Bactericidal Activity of Ready-To-Use Alcohol-Based Commercial Wipes According to EN 16615 Carrier Standard
Abstract
1. Introduction
2. Materials and Methods
2.1. Disinfection Wipes
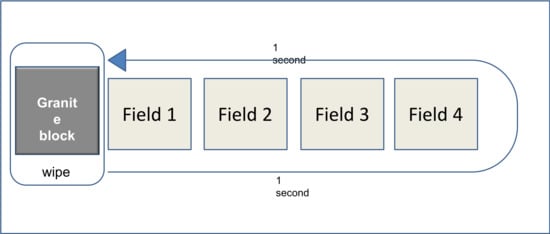
2.2. 4-Field Test
2.3. Controls for the 4-Field Test
2.4. Statistical Analyses
3. Results
3.1. Release of Disinfectant Solution from Wipes
3.2. Recovery of Bacteria from Test Fields
3.3. Reduction of Bacterial Load after Wiping
3.4. Spreading of Bacteria after Wiping
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloomfield, S.F.; Arthur, M.; Begun, K.; Patel, H. Comparative testing of disinfectants using proposed European surface test method. Lett. Appl. Microbiol. 1993, 17, 119–125. [Google Scholar] [CrossRef]
- Sattar, S.A.; Maillard, J.Y. The crucial role of wiping in decontamination of high-touch environmental surfaces: Review of current status and directions for the future. Am. J. Infect. Control 2013, 41, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A.; Bradley, C.; Kibbee, R.; Wesgate, R.; Wilkinson, M.A.C.; Sharpe, T.; Maillard, J.Y. Disinfectant wipes are appropriate to control microbial bioburden from surfaces: Use of a new ASTM standard test protocol to demonstrate efficacy. J. Hosp. Infect. 2015, 91, 319–325. [Google Scholar] [CrossRef] [PubMed]
- EN 13727+A2:2015. Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants for Instruments Used in the Medical Area. Test Method and Requirements (Phase 2/Step 1); Polish Standards Institution: Warsaw, Poland, 2015; Available online: sklep.pkn.pl/pn-en-13727-a2-2015-12e.
- Royal College of Nursing. The Selection and Use of Disinfectant Wipes. 2011. Available online: http://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/Infection-Prevention-Boot-Camp/How%20to%20choose%20a%20disinfectant%20wipe.pdf (accessed on 25 January 2019).
- Gemein, S.; Gebel, J.; Steinhauer, K.; Christiansen, B.; Martiny, H.; Meyer, B.; Ostermeyer, C.; Rödger, H.J.; Vossebein, L.; Paßvogel, L.; et al. Interlaboratory reproducibility of test method following four-field test methodology to evaluate the susceptibility of Clostridium difficile spores. J. Hosp. Infect. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- EN 16615:2015. Chemical Disinfectants and Antiseptics. Quantitative Test Method for the Evaluation of Bactericidal and Yeasticidal Activity on Non-Porous Surfaces with Mechanical Action Employing Wipes in the Medical Area (4-Field Test)—Test Method and Requirements (Phase 2/Step 2); Polish Standards Institution: Warsaw, Poland, 2015. Available online: sklep.pkn.pl/pn-en-16615-2015-06e.
- EN 14885:2015. Chemical Disinfectants and Antiseptics. Application of European Standards for Chemical Disinfectants and Antiseptics; Polish Standards Institution: Warsaw, Poland, 2018; Available online: sklep.pkn.pl/pn-en-14885-2019-01e.html.
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.U.; Kitajima, M.; Havas, A.; Gerba, C.P.; Reynolds, K.A. Evaluation of a disinfectant wipe intervention of fomite-to-finger microbial transfer. Appl. Environ. Microbiol. 2014, 80, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.J.; Denyer, S.P.; Hosein, I.K.; Hill, A.W.; Maillard, J.Y. The development of a new three-step protocol to determine the efficacy of disinfection wipes on surfaces contaminated with Staphylococcus aureus. J. Hosp. Infect. 2007, 67, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Yezli, S.; French, G.L. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect. Control Hosp. Epidemiol. 2011, 32, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Wesgate, R.; Robertson, A.; Barrell, M.; Teska, P.; Maillard, J.Y. Impact of test protocols and material binding on the efficacy of antimicrobial wipes. J. Hosp. Infect. 2019, 103, e25–e32. [Google Scholar] [CrossRef] [PubMed]
- Panousi, M.N.; Williams, G.J.; Girdlestone, S.; Hiom, S.J.; Mailard, J.Y. Evaluation of alcohol wipes used during aseptic manufacturing. Lett. Appl. Microbiol. 2009, 48, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Gebel, J.; Exner, M.; French, G.; Chartier, Y.; Christiansen, B.; Gemein, S.; Goroncy-Bermes, P.; Hartemann, P.; Heudorf, U.; Kramer, A.; et al. The role of surface disinfection in infection prevention. GMS Hyg. Infect. Control 2013, 8. [Google Scholar] [CrossRef]
- McDonnell, G.; Denver, R. Antiseptics and Disinfectants: Activity, action and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Block, S.S. Disinfection, Sterilization, and Preservation, 5th ed.; Alcohols; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 12, pp. 229–253. [Google Scholar]
- Gebel, J.; Gemein, S.; Kampf, G.; Pidot, S.J.; Buetti, N.; Exner, M. Isopropanol at 60% and at 70% are effective against isopropanol-tolerant Enterococcus faecium. J. Hosp. Infect. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Takeno, A. Relationship between bactericidal activity and the hydrophobicity-hydrophilicity balance of alcohol solution. Biocontrol Sci. 2001, 6, 107–111. [Google Scholar] [CrossRef][Green Version]
- Kampf, G.; Kramer, A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004, 17, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Jensen, C.S.; Kristensen, B. The Structure and Absorption Capacity of Non-Woven Fabrics Influences the Disinfection Efficiency of Commercially Manufactured Disinfection Wipes. In The Danish Microbiological Society Annual Congress 2016; American Society of Microbiology, FEMS: Copenhagen, Denmark, 2016; Available online: http://orbit.dtu.dk/files/127256055/FINAL_DMS_final_programme_abstract_book.pdf8KS_v_T.pdf (accessed on 25 November 2019).
- Ramm, L.; Siani, H.; Wesgate, R.; Maillard, J.Y. Pathogen transfer and high variability in pathogen removal by detergent wipes. Am. J. Infect. Control 2015, 43, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Block, S.S. Disinfection, Sterilization, and Preservation, 5th ed.; Synergism in Disinfectant Formulation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 22, pp. 459–473. [Google Scholar]




| Wipes | Formulations of Ready-to-Use Commercial Wipes in 100 g Solution | Test Method | Organic Load | Contact Time |
|---|---|---|---|---|
| A | 25 g ethyl alcohol, 35 g propan-1-ol | No information | No information on the label* | 1 min |
| B | 50 g propan-1-ol, 0.075 g didecyldimethylammonium chloride | No information | No information on the label * | 1 min |
| C | 35 g propan-2-ol, 25 g propan-1-ol | EN 13727 | Clean condition 0.3 g/L bovine albumin solution Dirty condition 3 g/L bovine albumin solution + 3 mL/l sheep erythrocytes | 1 min |
| D | 45 g ethyl alcohol, 30 g propan-2-ol, 0.5 g didecyldimethylammonium chloride | No information | No information on the label* | 30 s |
| Wipes/Test Organisms | Contact Times | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 Min | 5 Min | 10 Min | 15 Min | |||||
| lgR | T2-T4 | lgR | T2-T4 | lgR | T2-T4 | lgR | T2-T4 | |
| A/Eh | 2.48 | 800 | 4.26 | 3 | 2.78 | 0 | 2.84 | 5 |
| A/Ps | 2.69 | 2 | 1.32 | 7 | 4.20 | 0 | 2.90 | 460 |
| A/Sa | 2.36 | 3 | 2.65 | 0 | 2.72 | 0 | 2.74 | 0 |
| B/Eh | 2.60 | 550 | 4.60 | 0 | 4.18 | 146 | 2.85 | 8 |
| B/Ps | 5.05 | 28 | 5.37 | 0 | 5.37 | 0 | 5,39 | 0 |
| B/Sa | 3.87 | 1 | 3.78 | 0 | 3,44 | 44 | 3.32 | 5 |
| C/Eh | 2.82 | 2 | 3.08 | 1 | 2.96 | 2 | 2.77 | 38 |
| C/Ps | 1.78 | 0 | 2.57 | 2 | 4.30 | 3 | 5.25 | 3 |
| C/Sa | 3.06 | 138 | 3.06 | 138 | 2.59 | 3 | 3.30 | 5 |
| D/Eh | 2.52 | 447 | 2.39 | 13 | 2.88 | 7 | 2.96 | 0 |
| D/Ps | 2.04 | 92 | 2.56 | 0 | 3.54 | 0 | 2.60 | 35 |
| D/Sa | 2.35 | 125 | 2.67 | 108 | 2.99 | 17 | 3.32 | 0 |
| Water Control (Nw)/ Test Organisms | Contact Times | |||
|---|---|---|---|---|
| 1 Min | 5 Min | 10 Min | 15 Min | |
| T2-T4 cfu/25 cm2 | T2-T4 cfu/25 cm2 | T2-T4 cfu/25 cm2 | T2-T4 cfu/25 cm2 | |
| Nw/Eh | 16,500 | 16,500 | 2947 | 2660 |
| Nw/Ps | 2121 | 277 | 3667 | 1216 |
| Nw/Sa | 9490 | 11,133 | 4204 | 13,750 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarka, P.; Chojecka, A.; Paduch, O.; Nitsch-Osuch, A.; Kanecki, K.; Kierzkowska, A. Bactericidal Activity of Ready-To-Use Alcohol-Based Commercial Wipes According to EN 16615 Carrier Standard. Int. J. Environ. Res. Public Health 2019, 16, 3475. https://doi.org/10.3390/ijerph16183475
Tarka P, Chojecka A, Paduch O, Nitsch-Osuch A, Kanecki K, Kierzkowska A. Bactericidal Activity of Ready-To-Use Alcohol-Based Commercial Wipes According to EN 16615 Carrier Standard. International Journal of Environmental Research and Public Health. 2019; 16(18):3475. https://doi.org/10.3390/ijerph16183475
Chicago/Turabian StyleTarka, Patryk, Agnieszka Chojecka, Olga Paduch, Aneta Nitsch-Osuch, Krzysztof Kanecki, and Anna Kierzkowska. 2019. "Bactericidal Activity of Ready-To-Use Alcohol-Based Commercial Wipes According to EN 16615 Carrier Standard" International Journal of Environmental Research and Public Health 16, no. 18: 3475. https://doi.org/10.3390/ijerph16183475
APA StyleTarka, P., Chojecka, A., Paduch, O., Nitsch-Osuch, A., Kanecki, K., & Kierzkowska, A. (2019). Bactericidal Activity of Ready-To-Use Alcohol-Based Commercial Wipes According to EN 16615 Carrier Standard. International Journal of Environmental Research and Public Health, 16(18), 3475. https://doi.org/10.3390/ijerph16183475