Mapping the Potential Distribution of Major Tick Species in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing Tick Occurrence Data
2.2. Preparing Environmental Data
2.3. Identifying Key Factors
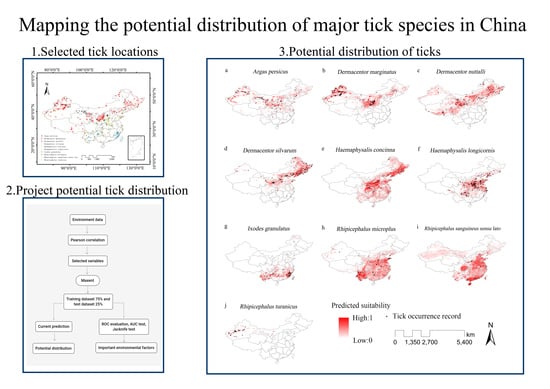
2.4. Projecting Potential Tick Distribution
3. Results
3.1. Environmental Determinants of Tick Occurrence
3.2. Predicted Potential Distribution of Major Tick Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prudencio, C.R.; De la Lastra, J.M.P.; Canales, M.; Villar, M.; De la Fuente, J. Mapping protective epitopes in the tick and mosquito subolesin ortholog proteins. Vaccine 2010, 28, 5398–5406. [Google Scholar] [CrossRef]
- De la Fuente, J. Controlling ticks and tick-borne diseases…looking forward. Ticks Tick Borne Dis. 2018, 9, 1354–1357. [Google Scholar] [CrossRef]
- Rauter, C.; Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 2005, 71, 7203–7216. [Google Scholar] [CrossRef] [Green Version]
- Hayasaka, D.; Ivanov, L.; Leonova, G.N.; Goto, A.; Yoshii, K.; Mizutani, T.; Kariwa, H.; Takashima, I. Distribution and characterization of tick-borne encephalitis viruses from Siberia and far-eastern Asia. J. Gen. Virol. 2001, 82, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Sharifah, N.; Heo, C.C.; Ehlers, J.; Houssaini, J.; Tappe, D. Ticks and tick-borne pathogens in animals and humans in the island nations of Southeast Asia: A review. Acta Trop. 2020, 209, 105527. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.-M.; Egan, S.; Gillett, A.; Banks, P.B.; Ryan, U.M.; Irwin, P.J.; Oskam, C.L. Molecular surveillance of piroplasms in ticks from small and medium-sized urban and peri-urban mammals in Australia. Int. J. Parasitol. Parasites Wildl. 2018, 7, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bajer, A.; Rodo, A.; Alsarraf, M.; Dwużnik, D.; Behnke, J.M.; Mierzejewska, E.J. Abundance of the tick Dermacentor reticulatus in an ecosystem of abandoned meadows: Experimental intervention and the critical importance of mowing. Vet. Parasitol. 2017, 246, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, R.; Siljander, M.; Dub, T.; Sane, J.; Sormunen, J.J.; Pellikka, P.; Vapalahti, O. Modelling habitat suitability for occurrence of human tick-borne encephalitis (TBE) cases in Finland. Ticks Tick Borne Dis. 2020, 11, 101457. [Google Scholar] [CrossRef]
- Boeckmann, M.; Joyner, T.A. Old health risks in new places? An ecological niche model for I. ricinus tick distribution in Europe under a changing climate. Health Place 2014, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Alkishe, A.A.; Peterson, A.T.; Samy, A.M. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes Ricinus. PLoS ONE 2017, 12, e0189092. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Heyman, P.; Cochez, C.; Simons, L.; Vanwambeke, S.O. A multi-level analysis of the relationship between environmental factors and questing Ixodes ricinus dynamics in Belgium. Parasit. Vectors 2012, 5, 149. [Google Scholar] [CrossRef] [Green Version]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit. Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groisman, P.; Soja, A. Northern Hemisphere high latitude climate and environmental change. Environ. Res. Lett. 2007, 2. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vourc’h, G.; Cislo, P.; Hoen, A.G.; Melton, F.; Hamer, S.A.; Rowland, M.; Cortinas, R.; Hickling, G.J.; Tsao, J.I.; et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob. Ecol. Biogeogr. 2010, 19, 504–514. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Holford, T.R.; Fish, D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 2003, 111, 1152–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Danielová, V.; Schwarzová, L.; Materna, J.; Daniel, M.; Metelka, L.; Holubová, J.; Kříž, B. Tick-borne encephalitis virus expansion to higher altitudes correlated with climate warming. Int. J. Med. Microbiol. 2008, 298, 68–72. [Google Scholar] [CrossRef]
- Li, S.; Vanwambeke, S.O.; Licoppe, A.M.; Speybroeck, N. Impacts of deer management practices on the spatial dynamics of the tick Ixodes ricinus: A scenario analysis. Ecol. Model. 2014, 276, 1–13. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Vanwambeke, S.O.; Yu, J.J.; Purse, B.V.; Harrison, P.A. Lyme disease risks in Europe under multiple uncertain drivers of change. Environ. Health Perspect. 2019, 127. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Harrison, P.A.; Rounsevell, M.D.A. Modelling the seasonality of Lyme disease risk and the potential impacts of a warming climate within the heterogeneous landscapes of Scotland. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Minigan, J.N.; Hager, H.A.; Peregrine, A.S.; Newman, J.A. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis. 2018, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; De la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Elaagip, A.H.; Kenawy, M.A.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile Virus and Lymphatic filariasis. PLoS ONE 2016, 11, 3863. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasit. Vectors 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Pena, A.; Venzal, J.M. Climate niches of tick species in the Mediterranean region: Modeling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2007, 44, 1130–1138. [Google Scholar] [CrossRef]
- Benítez-López, A.; Santini, L.; Schipper, A.M.; Busana, M.; Huijbregts, M.A.J. Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS Biol. 2019, 17, e3000247. [Google Scholar] [CrossRef] [Green Version]
- Verburg, P.H.; Neumann, K.; Nol, L. Challenges in using land use and land cover data for global change studies. Glob. Chang. Biol. 2011, 17, 974–989. [Google Scholar] [CrossRef] [Green Version]
- Pielke, R.A.; Avissar, R.; Raupach, M.; Dolman, A.J.; Zeng, X.B.; Denning, A.S. Interactions between the atmosphere and terrestrial ecosystems: Influence on weather and climate. Glob. Chang. Biol. 1998, 4, 461–475. [Google Scholar] [CrossRef]
- Chapin, F.S.; McGuire, A.D.; Randerson, J.; Pielke, R.; Baldocchi, D.; Hobbie, S.E.; Roulet, N.; Eugster, W.; Kasischke, E.; Rastetter, E.B.; et al. Arctic and boreal ecosystems of western North America as components of the climate system. Glob. Chang. Biol. 2000, 6, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Lembrechts, J.J.; Alexander, J.M.; Cavieres, L.A.; Haider, S.; Lenoir, J.; Kueffer, C.; McDougall, K.; Naylor, B.J.; Nunez, M.A.; Pauchard, A.; et al. Mountain roads shift native and non-native plant species’ ranges. Ecography 2017, 40, 353–364. [Google Scholar] [CrossRef]
- Randin, C.F.; Ashcroft, M.B.; Bolliger, J.; Cavender-Bares, J.; Coops, N.C.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Fernández, N.; et al. Monitoring biodiversity in the Anthropocene using remote sensing in species distribution models. Remote Sens. Environ. 2020, 239, 111626. [Google Scholar] [CrossRef]
- Jiang, J.; An, H.; Lee, J.S.; O’Guinn, M.L.; Kim, H.-C.; Chong, S.-T.; Zhang, Y.; Song, D.; Burrus, R.G.; Bao, Y.; et al. Molecular characterization of Haemaphysalis longicornis-borne rickettsiae, Republic of Korea and China. Ticks Tick Borne Dis. 2018, 9, 1606–1613. [Google Scholar] [CrossRef]
- Guo, H.; Yin, C.; Galon, E.M.; Du, J.; Gao, Y.; Adjou Moumouni, P.F.; Liu, M.; Efstratiou, A.; Lee, S.-H.; Li, J.; et al. Molecular survey and characterization of Theileria annulata and Ehrlichia ruminantium in cattle from Northwest China. Parasitol. Int. 2018, 67, 679–683. [Google Scholar] [CrossRef]
- Nüchel, J.; Bøcher, P.K.; Svenning, J.-C. Topographic slope steepness and anthropogenic pressure interact to shape the distribution of tree cover in China. Appl. Geogr. 2019, 103, 40–55. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Eisen, L.; Comstedt, P.; Mejlon, H.A.; Lindgren, E.; Bergstrom, S.; Olsen, B. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med. Vet. Entomol. 2009, 23, 226–237. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Species diversity and abundance of ticks in three habitats in southern Italy. Ticks Tick Borne Dis. 2013, 4, 251–255. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J. Development of outdoor recreation in Beijing, China between 1990 and 2010. Cities 2014, 37, 57–65. [Google Scholar] [CrossRef]
- Sheng, J.; Jiang, M.; Yang, M.; Bo, X.; Zhao, S.; Zhang, Y.; Wureli, H.; Wang, B.; Tu, C.; Wang, Y. Tick distribution in border regions of Northwestern China. Ticks Tick Borne Dis. 2019, 10, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Guanshi, Z.; Duo, Z.; Yuqin, T.; Sen, L. A dataset of distribution and diversity of ticks in China. Sci. Data 2019, 6, 105. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Zhang, X.-Y.; Liu, J.-Z. Ticks (Acari: Ixodoidea) in China: Geographical distribution, host diversity, and specificity. Arch. Insect Biochem. Physiol. 2019, 102, e21544. [Google Scholar] [CrossRef]
- Fang, L.-Q.; Liu, K.; Li, X.-L.; Liang, S.; Yang, Y.; Yao, H.-W.; Sun, R.-X.; Sun, Y.; Chen, W.-J.; Zuo, S.-Q.; et al. Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis. 2015, 15, 1467–1479. [Google Scholar] [CrossRef] [Green Version]
- Van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Yin, Q.; Liu, W.; Fu, S.; He, Y.; Lei, W.; Xu, S.; Liang, G.; Wang, S.; et al. Epidemiology of tick-borne encephalitis in China, 2007–2018. PLoS ONE 2019, 14, e0226712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; Estrada-Sánchez, A.; Estrada-Sánchez, D. Methodological caveats in the environmental modelling and projections of climate niche for ticks, with examples for Ixodes Ricinus (Ixodidae). Vet. Parasitol. 2015, 208, 14–25. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.M.; Leta, S.; Estrada-Peña, A.; Madder, M.; Adehan, S.; Vanwambeke, S.O. Species distribution modelling for Rhipicephalus microplus (Acari: Ixodidae) in Benin, West Africa: Comparing datasets and modelling algorithms. Prev. Vet. Med. 2015, 118, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Pang, Z.; Yu, X. Spatial-Temporal Pattern Analysis of Land Use/Cover; Science and Technology Academic Press: Beijing, China, 2014. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Sokolowska, M.; Mazurek, M.; Majer, M.; Podpora, M. Classification of user attitudes in Twitter -beginners guide to selected Machine Learning libraries. IFAC-PapersOnLine 2019, 52, 394–399. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Schwarz, A.; Maier, W.A.; Kistemann, T.; Kampen, H. Analysis of the distribution of the tick Ixodes ricinus L. (Acari: Ixodidae) in a nature reserve of western Germany using Geographic Information Systems. Int. J. Hyg. Environ. Health 2009, 212, 87–96. [Google Scholar] [CrossRef]
- Dobson, A.D.M.; Taylor, J.L.; Randolph, S.E. Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: Hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick Borne Dis. 2011, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Slater, H.; Michael, E. Predicting the current and future potential distributions of Lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ma, Y.; Hu, Z.; Li, Y.; Li, M.; Wu, L.; Li, C.; Dao, E.; Fan, X.; Hao, Y.; et al. MaxEnt Modeling of Dermacentor marginatus (Acari: Ixodidae) Distribution in Xinjiang, China. J. Med. Entomol. 2020. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Zhou, X.; Yu, H.; Li, W. Enhancing protected areas for biodiversity and ecosystem services in the Qinghai–Tibet Plateau. Ecosyst. Serv. 2020, 43, 101090. [Google Scholar] [CrossRef]
- Zamora, E.J.; Leal, B.; Thomas, D.B.; Dearth, R.K. Survival of off-host unfed Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) larvae in study arenas in relation to climatic factors and habitats in South Texas, USA. Ticks Tick Borne Dis. 2020, 11, 101317. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Reuben Kaufman, W. Ticks: Physiological aspects with implications for pathogen transmission. Ticks Tick Borne Dis. 2010, 1, 11–22. [Google Scholar] [CrossRef]
- Do Nascimento Ramos, V.; Osava, C.F.; Piovezan, U.; Szabó, M.P.J. Ambush behavior of the tick Amblyomma sculptum (Amblyomma cajennense complex) (Acari: Ixodidae) in the Brazilian Pantanal. Ticks Tick Borne Dis. 2017, 8, 506–510. [Google Scholar] [CrossRef]
- De Oliveira, S.V.; Romero-Alvarez, D.; Martins, T.F.; Santos, J.P.D.; Labruna, M.B.; Gazeta, G.S.; Escobar, L.E.; Gurgel-Gonçalves, R. Amblyomma ticks and future climate: Range contraction due to climate warming. Acta Trop. 2017, 176, 340–348. [Google Scholar] [CrossRef]
- Kiewra, D.; Stefańska-Krzaczek, E.; Szymanowski, M.; Szczepańska, A. Local-scale spatio-temporal distribution of questing Ixodes ricinus L. (Acari: Ixodidae)-A case study from a riparian urban forest in Wrocław, SW Poland. Ticks Tick Borne Dis. 2017, 8, 362–369. [Google Scholar] [CrossRef]
- Herrmann, C.; Gern, L. Search for blood or water is influenced by Borrelia burgdorferi in Ixodes ricinus. Parasit. Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauck, D.; Springer, A.; Chitimia-Dobler, L.; Strube, C. Two-year monitoring of tick abundance and influencing factors in an urban area (city of Hanover, Germany). Ticks Tick Borne Dis. 2020, 11, 101464. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Amiri, M.; Tarkesh, M.; Jafari, R.; Jetschke, G. Bioclimatic variables from precipitation and temperature records vs. remote sensing-based bioclimatic variables: Which side can perform better in species distribution modeling? Ecol. Inform. 2020, 57, 101060. [Google Scholar] [CrossRef]
- Klingseisen, B.; Stevenson, M.; Corner, R. Prediction of Bluetongue virus seropositivity on pastoral properties in northern Australia using remotely sensed bioclimatic variables. Prev. Vet. Med. 2013, 110, 159–168. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Variables | Unit |
|---|---|---|
| BIO1 | Annual Mean Temperature | ℃ |
| BIO2 | Mean Diurnal Temperature Range (Mean of monthly) | ℃ |
| BIO3 | Isothermality (BIO2/BIO7) (×100) | / |
| BIO4 | Temperature Seasonality (standard deviation × 100) | / |
| BIO5 | Maximum Temperature of the Warmest Month | ℃ |
| BIO6 | Minimum Temperature of the Coldest Month | ℃ |
| BIO7 | Temperature Annual Range (BIO5–BIO6) | ℃ |
| BIO10 | Mean Temperature of the Warmest Quarter | ℃ |
| BIO11 | Mean Temperature of the Coldest Quarter | ℃ |
| BIO12 | Annual Precipitation | mm |
| BIO13 | Precipitation of the Wettest Month | mm |
| BIO14 | Precipitation of the Driest Month | mm |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) | / |
| BIO16 | Precipitation of the Wettest Quarter | mm |
| PREC | Monthly Precipitation | mm |
| TMAX | Monthly Maximum Temperature | ℃ |
| TMIN | Monthly Minimum Temperature | ℃ |
| TMEAN | Monthly Mean Temperature | ℃ |
| CROP | Extent of Cropland | m2 |
| FOREST | Extent of Forest | m2 |
| GRASS | Extent of Grassland | m2 |
| SHRUB | Extent of Shrubland | m2 |
| URBAN | Extent of Urban Fabric | m2 |
| OLU | Extent of other Land Use Types | m2 |
| T_PH_H2O | PH Value of the Topsoil | -−Log(H+) |
| T_OC | Organic Carbon Content of the Topsoil | %weight |
| AWC_CLASS | Soil Available Water Content | / |
| T_TEXTURE | Soil Texture of the Topsoil | / |
| NDVI | Normalized Vegetation Index | / |
| Species | Models | Environmental Variables in Order of Importance | ||||
|---|---|---|---|---|---|---|
| 1st Contributor | 2nd Contributor | 3rd Contributor | 4th Contributor | 5th Contributor | ||
| Argas persicus | MaxEnt | URBAN | BIO13 | CROP | SHRUB | GRASS |
| GBDT | URBAN | BIO13 | CROP | BIO16 | BIO15 | |
| ERT | URBAN | CROP | BIO15 | BIO13 | FOREST | |
| RF | URBAN | CROP | BIO13 | BIO15 | BIO16 | |
| SVM_L1 | BIO13 | BIO14 | BIO16 | URBAN | BIO7 | |
| SVM_L2 | BIO14 | BIO13 | URBAN | BIO5 | BIO16 | |
| Dermacentor marginatus | MaxEnt | BIO13 | CROP | URBAN | TMIN | T_PH_H2O |
| GBDT | BIO13 | CROP | FOREST | BIO15 | T_PH_H2O | |
| ERT | CROP | BIO15 | BIO13 | OLU | BIO16 | |
| RF | CROP | BIO15 | BIO13 | BIO16 | URBAN | |
| SVM_L1 | BIO14 | BIO5 | BIO11 | BIO3 | BIO13 | |
| SVM_L2 | BIO14 | BIO5 | BIO3 | BIO13 | BIO16 | |
| Dermacentor Nuttalli | MaxEnt | URBAN | BIO6 | CROP | TMAX | BIO14 |
| GBDT | URBAN | CROP | TMEAN | TMAX | BIO12 | |
| ERT | URBAN | CROP | TMEAN | BIO1 | TMIN | |
| RF | URBAN | CROP | BIO1 | TMIN | TMEAN | |
| SVM_L1 | BIO14 | BIO7 | BIO4 | BIO16 | BIO2 | |
| SVM_L2 | BIO14 | BIO7 | BIO2 | BIO6 | BIO4 | |
| Dermacentor silvarum | MaxEnt | URBAN | TMEAN | FOREST | CROP | TMAX |
| GBDT | URBAN | BIO1 | CROP | BIO16 | GRASS | |
| ERT | CROP | GRASS | URBAN | OLU | BIO12 | |
| RF | URBAN | CROP | PREC | BIO12 | GRASS | |
| SVM_L1 | BIO14 | BIO10 | BIO1 | BIO7 | BIO13 | |
| SVM_L2 | BIO14 | BIO10 | BIO4 | BIO13 | BIO3 | |
| Haemaphysalis concinna | MaxEnt | PREC | CROP | FOREST | URBAN | BIO4 |
| GBDT | BIO12 | FOREST | OLU | PREC | SHRUB | |
| ERT | FOREST | PREC | CROP | BIO12 | OLU | |
| RF | PREC | BIO12 | FOREST | SHRUB | BIO16 | |
| SVM_L1 | BIO4 | BIO14 | BIO7 | TMAX | BIO3 | |
| SVM_L2 | BIO14 | BIO4 | BIO3 | FOREST | BIO7 | |
| Haemaphysalis longicornis | MaxEnt | CROP | URBAN | BIO12 | BIO7 | TMAX |
| GBDT | BIO6 | BIO11 | CROP | BIO16 | URBAN | |
| ERT | CROP | BIO6 | URBAN | BIO11 | BIO7 | |
| RF | BIO6 | BIO11 | CROP | URBAN | TMIN | |
| SVM_L1 | BIO10 | BIO13 | BIO4 | BIO14 | BIO16 | |
| SVM_L2 | BIO14 | BIO3 | BIO13 | BIO4 | URBAN | |
| Ixodes granulatus | MaxEnt | BIO14 | FOREST | GRASS | URBAN | SHRUB |
| GBDT | GRASS | BIO6 | PREC | TMEAN | FOREST | |
| ERT | FOREST | GRASS | BIO6 | BIO11 | TMIN | |
| RF | GRASS | BIO11 | BIO6 | FOREST | BIO16 | |
| SVM_L1 | BIO2 | OLU | BIO7 | BIO14 | BIO3 | |
| SVM_L2 | BIO2 | OLU | BIO14 | BIO3 | BIO7 | |
| Rhipicephalus microplus | MaxEnt | CROP | BIO14 | BIO6 | URBAN | FOREST |
| GBDT | BIO11 | URBAN | TMIN | BIO14 | BIO6 | |
| ERT | BIO11 | BIO6 | URBAN | TMIN | BIO2 | |
| RF | BIO6 | BIO11 | TMIN | URBAN | PREC | |
| SVM_L1 | BIO4 | BIO13 | BIO7 | URBAN | T_OC | |
| SVM_L2 | BIO13 | BIO4 | URBAN | OLU | T_OC | |
| Rhipicephalus sanguineus sensu lato | MaxEnt | URBAN | TMEAN | GRASS | BIO1 | SHRUB |
| GBDT | TMEAN | TMIN | BIO6 | BIO5 | SHRUB | |
| ERT | TMIN | CROP | TMEAN | BIO1 | BIO6 | |
| RF | TMIN | BIO1 | TMEAN | SHRUB | BIO11 | |
| SVM_L1 | BIO14 | BIO11 | URBAN | BIO10 | SHRUB | |
| SVM_L2 | BIO14 | URBAN | SHRUB | BIO6 | FOREST | |
| Rhipicephalus turanicus | MaxEnt | BIO13 | CROP | URBAN | BIO4 | AWC_CLASS |
| GBDT | CROP | BIO16 | BIO13 | FOREST | BIO4 | |
| ERT | CROP | OLU | BIO12 | BIO16 | BIO13 | |
| RF | CROP | URBAN | BIO16 | BIO13 | FOREST | |
| SVM_L1 | BIO13 | PREC | BIO12 | URBAN | SHRUB | |
| SVM_L2 | BIO13 | PREC | BIO12 | BIO16 | SHRUB | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Gao, Z.; Zhou, T.; Zhang, J.; Wang, L.; Xiao, L.; Wu, H.; Li, S. Mapping the Potential Distribution of Major Tick Species in China. Int. J. Environ. Res. Public Health 2020, 17, 5145. https://doi.org/10.3390/ijerph17145145
Yang X, Gao Z, Zhou T, Zhang J, Wang L, Xiao L, Wu H, Li S. Mapping the Potential Distribution of Major Tick Species in China. International Journal of Environmental Research and Public Health. 2020; 17(14):5145. https://doi.org/10.3390/ijerph17145145
Chicago/Turabian StyleYang, Xin, Zheng Gao, Tianli Zhou, Jian Zhang, Luqi Wang, Lingjun Xiao, Hongjuan Wu, and Sen Li. 2020. "Mapping the Potential Distribution of Major Tick Species in China" International Journal of Environmental Research and Public Health 17, no. 14: 5145. https://doi.org/10.3390/ijerph17145145