The Interaction between Lockdown-Specific Conditions and Family-Specific Variables Explains the Presence of Child Insomnia during COVID-19: A Key Response to the Current Debate
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Materials and Design
2.3. Data Pre-Processing
2.4. Data Analysis
3. Results
3.1. Participants
3.2. Linear Multiple Regression on Child Sleep Disorder and Insomnia (DIMS)
3.2.1. French Sample
3.2.2. Swiss Sample
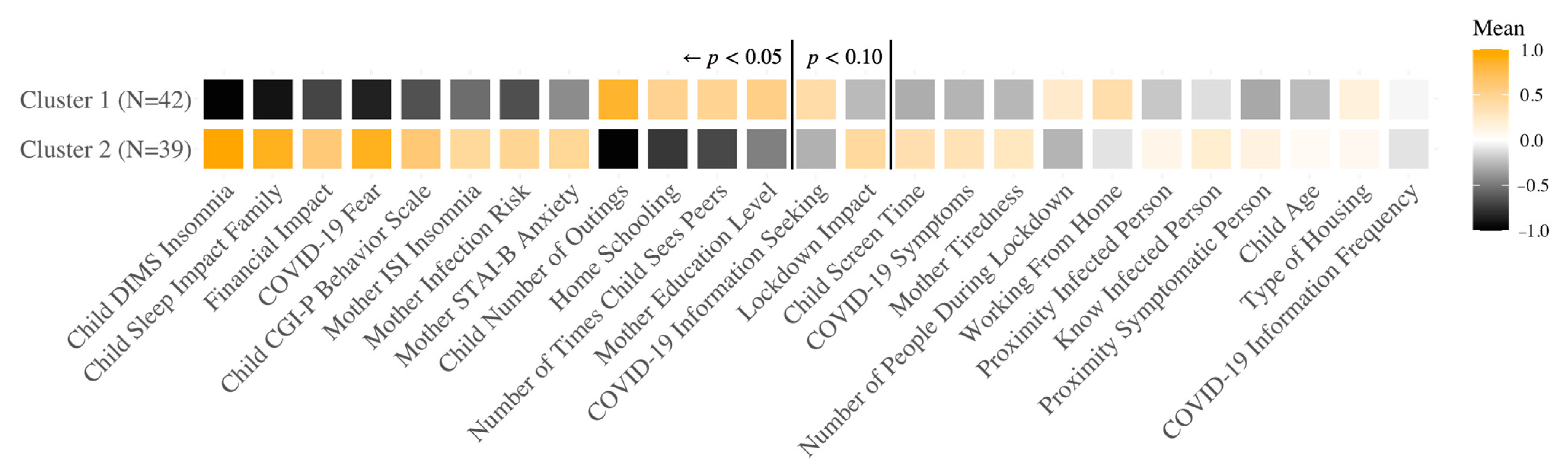
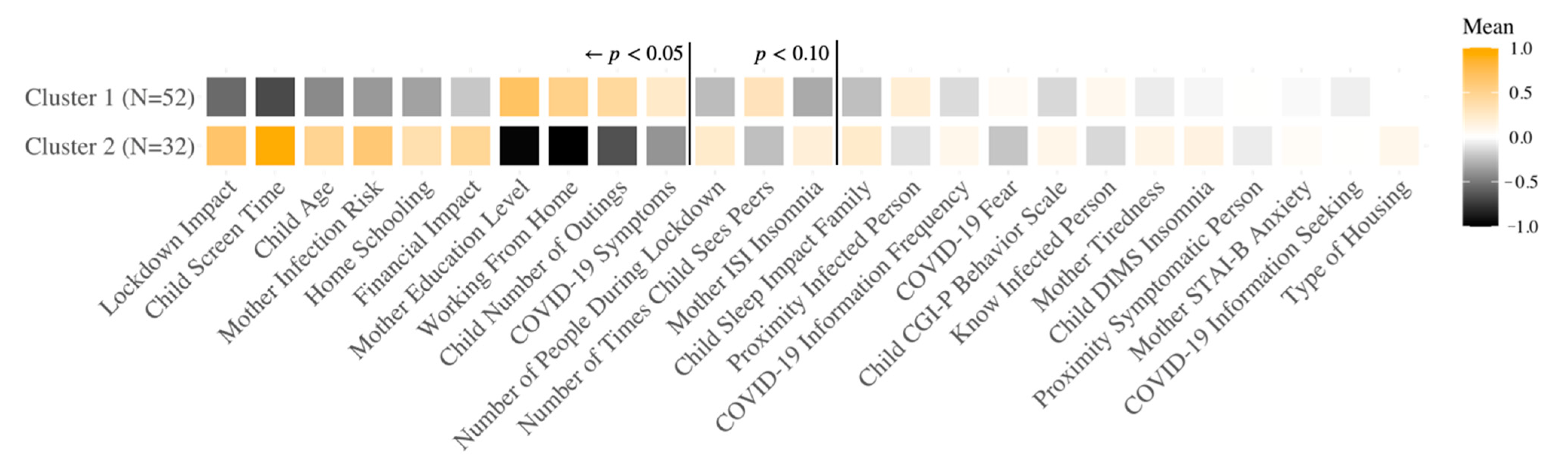
3.3. Different COVID-19 Response Groups Based on Clustering Analysis
3.3.1. French Sample
3.3.2. Swiss Sample
3.4. Integrative Model of COVID-19 Variable Relationships through Structural Equation Modeling
French Sample
4. Discussion
4.1. Identifying a Potential Source of the Controversial Findings
4.2. Interaction between Lockdown and Family Variables Predicts Insomnia in Young Children
4.3. Explaining the Main Factors Influencing Child Sleep Disturbance during a Pandemic Response
4.4. Further Identifying the Causal Links through Structural Equation Modeling
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Giorgio, E.; Di Riso, D.; Mioni, G.; Cellini, N. The interplay between mothers’ and children behavioral and psychological factors during COVID-19: An Italian study. Eur. Child Adolesc. Psychiatry 2020, 30, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Dellagiulia, A.; Lionetti, F.; Fasolo, M.; Verderame, C.; Sperati, A.; Alessandri, G. Early impact of COVID-19 lockdown on children’s sleep: A four-week longitudinal study. J. Clin. Sleep Med. 2020, 16, 1639–1640. [Google Scholar] [CrossRef]
- Cellini, N.; Di Giorgio, E.; Mioni, G.; Di Riso, D. Sleep and Psychological Difficulties in Italian School-Age Children during COVID-19 Lockdown. J. Pediatr. Psychol. 2021, 46, 153–167. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Ohler, A.; Gileles-Hillel, A.; Choshen-Hillel, S.; Gozal, D.; Bruni, O.; Aydinoz, S.; Cortese, R.; Kheirandish-Gozal, L. Effects of the COVID-19 lockdown on sleep duration in children and adolescents: A survey across different continents. Pediatr. Pulmonol. 2021, 56, 2265–2273. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, H.; Jin, Q.; Wang, G.; Yang, Z.; Chen, H.; Yan, H.; Rao, W.; Owens, J. Sleep of preschoolers during the coronavirus disease 2019 (COVID-19) outbreak. J. Sleep Res. 2021, 30, e13142. [Google Scholar] [CrossRef] [PubMed]
- Cerasuolo, M.; Malloggi, S.; Conte, F.; Albinni, B.; De Rosa, O.; Rescott, M.L.; Giganti, F.; Ficca, G. The Effects of the COVID19-Related Lockdown Are Modulated by Age: An Italian Study in Toddlers and Pre-Schoolers. Brain Sci. 2021, 11, 1051. [Google Scholar] [CrossRef]
- Lecuelle, F.; Leslie, W.; Huguelet, S.; Franco, P.; Putois, B. Did the COVID-19 lockdown really have no impact on young children’s sleep? J. Clin. Sleep Med. 2020, 16, 2121. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Malorgio, E.; Doria, M.; Finotti, E.; Spruyt, K.; Melegari, M.; Villa, M.; Ferri, R. Changes in sleep patterns and disturbances in children and adolescents in Italy during the Covid-19 outbreak. Sleep Med. 2021, in press. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, A.; Gorwood, P. The consequences of the COVID-19 pandemic on mental health and implications for clinical practice. Eur. Psychiatry 2020, 63, e32. [Google Scholar] [CrossRef] [Green Version]
- Palacio-Ortiz, J.D.; Londoño-Herrera, J.P.; Nanclares-Márquez, A.; Robledo-Rengifo, P.; Quintero-Cadavid, C.P. Psychiatric disorders in children and adolescents during the COVID-19 pandemic. Rev. Colomb. Psiquiatr. (English ed.) 2020, 49, 279–288. [Google Scholar] [CrossRef]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiatry 2020, 33, e100213. [Google Scholar] [CrossRef] [Green Version]
- Gualano, M.R.; Lo Moro, G.; Voglino, G.; Bert, F.; Siliquini, R. Effects of Covid-19 Lockdown on Mental Health and Sleep Disturbances in Italy. Int. J. Environ. Res. Public Health 2020, 17, 4779. [Google Scholar] [CrossRef]
- Hidalgo, M.; Balluerka, N.; Gorostiaga, A.; Espada, J.; Santed, M.; Padilla, J.; Gómez-Benito, J. The Psychological Consequences of COVID-19 and Lockdown in the Spanish Population: An Exploratory Sequential Design. Int. J. Environ. Res. Public Health 2020, 17, 8578. [Google Scholar] [CrossRef]
- Franceschini, C.; Musetti, A.; Zenesini, C.; Palagini, L.; Scarpelli, S.; Quattropani, M.; Lenzo, V.; Freda, M.; Lemmo, D.; Vegni, E.; et al. Poor Sleep Quality and Its Consequences on Mental Health During the COVID-19 Lockdown in Italy. Front. Psychol. 2020, 11, 574475. [Google Scholar] [CrossRef]
- Ausín, B.; González-Sanguino, C.; Castellanos, M.Á.; Muñoz, M. Gender-related differences in the psychological impact of confinement as a consequence of COVID-19 in Spain. J. Gend. Stud. 2020, 30, 29–38. [Google Scholar] [CrossRef]
- Özdin, S. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: The importance of gender. Int. J. Soc. Psychiatry 2020, 66, 504–511. [Google Scholar] [CrossRef]
- Casagrande, M.; Favieri, F.; Tambelli, R.; Forte, G. The enemy who sealed the world: Effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020, 75, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Sampogna, G.; Giallonardo, V.; Vecchio, V.; Del Luciano, M.; Albert, U.; Carmassi, C.; Carrà, G.; Cirulli, F.; Dell’Osso, B.; et al. Effects of the lockdown on the mental health of the general population during the COVID-19 pandemic in Italy: Results from the COMET collaborative network. Eur. Psychiatry 2020, 63, e87. [Google Scholar] [CrossRef] [PubMed]
- Markovic, A.; Mühlematter, C.; Beaugrand, M.; Camos, V.; Kurth, S. Severe effects of the COVID-19 confinement on young children’s sleep: A longitudinal study identifying risk and protective factors. J. Sleep Res. 2021, 30, e13314. [Google Scholar] [CrossRef]
- Kokou-Kpolou, C.; Megalakaki, O.; Laimou, D.; Kousouri, M. Insomnia during COVID-19 pandemic and lockdown: Prevalence, severity, and associated risk factors in French population. Psychiatry Res. 2020, 290, 113128. [Google Scholar] [CrossRef] [PubMed]
- Voitsidis, P.; Gliatas, I.; Bairachtari, V.; Papadopoulou, K.; Papageorgiou, G.; Parlapani, E.; Syngelakis, M.; Holeva, V.; Diakogiannis, I. Insomnia during the COVID-19 pandemic in a Greek population. Psychiatry Res. 2020, 289, 113076. [Google Scholar] [CrossRef]
- Lin, C.; Broström, A.; Griffiths, M.; Pakpour, A. Investigating mediated effects of fear of COVID-19 and COVID-19 misunderstanding in the association between problematic social media use, psychological distress, and insomnia. Internet Interv. 2020, 21, 100345. [Google Scholar] [CrossRef]
- Trnka, R.; Lorencova, R. Fear, anger, and media-induced trauma during the outbreak of COVID-19 in the Czech Republic. Psychol. Trauma 2020, 12, 546–549. [Google Scholar] [CrossRef]
- Yoshioka, T.; Maeda, Y. COVID-19 Stigma Induced by Local Government and Media Reporting in Japan: It’s Time to Reconsider Risk Communication Lessons from the Fukushima Daiichi Nuclear Disaster. J. Epidemiol. 2020, 30, 372–373. [Google Scholar] [CrossRef]
- Feldman, R. Parent-infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monogr. Soc. Res. Child Dev. 2012, 77, 42–51. [Google Scholar] [CrossRef]
- Feldman, R.; Magori-Cohen, R.; Galili, G.; Singer, M.; Louzoun, Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. 2011, 34, 569–577. [Google Scholar] [CrossRef]
- Mills-Koonce, W.R.; Propper, C.; Gariepy, J.L.; Barnett, M.; Moore, G.A.; Calkins, S.; Cox, M.J. Psychophysiological Correlates of Parenting Behavior in Mothers of Young Children. Dev. Psychobiol. 2009, 51, 650–661. [Google Scholar] [CrossRef]
- Ham, J.; Tronick, E. Relational psychophysiology: Lessons from mother-infant physiology research on dyadically expanded states of consciousness. Psychother. Res. 2009, 19, 619–632. [Google Scholar] [CrossRef]
- Feldman, R. Parent–Infant Synchrony: Biological Foundations and Developmental Outcomes. Curr. Dir. Psychol. Sci. 2016, 16, 340–345. [Google Scholar] [CrossRef]
- Azhari, A.; Leck, W.Q.; Gabrieli, G.; Bizzego, A.; Rigo, P.; Setoh, P.; Bornstein, M.H.; Esposito, G. Parenting Stress Undermines Mother-Child Brain-to-Brain Synchrony: A Hyperscanning Study. Sci. Rep. 2019, 9, 11407. [Google Scholar] [CrossRef] [Green Version]
- Tikotzky, L. Parenting and sleep in early childhood. Curr. Opin. Psychol. 2017, 15, 118–124. [Google Scholar] [CrossRef]
- Camerota, M.; Propper, C.B.; Teti, D.M. Intrinsic and extrinsic factors predicting infant sleep: Moving beyond main effects. Dev. Rev. 2019, 53, 100871. [Google Scholar] [CrossRef]
- Teti, D.; Crosby, B. Maternal depressive symptoms, dysfunctional cognitions, and infant night waking: The role of maternal nighttime behavior. Child Dev. 2012, 83, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Zreik, G.; Asraf, K.; Haimov, I.; Tikotzky, L. Maternal perceptions of sleep problems among children and mothers during the coronavirus disease 2019 (COVID-19) pandemic in Israel. J. Sleep Res. 2021, 30, e13201. [Google Scholar] [CrossRef] [PubMed]
- Suveg, C.; Shaffer, A.; Davis, M. Family stress moderates relations between physiological and behavioral synchrony and child self-regulation in mother–preschooler dyads. Dev. Psychobiol. 2016, 58, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Panchal, U.; Salazar de Pablo, G.; Franco, M.; Moreno, C.; Parellada, M.; Arango, C.; Fusar-Poli, P. The impact of COVID-19 lockdown on child and adolescent mental health: Systematic review. Eur. Child Adolesc. Psychiatry 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Suffren, S.; Dubois-Comtois, K.; Lemelin, J.; St-Laurent, D.; Milot, T. Relations between Child and Parent Fears and Changes in Family Functioning Related to COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 1786. [Google Scholar] [CrossRef]
- Aguilar-Farias, N.; Toledo-Vargas, M.; Miranda-Marquez, S.; Cortinez-O’Ryan, A.; Cristi-Montero, C.; Rodriguez-Rodriguez, F.; Martino-Fuentealba, P.; Okely, A.D.; del Pozo Cruz, B. Sociodemographic Predictors of Changes in Physical Activity, Screen Time, and Sleep among Toddlers and Preschoolers in Chile during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 18, 176. [Google Scholar] [CrossRef]
- Susilowati, I.H.; Nugraha, S.; Alimoeso, S.; Hasiholan, B.P. Screen Time for Preschool Children: Learning from Home during the COVID-19 Pandemic. Glob. Pediatr. Health 2021, 8, 2333794X211017836. [Google Scholar] [CrossRef]
- Marinelli, M.; Sunyer, J.; Alvarez-Pedrerol, M.; Iñiguez, C.; Torrent, M.; Vioque, J.; Turner, M.C.; Julvez, J. Hours of Television Viewing and Sleep Duration in Children: A Multicenter Birth Cohort Study. JAMA Pediatr. 2014, 168, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Christakis, D.A. The Association between Television Viewing and Irregular Sleep Schedules Among Children Less Than 3 Years of Age. Pediatrics 2005, 116, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Anders, R.; Chaussoy, L.; Herbillon, V.; Franco, P.; Putois, B. Screen exposure exacerbates ADHD symptoms indirectly through increased sleep disturbance. Sleep Med. 2021, 83, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Madigan, S.; Browne, D.; Racine, N.; Mori, C.; Tough, S. Association between Screen Time and Children’s Performance on a Developmental Screening Test. JAMA Pediatr. 2019, 173, 244–250. [Google Scholar] [CrossRef]
- Cellini, N.; Di Giorgio, E.; Mioni, G.; Di Riso, D. Sleep quality, timing, and psychological difficulties in Italian school-age children and their mothers during COVID-19 lockdown. PsyArXiv Prepr. 2020. [Google Scholar] [CrossRef]
- Lam, P.; Hiscock, H.; Wake, M. Outcomes of Infant Sleep Problems: A Longitudinal Study of Sleep, Behavior, and Maternal Well-Being. Pediatrics 2003, 111, e203–e207. [Google Scholar] [CrossRef] [Green Version]
- Basta, M.; Chrousos, G.P.; Vela-Bueno, A.; Vgontzas, A.N. Chronic insomnia and stress system. Sleep Med. Clin. 2007, 2, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Mendoza, J.; Vgontzas, A.N.; Calhoun, S.L.; Vgontzas, A.; Tsaoussoglou, M.; Gaines, J.; Liao, D.; Chrousos, G.P.; Bixler, E.O. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur. J. Clin. Investig. 2014, 44, 493–500. [Google Scholar] [CrossRef]
- Wu, K.H.; Tsai, C.; Wu, H.P.; Sieber, M.; Peng, C.T.; Chao, Y.H. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: Enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013, 22, 2041–2051. [Google Scholar] [CrossRef] [Green Version]
- Hankins, J.S.; Verevkina, N.I.; Smeltzer, M.P.; Wu, S.; Aygun, B.; Clarke, D.F. Assessment of sleep-related disorders in children with sickle cell disease. Hemoglobin 2014, 38, 244–251. [Google Scholar] [CrossRef]
- Abidin, R.R. Introduction to the Special issue: The Stresses of Parenting. J. Clin. Child Psychol. 1990, 19, 298–301. [Google Scholar] [CrossRef]
- Lacharité, C.; Ethier, L.; Piché, C. Parental stress in mothers of preschool children: Validation and Quebec norms for the Parental Stress Inventory. Sante Ment. Que. 1992, 17, 183–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, W.; Lucas-Thompson, R.; Germo, G.; Keller, M.; Davis, E.; Sandman, C. Eye of the beholder? Maternal mental health and the quality of infant sleep. Soc. Sci. Med. 2013, 79, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byars, K.C.; Yeomans-Maldonado, G.; Noll, J.G. Parental functioning and pediatric sleep disturbance: An examination of factors associated with parenting stress in children clinically referred for evaluation of insomnia. Sleep Med. 2011, 12, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, E. The Relative Influence of Child and Environmental characteristics on sleep disturbances in the first and second years of life. J. Dev. Behav. Pediatr. 1985, 6, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.; Lacks, P.; Futterman, A. Effects of parent training on infant sleeping patterns, parents’ stress, and perceived parental competence. J. Consult. Clin. Psychol. 1992, 60, 41–48. [Google Scholar] [CrossRef]
- Sinai, D.; Tikotzky, L. Infant sleep, parental sleep and parenting stress in families of mothers on maternity leave and in families of working mothers. Infant Behav. Dev. 2012, 35, 179–186. [Google Scholar] [CrossRef]
- Mindell, J. Insomnia in children and adolescents. Princ. Manag. Insomnia 2003, 8, 125–135. [Google Scholar]
- Schlarb, A.A.; Bihlmaier, I.; Velten-Schurian, K.; Poets, C.F.; Hautzinger, M. Short- and Long-Term Effects of CBT-I in Groups for School-Age Children Suffering from Chronic Insomnia: The KiSS-Program. Behav. Sleep Med. 2018, 16, 380–397. [Google Scholar] [CrossRef]
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef]
- Owens, J.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Gagnon, C. Validation de L’INDEX de Sévérité de L’insomnie Dans les Cliniques de Médecine Générale. Ph.D. Thesis, Université Laval, Quebec City, QC, Canada, 2012. [Google Scholar]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Lecuelle, F.; Gustin, M.; Leslie, W.; Mindell, J.; Franco, P.; Putois, B. French validation of the sleep disturbance scale for children (SDSC) in young children (aged 6 months to 4 years). Sleep Med. 2020, 67, 56–65. [Google Scholar] [CrossRef]
- Spruyt, K.; Gozal, D. Development of pediatric sleep questionnaires as diagnostic or epidemiological tools: A brief review of dos and don’ts. Sleep Med. Rev. 2011, 15, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPaul, G.J. Parent and Teacher Ratings of ADHD Symptoms: Psychometric Properties in a Community-Based Sample. J. Clin. Child Psychol. 1991, 20, 245–253. [Google Scholar] [CrossRef]
- Fumeaux, P.; Mercier, C.; Roche, S.; Iwaz, J.; Bader, M.; Stéphan, P.; Ecochard, R.; Revol, O. Validation of the French Version of Conners’ Parent Rating Scale Revised, Short Version: Factorial Structure and Reliability. Can. J. Psychiatry 2016, 61, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruchon-Schweitzer, M.; Paulhan, I. Manuel du STAI-Y de CD Spielberger, Adaptation Française; ECPA: Paris, France, 1993.
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Yeo, I.; Johnson, R.A. A new family of power transformations to improve normality or symmetry. Biometrika 2000, 87, 954–959. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. Wiley Interdiscip. Rev. Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Sharma, S.; Mukherjee, S.; Kumar, A.; Dillon, W.R. A simulation study to investigate the use of cutoff values for assessing model fit in covariance structure models. J. Bus. Res. 2005, 58, 935–943. [Google Scholar] [CrossRef]
- Jöreskog, K.G. A general approach to confirmatory maximum likelihood factor analysis. Psychom 1969, 34, 183–202. [Google Scholar] [CrossRef]
- Bentler, P. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238–246. [Google Scholar] [CrossRef]
- Marsh, H.W.; Balla, J.R.; McDonald, R.P. Goodness-of-Fit Indexes in Confirmatory Factor Analysis: The Effect of Sample Size. Psychol. Bull. 1988, 103, 391–410. [Google Scholar] [CrossRef]
- Bollen, K.A. A New Incremental Fit Index for General Structural Equation Models. Sociol. Methods Res. 1989, 17, 303–316. [Google Scholar] [CrossRef]
- Tucker, L.R.; Lewis, C. A reliability coefficient for maximum likelihood factor analysis. Psychom 1973, 38, 1–10. [Google Scholar] [CrossRef]
- Steiger, J.H.; Lind, J.C. Statistically based tests for the number of common factors. In Proceedings of the Annual Meeting of the Psychometric Society, Iowa City, IA, USA, 28 May 1980. [Google Scholar]
- Maccallum, R.C.; Browne, M.; Sugawara, H. Power analysis and determination of sample size for covariance structure modeling. Psychol. Methods 1996, 1, 130–149. [Google Scholar] [CrossRef]
- Jöreskog, K.; Sörbom, D. Analysis of Linear Structural Relationships by Maximum Likelihood and Least Squares Methods; University of Uppsala: Uppsala, Sweden, 1981. [Google Scholar]
- Schermelleh-engel, K.; Moosbrugger, H.; Müller, H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-offit measures. METHODS Psychol. Res. 2003, 8, 23–74. [Google Scholar]
- Putois, B.; Leslie, W.; Gustin, M.P.; Challamel, M.-J.; Raoux, A.; Guignard-Perret, A.; Weick, D.; Sauzeau, J.-B.; Herbillon, V.; Zourou, F.; et al. The French Sleep Disturbance Scale for Children. Sleep Med. 2017, 32, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Symon, B.; Crichton, G. The joy of parenting: Infant sleep intervention to improve maternal emotional well-being and infant sleep. Singapore Med. J. 2017, 58, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Newby, J.; O’Moore, K.; Tang, S.; Christensen, H.; Faasse, K. Acute mental health responses during the COVID-19 pandemic in Australia. PLoS ONE 2020, 15, e0236562. [Google Scholar] [CrossRef]
- Goldberger-Raskin, B.; Gothelf, D.; Bachner-Melman, R.; Lang, C.; Kushnir, J. The association between sleep disturbances of children with anxiety disorders and those of their mothers. Sleep Med. 2018, 43, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.; Ho, R. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.R.; Carvalho, L.B.C.; Ruotolo, F.; de Morais, J.F.; Prado, L.B.F.; Prado, G.F. Sleep disturbance scale for children: Translation, cultural adaptation, and validation. Sleep Med. 2009, 10, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-M.; Qian, Z.; Wang, J.; Vaughn, M.G.; Lee, Y.L.; Dong, G.-H. Validation of the Sleep Disturbance Scale for Children and prevalence of parent-reported sleep disorder symptoms in Chinese children. Sleep Med. 2014, 15, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Marriner, A.M.; Pestell, C.; Bayliss, D.M.; McCann, M.; Bucks, R.S. Confirmatory factor analysis of the Sleep Disturbance Scale for Children (SDSC) in a clinical sample of children and adolescents. J. Sleep Res. 2017, 26, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Bruni, O.; Brogna, C.; Ferri, R.; Galluccio, C.; De Clemente, V.; Di Jorio, M.; Quintiliani, M.; Ricci, D.; Mercuri, E. Application of the Sleep Disturbance Scale for Children (SDSC) in preschool age. Eur. J. Paediatr. Neurol. 2013, 17, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Spruyt, K.; Gozal, D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep Med. Rev. 2011, 15, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | β | CI | p |
|---|---|---|---|
| Child Sleep Impact Family | 0.59 | 0.46–0.73 | <0.001 |
| Child Behavior: Conners’ Global Index | 0.33 | 0.20–0.46 | <0.001 |
| Child Gender | 0.23 | 0.11–0.34 | <0.001 |
| Proximity Infected Person | 0.23 | 0.10–0.36 | 0.001 |
| Lockdown Impact | 0.20 | 0.07–0.33 | 0.003 |
| Mother Age | 0.19 | 0.06–0.32 | 0.004 |
| Number of People During Lockdown | −0.35 | −0.48–−0.22 | <0.001 |
| Number of Times Child Sees Peers | −0.29 | −0.41–−0.16 | <0.001 |
| Know Infected Person | −0.23 | −0.36–−0.10 | 0.001 |
| COVID-19 Information Seeking | 0.11 | −0.02–0.23 | 0.09 |
| Working From Home | 0.11 | −0.02–0.24 | 0.09 |
| Mother Infection Risk | −0.11 | −0.23–0.02 | 0.09 |
| Mother Insomnia Severity Index | 0.11 | −0.02–0.24 | 0.09 |
| Proximity Symptomatic Person | 0.10 | −0.03–0.23 | 0.14 |
| COVID-19 Information Frequency | −0.10 | −0.24–0.04 | 0.17 |
| Financial Impact | 0.09 | −0.04–0.22 | 0.16 |
| Education Level | −0.08 | −0.20–0.04 | 0.18 |
| Variable | β | CI | p |
|---|---|---|---|
| Child Sleep Impact Family | 0.61 | 0.47–0.74 | <0.001 |
| Financial Impact | 0.29 | 0.17–0.41 | <0.001 |
| Mother Insomnia Severity Index | 0.24 | 0.11–0.37 | <0.001 |
| Education Level | 0.19 | 0.06–0.33 | 0.01 |
| COVID-19 Fear | 0.14 | 0.02–0.26 | 0.03 |
| Work Duration | −0.15 | −0.27–−0.03 | 0.02 |
| Mother Infection Risk | −0.13 | −0.26–−0.01 | 0.03 |
| Proximity Infected Person | 0.11 | −0.00–0.23 | 0.06 |
| Mother Age | 0.12 | −0.01–0.24 | 0.07 |
| Working From Home | −0.10 | −0.23–0.03 | 0.12 |
| Indices | Observed Value | Acceptable Threshold |
|---|---|---|
| Model χ²/df [73] | 1.19 | <5.0 |
| CFI 1 [74] | 0.93 | >0.90 |
| IFI 2 [75,76] | 0.93 | >0.90 |
| NNFI 3 (TLI) [77] | 0.92 | >0.90 |
| RMSEA 4 [78] | 0.049 | <0.10 |
| RMSEA p Close Fit [79] | 0.505 | >0.10 |
| RMSEA 90% Confidence Interval [79] | [0.00; 0.079] | [0.00; Close to RMSEA] |
| GFI 5 [80] | 0.82 | >0.90 |
| AGFI 6 [80] | 0.74 | >0.90 |
| Model χ² (df = 105) 7 | 125.29, p = 0.09 | p > 0.05 |
| Baseline model χ² (df = 126) 7 | 414.47, p < 0.001 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, R.; Lecuelle, F.; Perrin, C.; Ruyter, S.; Franco, P.; Huguelet, S.; Putois, B. The Interaction between Lockdown-Specific Conditions and Family-Specific Variables Explains the Presence of Child Insomnia during COVID-19: A Key Response to the Current Debate. Int. J. Environ. Res. Public Health 2021, 18, 12503. https://doi.org/10.3390/ijerph182312503
Anders R, Lecuelle F, Perrin C, Ruyter S, Franco P, Huguelet S, Putois B. The Interaction between Lockdown-Specific Conditions and Family-Specific Variables Explains the Presence of Child Insomnia during COVID-19: A Key Response to the Current Debate. International Journal of Environmental Research and Public Health. 2021; 18(23):12503. https://doi.org/10.3390/ijerph182312503
Chicago/Turabian StyleAnders, Royce, Florian Lecuelle, Clément Perrin, Swann Ruyter, Patricia Franco, Stéphanie Huguelet, and Benjamin Putois. 2021. "The Interaction between Lockdown-Specific Conditions and Family-Specific Variables Explains the Presence of Child Insomnia during COVID-19: A Key Response to the Current Debate" International Journal of Environmental Research and Public Health 18, no. 23: 12503. https://doi.org/10.3390/ijerph182312503






