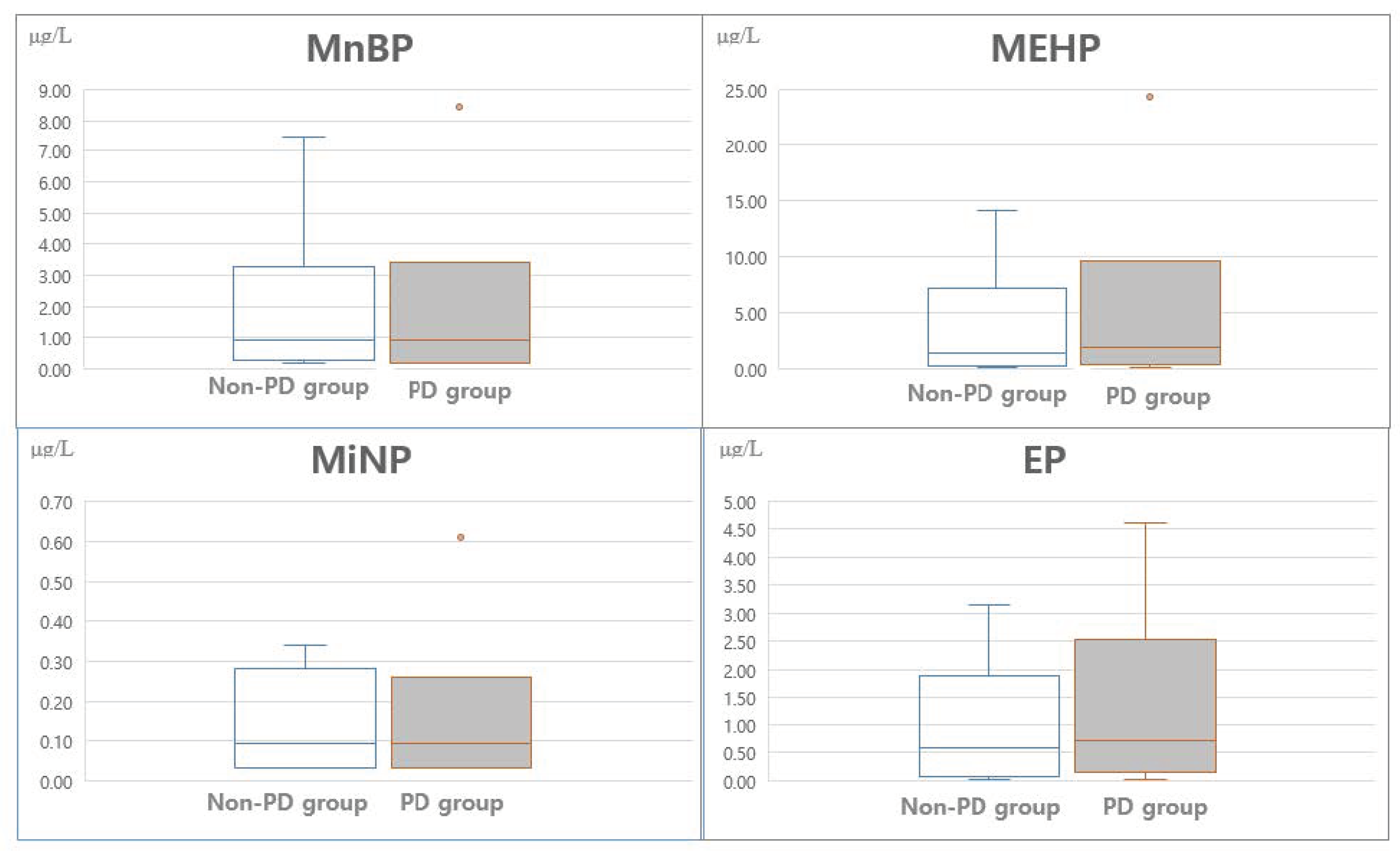
| MnBP | 0.83 (3.16) | 0.81 (2.05)) | 0.85 (2.96) | 1.35 | 0.178 |
| MEHP | 1.44 (6.00) | 1.05 (4.86) | 1.82 (6.11) | 1.79 | 0.076 |
| MiNP | 0.10 (2.84) | 0.04 (2.16) | 0.15 (2.37) | 2.56 | 0.112 |
| EP | 0.46 (5.23) | 0.44 (3.96) | 0.48 (4.98) | −0.62 | 0.534 |
| Crude OR | 95% CI | p | Adjusted OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| MnBP | 0.95 | 0.61–1.37 | 0.541 | 0.94 | 0.64–1.38 | 0.745 |
| MEHP | 1.12 | 1.04–1.14 | 0.050 | 1.13 | 1.03–1.12 | 0.053 |
| MiNP | 0.22 | 0.44–1.38 | 0.330 | 0.23 | 0.04–1.35 | 0.394 |
| EP | 1.18 | 0.99–1.40 | 0.089 | 1.17 | 0.99–1.32 | 0.091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Shin, H.-S.; Lee, W.-H. Impact of Endocrine-Disrupting Chemicals in Breast Milk on Postpartum Depression in Korean Mothers. Int. J. Environ. Res. Public Health 2021, 18, 4444. https://doi.org/10.3390/ijerph18094444
Kim J-H, Shin H-S, Lee W-H. Impact of Endocrine-Disrupting Chemicals in Breast Milk on Postpartum Depression in Korean Mothers. International Journal of Environmental Research and Public Health. 2021; 18(9):4444. https://doi.org/10.3390/ijerph18094444
Chicago/Turabian StyleKim, Ju-Hee, Hye-Sook Shin, and Woo-Hyoung Lee. 2021. "Impact of Endocrine-Disrupting Chemicals in Breast Milk on Postpartum Depression in Korean Mothers" International Journal of Environmental Research and Public Health 18, no. 9: 4444. https://doi.org/10.3390/ijerph18094444






