Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever
Abstract
:1. Introduction
2. Materials and Methods
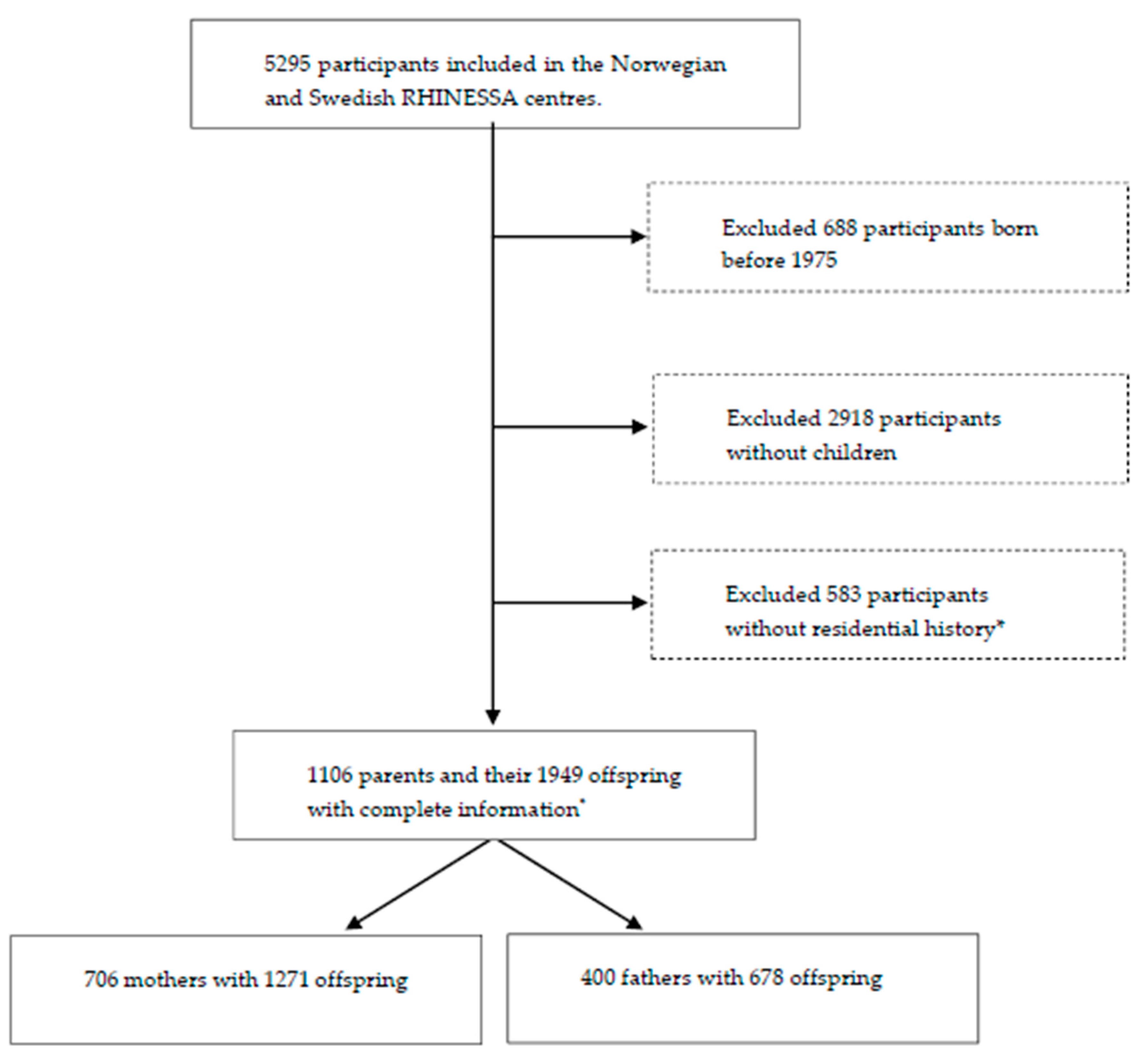
2.1. Study Design and Population
2.2. Residential Address History
2.3. Outcomes
2.4. Exposure Assessment
2.4.1. Air Pollution
2.4.2. Greenness
2.5. Time Windows for Exposures
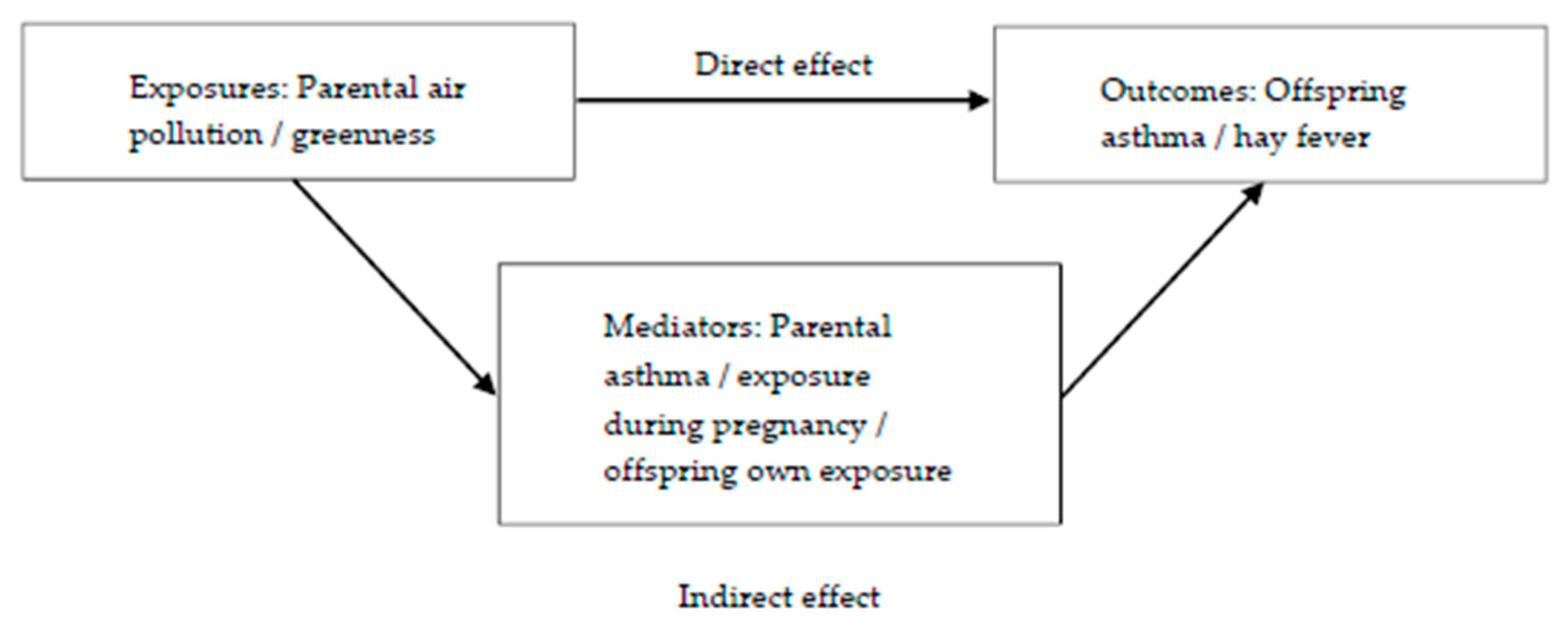
2.6. Covariates and Mediators
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Air Quality in Europe—2015 Report by the European Environment Agency (EEA). Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2015 (accessed on 11 August 2020).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [Green Version]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Molter, A.; Simpson, A.; Berdel, D.; Brunekreef, B.; Custovic, A.; Cyrys, J.; de Jongste, J.; de Vocht, F.; Fuertes, E.; Gehring, U.; et al. A multicentre study of air pollution exposure and childhood asthma prevalence: The ESCAPE project. Eur. Respir. J. 2015, 45, 610–624. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulphur Dioxide. Global Update 2005. Available online: https://www.who.int/airpollution/publications/aqg2005/en/ (accessed on 11 August 2020).
- Air Quality Standards under the Air Quality Directive from the European Environment Agency (EEA, 2016). Available online: https://www.eea.europa.eu/data-and-maps/figures/air-quality-standards-under-the (accessed on 11 August 2020).
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef]
- Lambert, K.A.; Bowatte, G.; Tham, R.; Lodge, C.; Prendergast, L.; Heinrich, J.; Abramson, M.J.; Dharmage, S.C.; Erbas, B. Residential greenness and allergic respiratory diseases in children and adolescents—A systematic review and meta-analysis. Environ. Res. 2017, 159, 212–221. [Google Scholar] [CrossRef]
- Eisenman, T.S.; Churkina, G.; Jariwala, S.P.; Kumar, P.; Lovasi, G.S.; Pataki, D.E.; Weinberger, K.R.; Whitlow, T.H. Urban trees, air quality, and asthma: An interdisciplinary review. Landsc. Urban Plan. 2019, 187, 47–59. [Google Scholar] [CrossRef]
- Lambert, K.A.; Bowatte, G.; Tham, R.; Lodge, C.J.; Prendergast, L.A.; Heinrich, J.; Abramson, M.J.; Dharmage, S.C.; Erbas, B. Greenspace and Atopic Sensitization in Children and Adolescents—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2539. [Google Scholar] [CrossRef] [Green Version]
- Squillacioti, G.; Bellisario, V.; Levra, S.; Piccioni, P.; Bono, R. Greenness Availability and Respiratory Health in a Population of Urbanised Children in North-Western Italy. Int. J. Environ. Res. Public Health 2019, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Vienneau, D.; de Hoogh, K.; Faeh, D.; Kaufmann, M.; Wunderli, J.M.; Roosli, M. More than clean air and tranquillity: Residential green is independently associated with decreasing mortality. Environ. Int. 2017, 108, 176–184. [Google Scholar] [CrossRef]
- Gascon, M.; Triguero-Mas, M.; Martinez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasencia, A.; Nieuwenhuijsen, M.J. Residential green spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef] [Green Version]
- James, P.; Hart, J.E.; Banay, R.F.; Laden, F. Exposure to Greenness and Mortality in a Nationwide Prospective Cohort Study of Women. Environ. Health Perspect. 2016, 124, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Fong, K.C.; Hart, J.E.; James, P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr. Environ. Health Rep. 2018, 5, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Dadvand, P.; Villanueva, C.M.; Font-Ribera, L.; Martinez, D.; Basagana, X.; Belmonte, J.; Vrijheid, M.; Grazuleviciene, R.; Kogevinas, M.; Nieuwenhuijsen, M.J. Risks and benefits of green spaces for children: A cross-sectional study of associations with sedentary behavior, obesity, asthma, and allergy. Environ. Health Perspect. 2014, 122, 1329–1335. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; von Berg, A.; Bauer, C.P.; Berdel, D.; Koletzko, S.; Sugiri, D.; Heinrich, J. Greenness and allergies: Evidence of differential associations in two areas in Germany. J. Epidemiol. Community Health 2014, 68, 787–790. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; Bowatte, G.; Gruzieva, O.; Gehring, U.; Becker, A.; Berdel, D.; von Berg, A.; Bergstrom, A.; Brauer, M.; et al. Residential greenness is differentially associated with childhood allergic rhinitis and aeroallergen sensitization in seven birth cohorts. Allergy 2016, 71, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; de Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J.; et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Svanes, C.; Omenaas, E.; Jarvis, D.; Chinn, S.; Gulsvik, A.; Burney, P. Parental smoking in childhood and adult obstructive lung disease: Results from the European Community Respiratory Health Survey. Thorax 2004, 59, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Dratva, J.; Zemp, E.; Dharmage, S.C.; Accordini, S.; Burdet, L.; Gislason, T.; Heinrich, J.; Janson, C.; Jarvis, D.; de Marco, R.; et al. Early Life Origins of Lung Ageing: Early Life Exposures and Lung Function Decline in Adulthood in Two European Cohorts Aged 28–73 Years. PLoS ONE 2016, 11, e0145127. [Google Scholar] [CrossRef] [Green Version]
- de Marco, R.; Pattaro, C.; Locatelli, F.; Svanes, C. Influence of early life exposures on incidence and remission of asthma throughout life. J. Allergy Clin. Immunol. 2004, 113, 845–852. [Google Scholar] [CrossRef]
- Morkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772. [Google Scholar] [CrossRef]
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Braback, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef]
- Johannessen, A.; Lonnebotn, M.; Calciano, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.; Franklin, K.A.; Gislason, T.; Holm, M.; et al. Being overweight in childhood, puberty, or early adulthood: Changing asthma risk in the next generation? J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, I.N.; Svanes, C.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.C.; Holm, M.; Janson, C.; Jogi, R.; Malinovschi, A.; et al. Agreement in reporting of asthma by parents or offspring—The RHINESSA generation study. BMC Pulm. Med. 2018, 18, 122. [Google Scholar] [CrossRef] [Green Version]
- RHINESSA Generation Study Homepage. Available online: www.rhinessa.net (accessed on 11 August 2020).
- Overview of Ethics Committees and Approval Numbers of RHINESSA Centers. Available online: https://helse-bergen.no/seksjon/RHINESSA/Documents/Ethic%20Committees%20list.pdf (accessed on 11 August 2020).
- Vienneau, D.; de Hoogh, K.; Bechle, M.J.; Beelen, R.; van Donkelaar, A.; Martin, R.V.; Millet, D.B.; Hoek, G.; Marshall, J.D. Western European land use regression incorporating satellite- and ground-based measurements of NO2 and PM10. Environ. Sci. Technol. 2013, 47, 13555–13564. [Google Scholar] [CrossRef]
- de Hoogh, K.; Gulliver, J.; Donkelaar, A.V.; Martin, R.V.; Marshall, J.D.; Bechle, M.J.; Cesaroni, G.; Pradas, M.C.; Dedele, A.; Eeftens, M.; et al. Development of West-European PM2.5 and NO2 land use regression models incorporating satellite-derived and chemical transport modelling data. Environ. Res. 2016, 151, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de Hoogh, K.; Chen, J.; Gulliver, J.; Hoffmann, B.; Hertel, O.; Ketzel, M.; Bauwelinck, M.; van Donkelaar, A.; Hvidtfeldt, U.A.; Katsouyanni, K.; et al. Spatial PM2.5, NO2, O3 and BC models for Western Europe—Evaluation of spatiotemporal stability. Environ. Int. 2018, 120, 81–92. [Google Scholar] [CrossRef]
- Procedure for Back-Extrapolation, Manual by the ESCAPE Project. Available online: http://www.escapeproject.eu/manuals/Procedure_for_extrapolation_back_in_time.pdf (accessed on 11 August 2020).
- Christensen, J.H. The Danish eulerian hemispheric model—A three-dimensional air pollution model used for the arctic. Atmos. Environ. 1997, 31, 4169–4191. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Satellite images, United States Geological Survey. Available online: http://earthexplorer.usgs.gov (accessed on 11 August 2020).
- Weier, J.H.; Herring, D. Measuring Vegeation (NDVI & EVI). Available online: https://earthobservatory.nasa.gov/features/MeasuringVegetation (accessed on 11 August 2020).
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar]
- DAGitty, a browser based environment to draw and analyze causal diagrams. Available online: www.dagitty.net (accessed on 11 August 2020).
- Buis, M.L. Direct and indirect effects in a logit model. Stata J. 2010, 10, 11–29. [Google Scholar]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Lee, A.; Leon Hsu, H.H.; Mathilda Chiu, Y.H.; Bose, S.; Rosa, M.J.; Kloog, I.; Wilson, A.; Schwartz, J.; Cohen, S.; Coull, B.A.; et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J. Allergy Clin. Immunol. 2018, 141, 1880–1886. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.H.; Chiu, Y.H.; Coull, B.A.; Kloog, I.; Schwartz, J.; Lee, A.; Wright, R.O.; Wright, R.J. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am. J. Respir. Crit. Care Med. 2015, 192, 1052–1059. [Google Scholar] [CrossRef]
- Zhou, C.; Baiz, N.; Zhang, T.; Banerjee, S.; Annesi-Maesano, I. Modifiable exposures to air pollutants related to asthma phenotypes in the first year of life in children of the EDEN mother-child cohort study. BMC Public Health 2013, 13, 506. [Google Scholar] [CrossRef] [Green Version]
- Arshad, S.H.; Karmaus, W.; Zhang, H.; Holloway, J.W. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol. 2017, 139, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. 2014, 22, 1382–1386. [Google Scholar] [CrossRef] [Green Version]
- Parmes, E.; Pesce, G.; Sabel, C.E.; Baldacci, S.; Bono, R.; Brescianini, S.; D’Ippolito, C.; Hanke, W.; Horvat, M.; Liedes, H.; et al. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: Evidence from 9 European cohorts. Environ. Res. 2019, 108953. [Google Scholar] [CrossRef]
- Mørkve Knudsen, G.T.; Rezwan, F.I.; Johannessen, A.; Skulstad, S.M.; Bertelsen, R.J.; Real, F.G.; Krauss-Etschmann, S.; Patil, V.; Jarvis, D.; Arshad, S.H.; et al. Epigenome-wide association of father’s smoking with offspring DNA methylation: A hypothesis-generating study. Environ. Epigenet. 2019, 5. [Google Scholar] [CrossRef]
- Khreis, H.; Nieuwenhuijsen, M.J. Traffic-Related Air Pollution and Childhood Asthma: Recent Advances and Remaining Gaps in the Exposure Assessment Methods. Int. J. Environ. Res. Public Health 2017, 14, 312. [Google Scholar] [CrossRef] [Green Version]
- Beelen, R.; Hoek, G.; Vienneau, D.; Eeftens, M.; Dimakopoulou, K.; Pedeli, X.; Tsai, M.-Y.; Künzli, N.; Schikowski, T.; Marcon, A.; et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—The ESCAPE project. Atmos. Environ. 2013, 72, 10–23. [Google Scholar] [CrossRef]
- Patel, C.J.; Ioannidis, J.P.A. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J. Epidemiol. Community Health 2014, 68, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Pape, K.; Svanes, C.; Malinovschi, A.; Benediktsdottir, B.; Lodge, C.; Janson, C.; Moratalla, J.; Sánchez-Ramos, J.L.; Bråbäck, L.; Holm, M.; et al. Agreement of offspring-reported parental smoking status: The RHINESSA generation study. BMC Public Health 2019, 19, 94. [Google Scholar] [CrossRef] [Green Version]
- Lonnebotn, M.; Svanes, C.; Igland, J.; Franklin, K.A.; Accordini, S.; Benediktsdottir, B.; Bentouhami, H.; Blanco, J.A.G.; Bono, R.; Corsico, A.; et al. Body silhouettes as a tool to reflect obesity in the past. PLoS ONE 2018, 13, e0195697. [Google Scholar] [CrossRef] [Green Version]
- Overview Funding RHINESSA. Available online: https://helse-bergen.no/seksjon/RHINESSA/Documents/Funding%20RHINESSA.pdf (accessed on 11 August 2020).
| Characteristics a | RHINESSA | |
|---|---|---|
| Fathers | Mothers | |
| N (%) | N (%) | |
| N | 400 (36.2) | 706 (63.8) |
| Umea | 88 (22.0) | 166 (23.5) |
| Uppsala | 85 (21.3) | 136 (19.3) |
| Gothenburg | 58 (14.5) | 93 (13.1) |
| Bergen | 169 (42.2) | 311 (44.1) |
| Offspring sex (male) | 327 (48.2) | 630 (49.6) |
| Offspring mean age (SD) | 5.4 (3.6) | 6.1 (4.2) |
| Offspring early-onset asthma (<10 years of age) | 60 (15.0) | 141 (20.0) |
| Offspring hay fever | 27 (6.8) | 70 (9.9) |
| Parental mean age (SD) | 35.0 (3.8) | 34.6 (3.9) |
| Parental asthma | 62 (15.5) | 128 (18.1) |
| Early-onset asthma | 31 (7.8) | 35 (5.0) |
| Late-onset asthma | 28 (7.0) | 88 (12.5) |
| Parental hay fever | 125 (31.3) | 193 (27.3) |
| Parental smoking onset | ||
| Never-smokers | 271 (67.8) | 427 (60.5) |
| Smokers before 18 years old | 103 (25.8) | 246 (34.8) |
| Smokers after 18 years old | 26 (6.5) | 31 (4.4) |
| Parental education | ||
| Primary school | 9 (2.3) | 22 (3.1) |
| Secondary school | 137 (34.3) | 185 (26.2) |
| College/university | 253 (63.3) | 498 (70.5) |
| Grandparental asthma | 45 (11.3) | 74 (10.5) |
| (a) Early-Onset Asthma. | |||||||||
| Univariable | Multivariable 3 | Univariable | Multivariable 3 | ||||||
| Exposure 1 | Exposure Level 2 | Fathers (OR, 95% CI) | p4 | Fathers (OR, 95% CI) | p4 | Mothers (OR, 95% CI) | p4 | Mothers (OR, 95% CI) | p4 |
| NO2 | Medium | 1.15 (0.58–2.30) | 0.690 | 1.09 (0.51–2.32) | 0.824 | 1.69 (1.05–2.73) | 0.032 | 1.78 (0.96–3.31) | 0.067 |
| High | 0.70 (0.34–1.44) | 0.332 | 0.50 (0.21–1.20) | 0.120 | 1.68 (1.04–2.72) | 0.034 | 1.79 (0.89–3.60) | 0.101 | |
| PM2.5 | Medium | 0.56 (0.27–1.14) | 0.111 | 0.48 (0.20–1.14) | 0.098 | 2.09 (1.30–3.37) | 0.002 | 2.23 (1.32–3.78) | 0.003 |
| High | 0.70 (0.35–1.41) | 0.320 | 0.53 (0.24–1.17) | 0.115 | 1.55 (0.94–2.57) | 0.088 | 1.66 (0.96–2.88) | 0.072 | |
| PM10 | Medium | 0.49 (0.23–1.04) | 0.064 | 0.46 (0.20–1.09) | 0.077 | 2.13 (1.35–3.38) | 0.001 | 2.27 (1.36–3.80) | 0.002 |
| High | 0.82 (0.42–1.62) | 0.567 | 0.65 (0.31–1.40) | 0.273 | 1.39 (0.83–2.31) | 0.209 | 1.46 (0.84–2.53) | 0.183 | |
| BC | Medium | 1.26 (0.64–2.46) | 0.501 | 0.86 (0.40–1.87) | 0.707 | 1.60 (1.00–2.58) | 0.051 | 1.45 (0.83–2.54) | 0.186 |
| High | 0.48 (0.22–1.04) | 0.064 | 0.31 (0.11–0.87) | 0.026 | 1.57 (0.98–2.53) | 0.060 | 1.33 (0.69–2.58) | 0.393 | |
| O3 | Medium | 1.90 (0.95–3.80) | 0.071 | 1.93 (0.93–4.01) | 0.079 | 0.81 (0.52–1.27) | 0.366 | 0.86 (0.53–1.39) | 0.542 |
| High | 1.25 (0.60–2.60) | 0.550 | 1.09 (0.42–2.82) | 0.852 | 0.67 (0.42–1.06) | 0.084 | 0.97 (0.52–1.82) | 0.923 | |
| NDVI (300 m) | Medium | 0.65 (0.30–1.42) | 0.279 | 0.56 (0.26–1.20) | 0.138 | 1.17 (0.74–1.85) | 0.505 | 1.25 (0.79–2.00) | 0.341 |
| High | 0.76 (0.39–1.47) | 0.411 | 0.67 (0.31–1.42) | 0.297 | 0.78 (0.46–1.31) | 0.341 | 1.00 (0.59–1.72) | 0.987 | |
| (b) Hay Fever. | |||||||||
| Univariable | Multivariable 3 | Univariable | Multivariable 3 | ||||||
| Exposure 1 | Exposure Level 2 | Fathers (OR, 95% CI) | p4 | Fathers (OR, 95% CI) | p4 | Mothers (OR, 95% CI) | p4 | Mothers (OR, 95% CI) | p4 |
| NO2 | Medium | 1.67 (0.65–4.26) | 0.285 | 2.72 (0.82–9.02) | 0.103 | 1.13 (0.55–2.34) | 0.740 | 1.52 (0.51–4.56) | 0.454 |
| High | 1.24 (0.45–3.40) | 0.680 | 2.41 (0.60–9.65) | 0.213 | 2.01 (1.04–3.90) | 0.039 | 2.84 (0.88–9.19) | 0.081 | |
| PM2.5 | Medium | 1.46 (0.48–4.45) | 0.510 | 1.72 (0.44–6.80) | 0.438 | 1.69 (0.83–3.46) | 0.151 | 1.85 (0.85–4.00) | 0.121 |
| High | 2.26 (0.75–6.85) | 0.149 | 2.78 (0.77–10.10) | 0.120 | 1.97 (0.99–3.91) | 0.052 | 1.90 (0.91–3.97) | 0.086 | |
| PM10 | Medium | 1.24 (0.40–3.88) | 0.708 | 1.90 (0.46–7.87) | 0.375 | 1.71 (0.83–3.52) | 0.147 | 1.85 (0.85–4.01) | 0.121 |
| High | 2.34 (0.78–7.00) | 0.127 | 3.41 (0.87–13.30) | 0.078 | 2.44 (1.26–4.72) | 0.008 | 2.66 (1.19–5.91) | 0.017 | |
| BC | Medium | 2.10 (0.75–5.89) | 0.160 | 2.52 (0.81–7.88) | 0.112 | 1.50 (0.74–3.04) | 0.257 | 1.70 (0.70–4.16) | 0.243 |
| High | 1.37 (0.46–4.05) | 0.575 | 2.56 (0.70–9.37) | 0.157 | 1.99 (1.00–3.97) | 0.052 | 2.71 (0.96–7.65) | 0.060 | |
| O3 | Medium | 3.30 (1.16–9.40) | 0.025 | 4.15 (1.28–13.50) | 0.018 | 1.33 (0.70–2.52) | 0.383 | 1.56 (0.79–3.06) | 0.198 |
| High | 1.91 (0.63–5.80) | 0.253 | 2.78 (0.58–13.26) | 0.199 | 0.84 (0.42–1.68) | 0.618 | 1.62 (0.54–4.82) | 0.389 | |
| NDVI (300 m) | Medium | 0.80 (0.27–2.36) | 0.683 | 0.72 (0.24–2.14) | 0.551 | 1.18 (0.60–2.33) | 0.629 | 1.29 (0.65–2.57) | 0.460 |
| High | 1.22 (0.48–3.13) | 0.681 | 1.35 (0.44–4.19) | 0.602 | 1.15 (0.58–2.30) | 0.683 | 1.57 (0.72–3.43) | 0.257 | |
| (a) Early-Onset Asthma. | ||||
| Mediator | Parental exposure | Offspring Early-onset Asthma | ||
| Total Effect | Indirect Effect | Direct Effect | ||
| OR (95% CI) * | OR (95% CI) * | OR (95% CI) * | ||
| Exposure during pregnancy (PM10) | PM10 (maternal) | |||
| Low | 1.00 | 1.00 | 1.00 | |
| Medium | 2.08 (1.31–3.31) | 1.10 (0.97–1.25) | 1.89 (1.17–3.06) | |
| High | 1.36 (0.85–2.19) | 1.20 (0.96–1.50) | 1.13 (0.67–1.93) | |
| (b) Hay Fever. | ||||
| Mediator | Parental exposure | Offspring Hay Fever | ||
| Total Effect | Indirect Effect | Direct Effect | ||
| OR (95% CI) * | OR (95% CI) * | OR (95% CI) * | ||
| Offspring own exposure (PM10) | PM10 (maternal) | |||
| Low | 1.00 | 1.00 | 1.00 | |
| Medium | 1.75 (0.75–4.04) | 1.24 (1.08–1.44) | 1.40 (0.60–3.27) | |
| High | 2.70 (1.20–6.08) | 1.73 (1.25–2.39) | 1.56 (0.66–3.69) | |
| Exposure during pregnancy (PM10) | PM10 (maternal) | |||
| Low | 1.00 | 1.00 | 1.00 | |
| Medium | 1.79 (0.79–4.08) | 1.49 (1.22–1.83) | 1.20 (0.52–2.74) | |
| High | 2.71 (1.24–5.93) | 2.02 (1.49–2.76) | 1.34 (0.61–2.94) | |
| Exposure during pregnancy (O3) | O3 (paternal) | |||
| Low | 1.00 | 1.00 | 1.00 | |
| Medium | 5.48 (1.50–20.1) | 1.10 (0.80–1.50) | 5.00 (1.31–19.1) | |
| High | 4.14 (0.69–24.9) | 1.16 (0.70–1.94) | 3.55 (0.53–24.0) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuiper, I.N.; Markevych, I.; Accordini, S.; Bertelsen, R.J.; Bråbäck, L.; Christensen, J.H.; Forsberg, B.; Halvorsen, T.; Heinrich, J.; Hertel, O.; et al. Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever. Int. J. Environ. Res. Public Health 2020, 17, 5828. https://doi.org/10.3390/ijerph17165828
Kuiper IN, Markevych I, Accordini S, Bertelsen RJ, Bråbäck L, Christensen JH, Forsberg B, Halvorsen T, Heinrich J, Hertel O, et al. Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever. International Journal of Environmental Research and Public Health. 2020; 17(16):5828. https://doi.org/10.3390/ijerph17165828
Chicago/Turabian StyleKuiper, Ingrid Nordeide, Iana Markevych, Simone Accordini, Randi J. Bertelsen, Lennart Bråbäck, Jesper Heile Christensen, Bertil Forsberg, Thomas Halvorsen, Joachim Heinrich, Ole Hertel, and et al. 2020. "Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever" International Journal of Environmental Research and Public Health 17, no. 16: 5828. https://doi.org/10.3390/ijerph17165828






