Detection and Quantification of Tire Particles in Sediments Using a Combination of Simultaneous Thermal Analysis, Fourier Transform Infra-Red, and Parallel Factor Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Tire Granules
2.2. Formulated Sediment
2.3. Sample Preparation
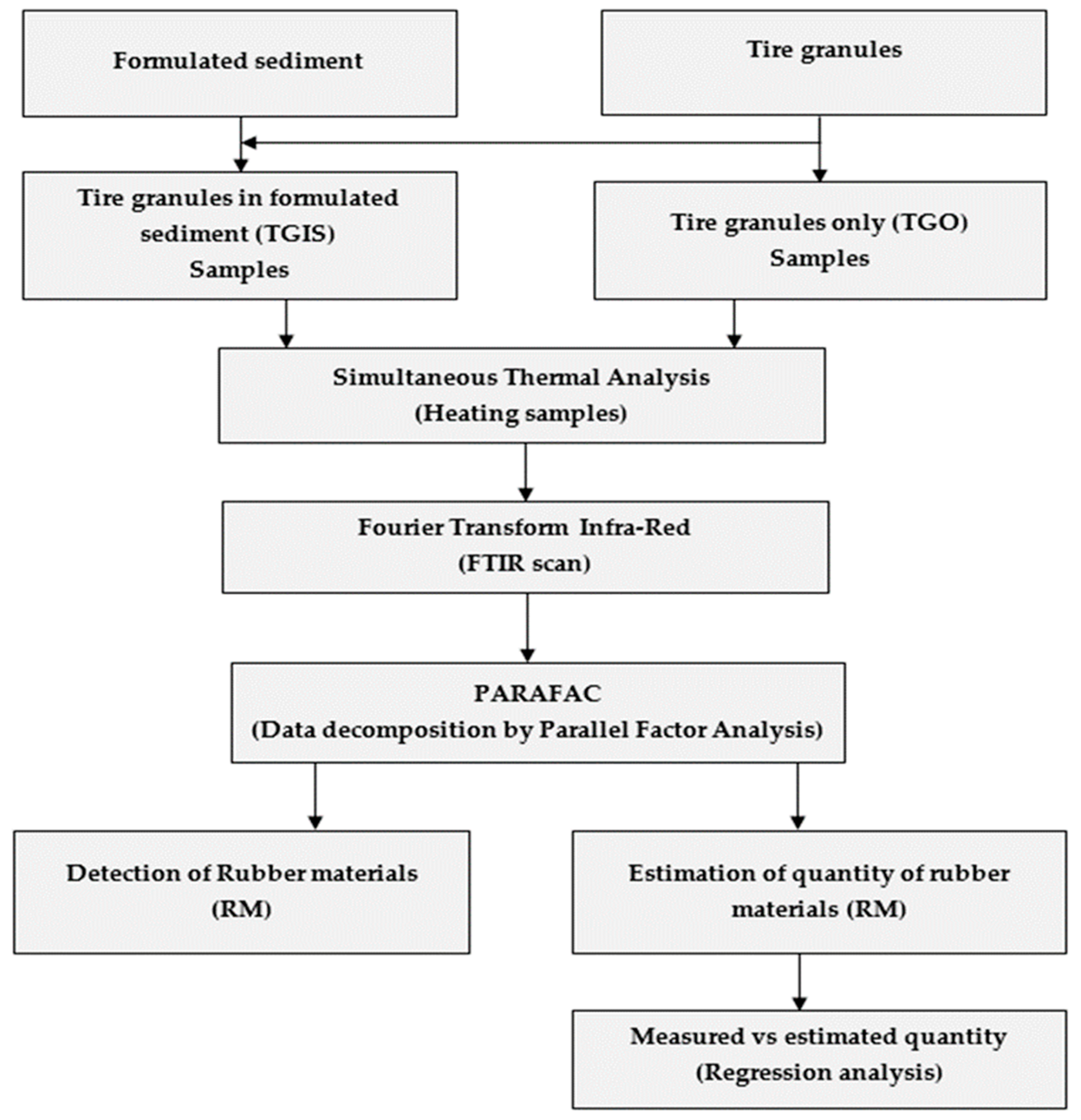
2.4. Experimental Flow and Procedure
2.5. Data and the PARAFAC Model
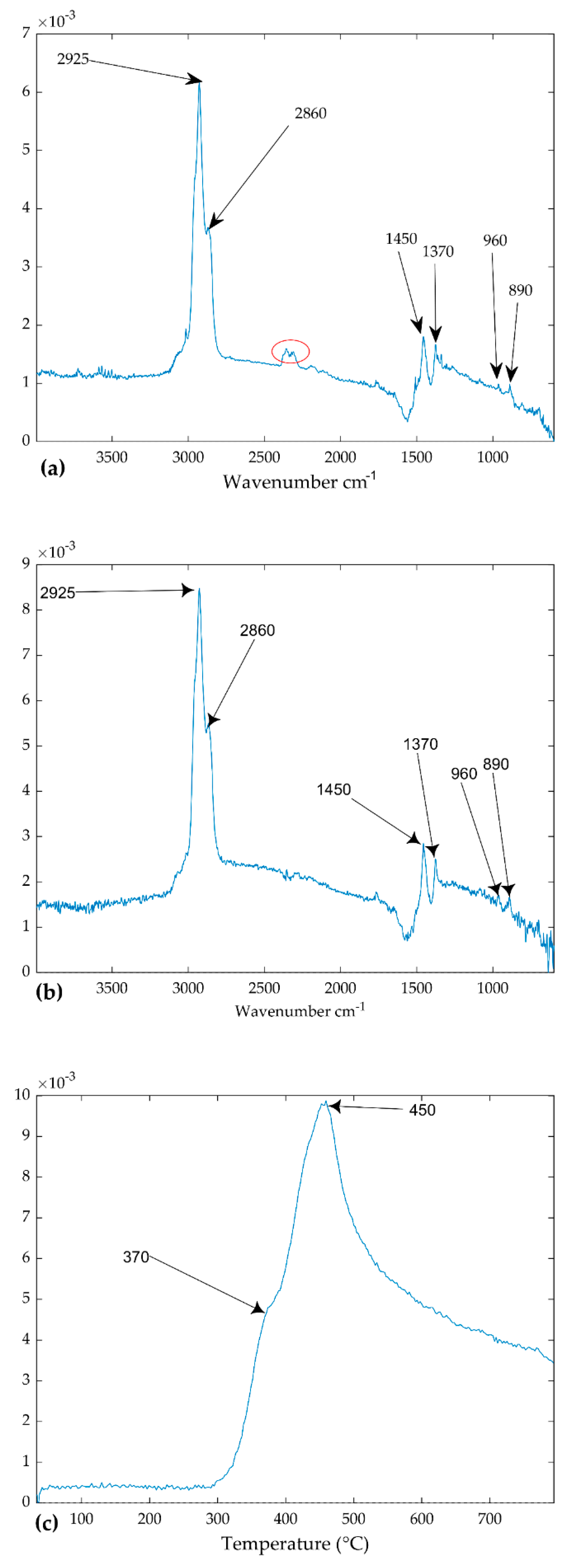
2.6. Rubber Material Identification
2.7. Quantity Estimation
3. Results
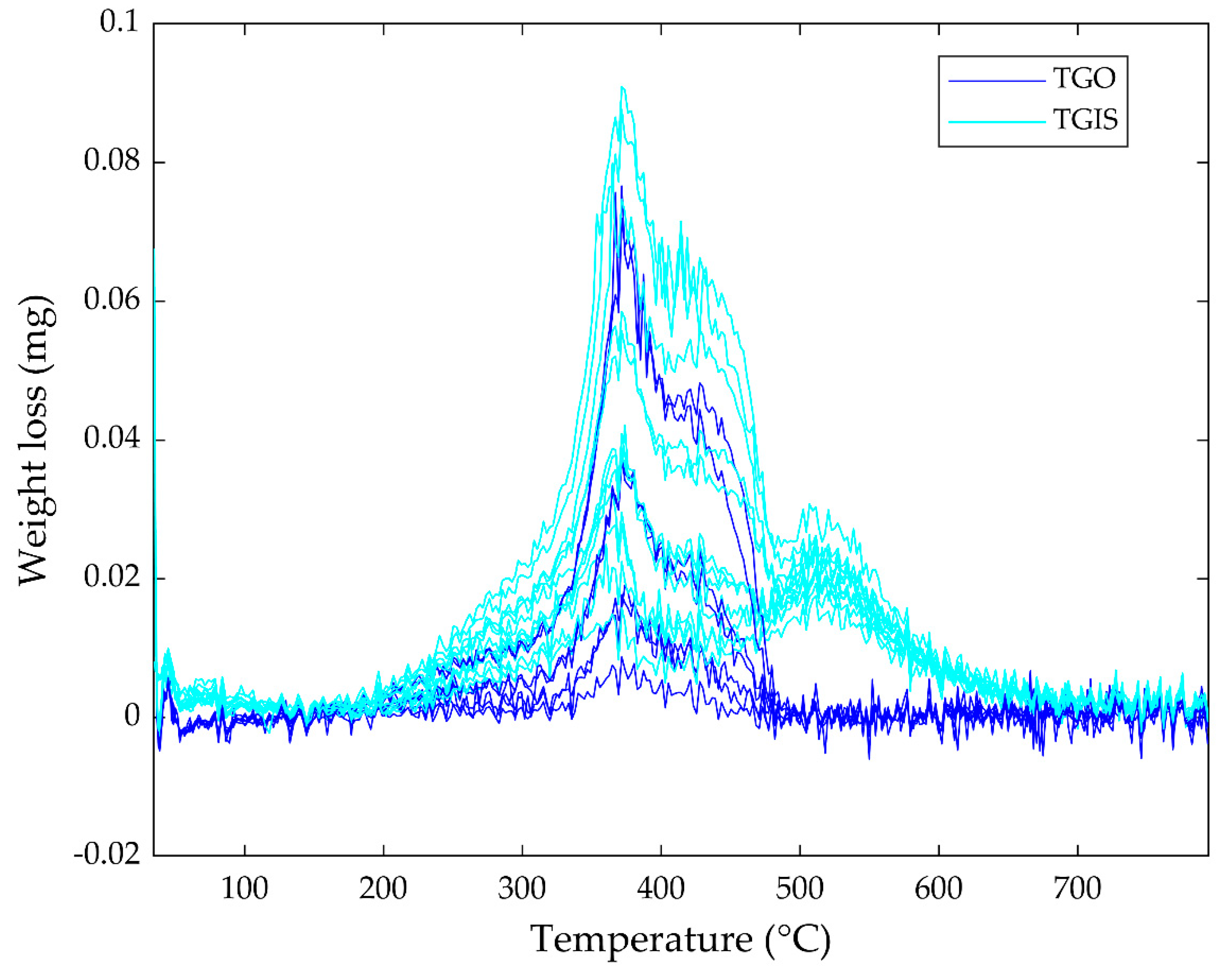
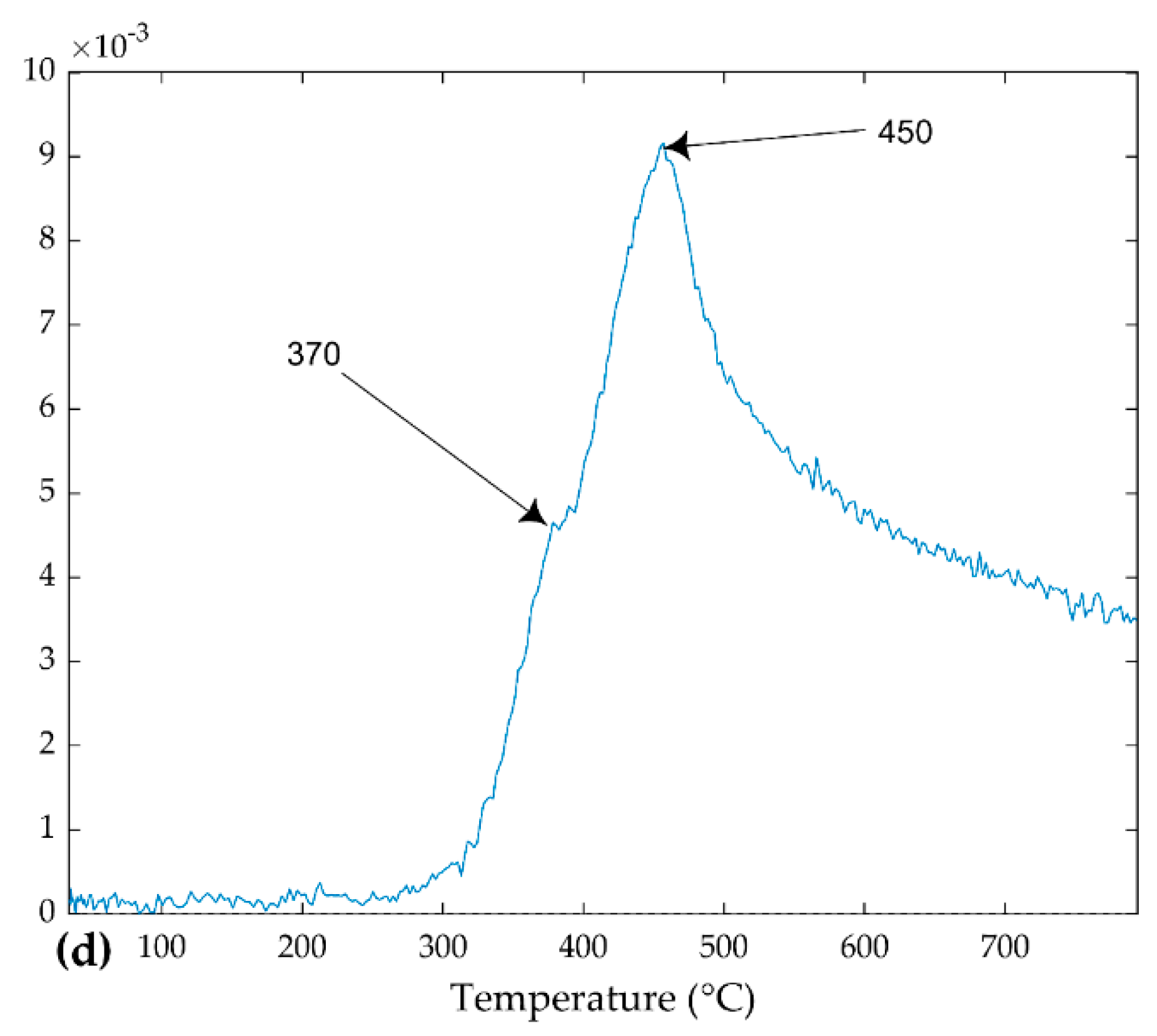
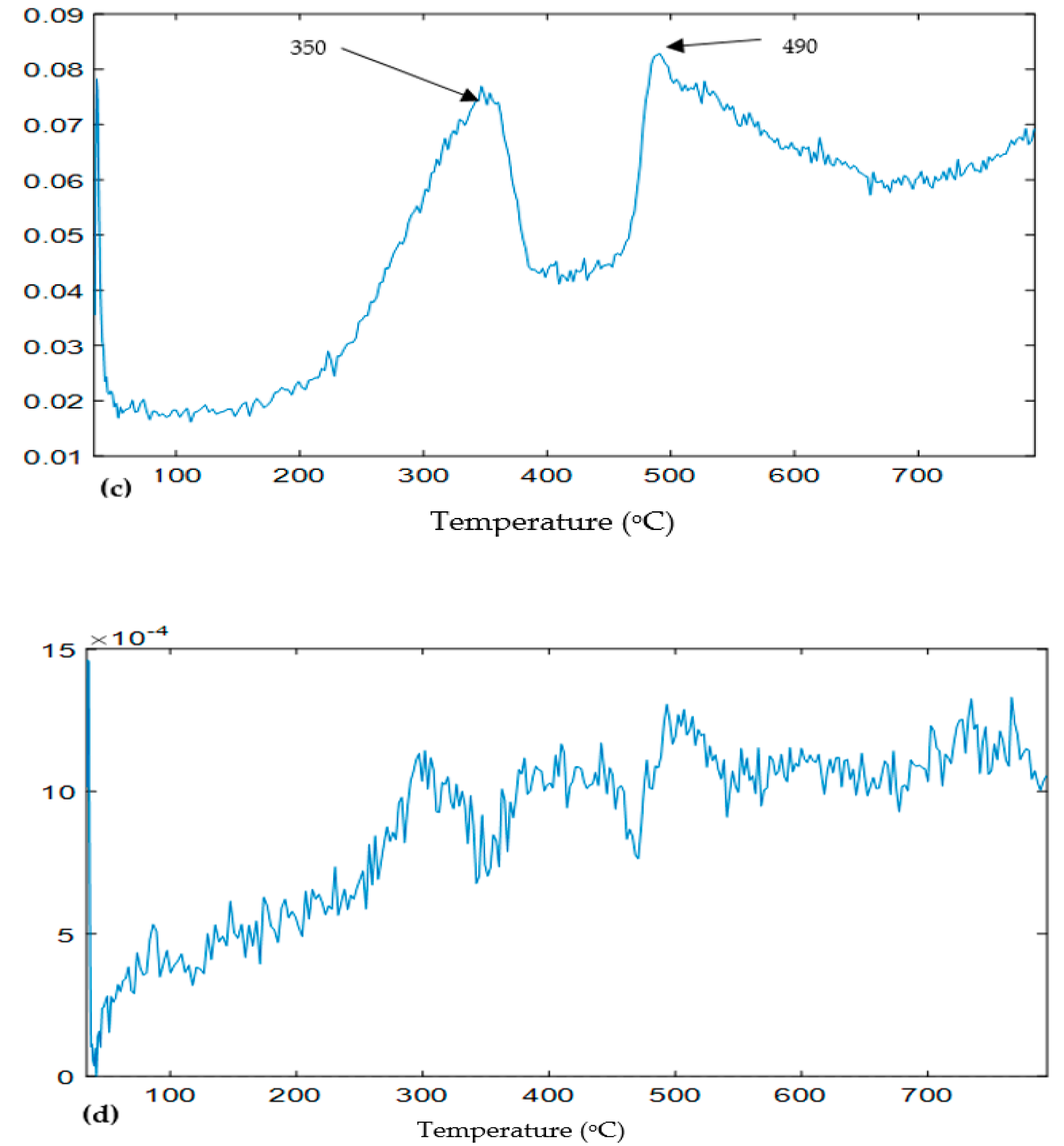
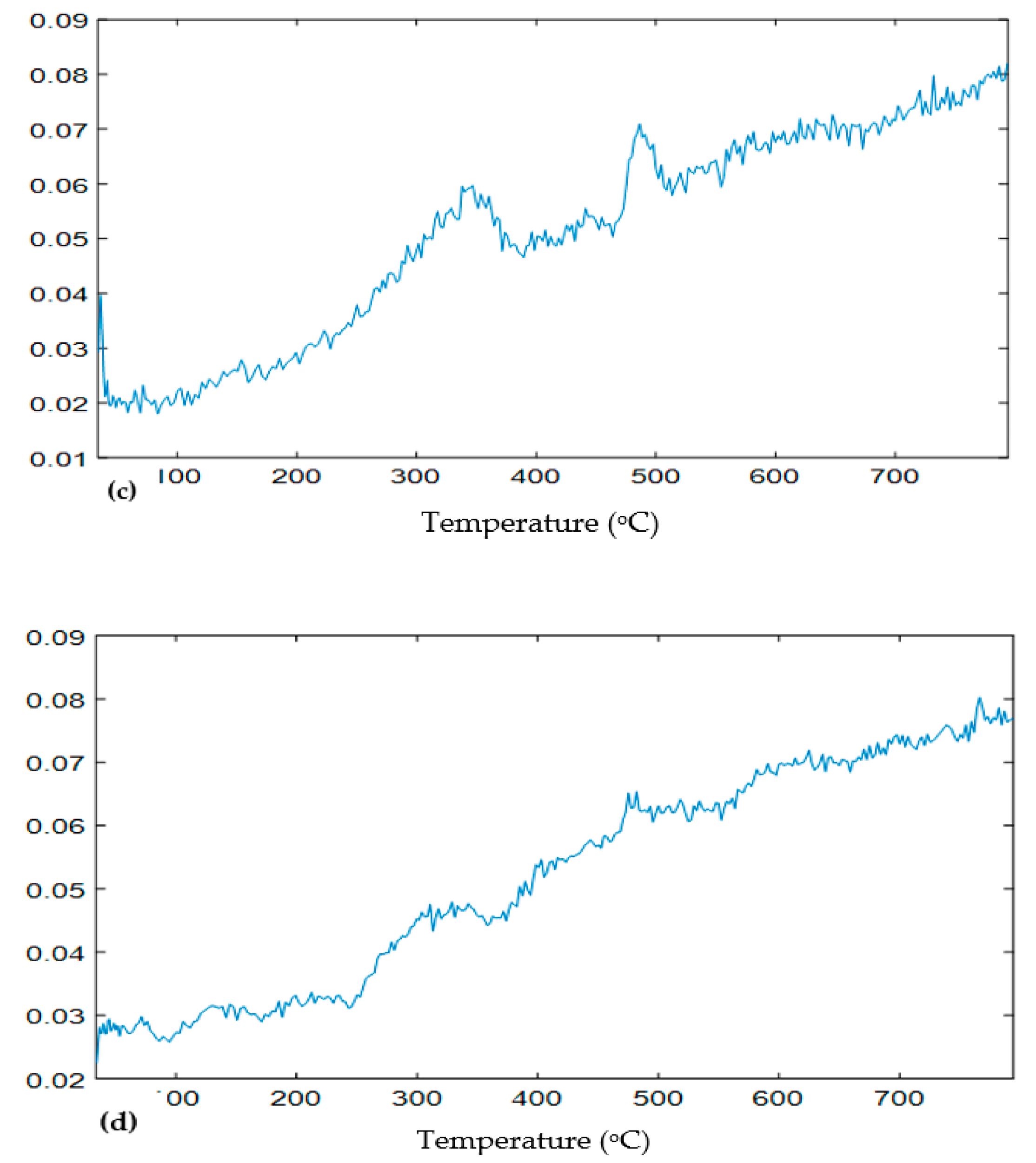
3.1. Sample Weight Loss in Simultaneous Thermal Analysis (STA)
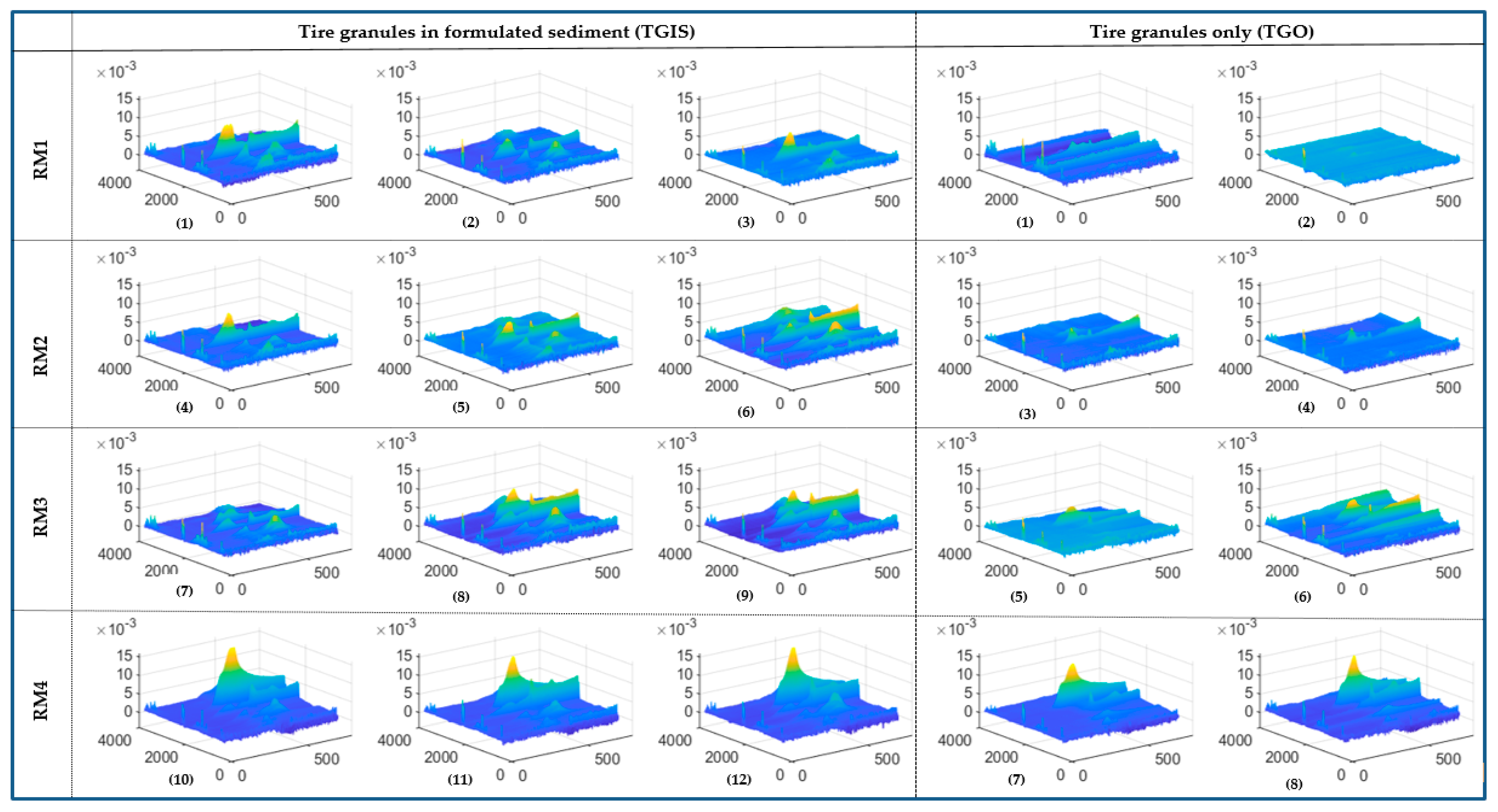
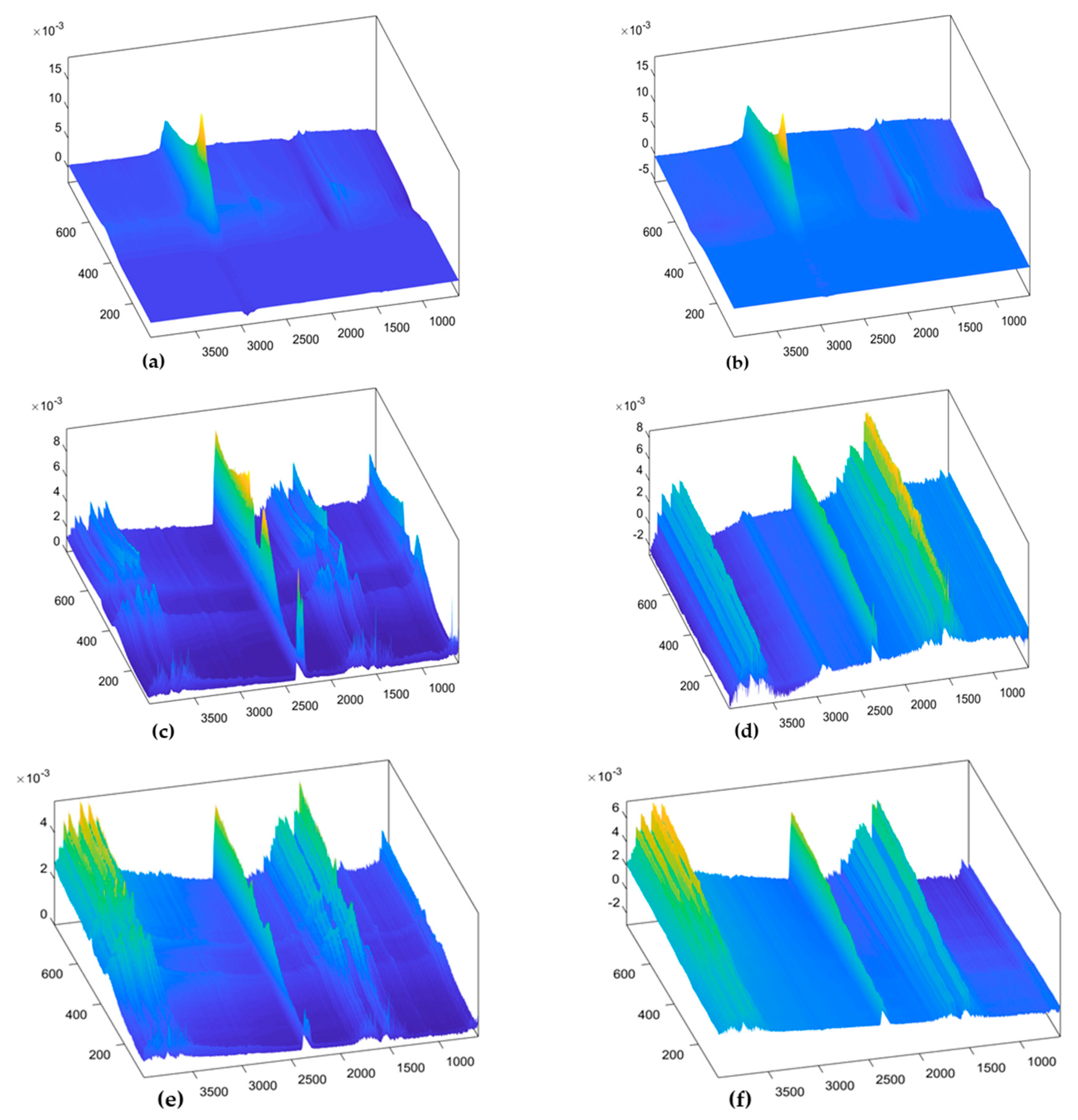
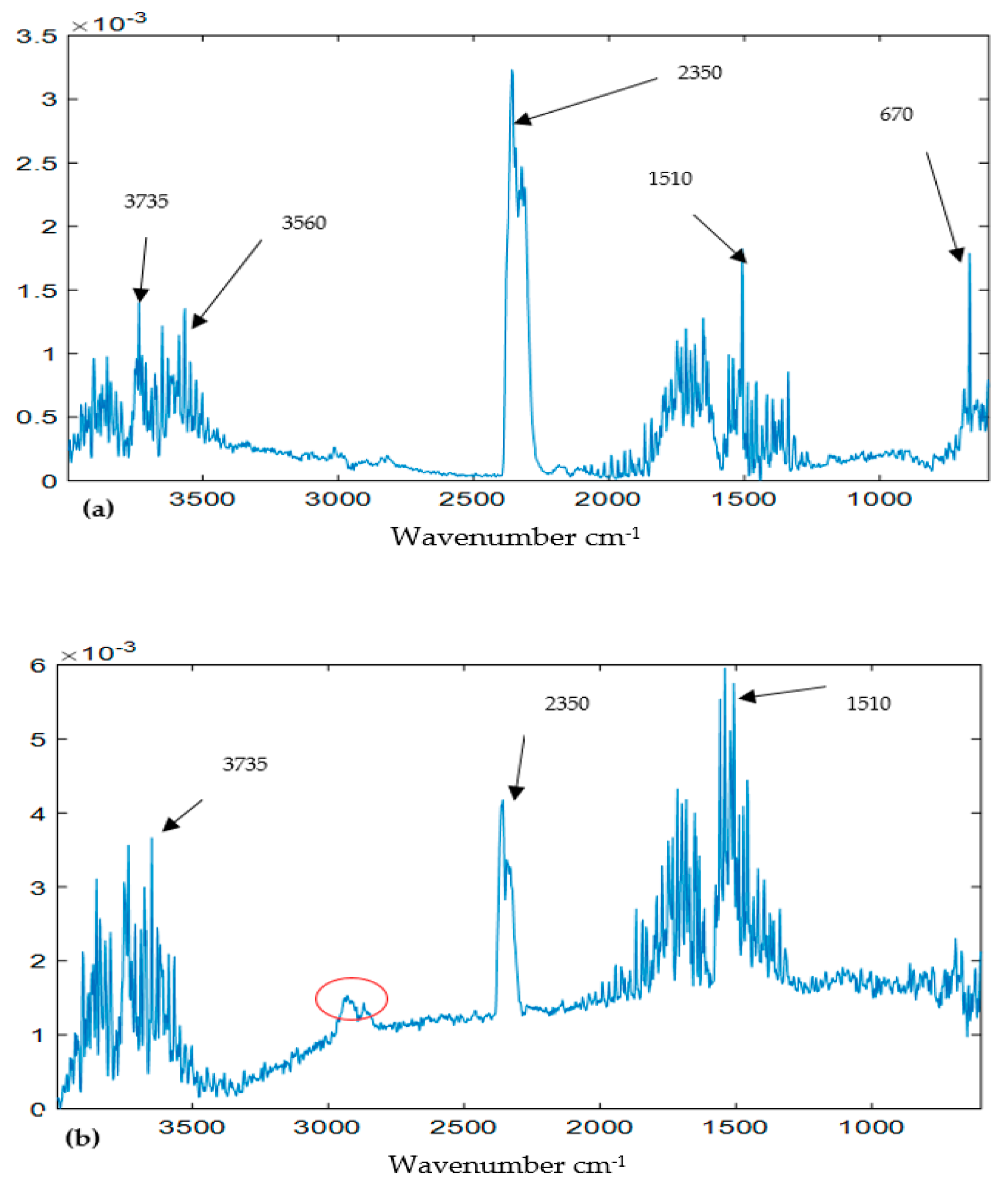
3.2. Landscape Surfaces of FTIR Data
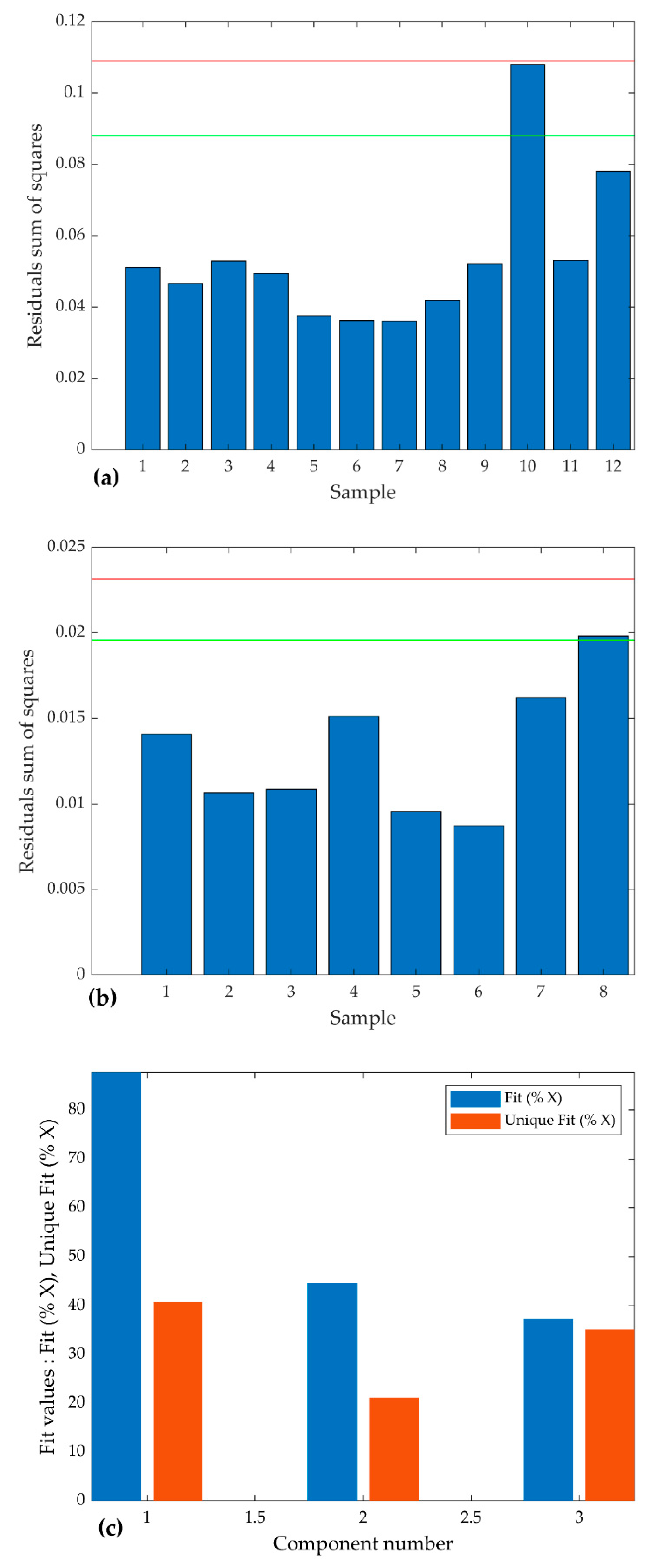
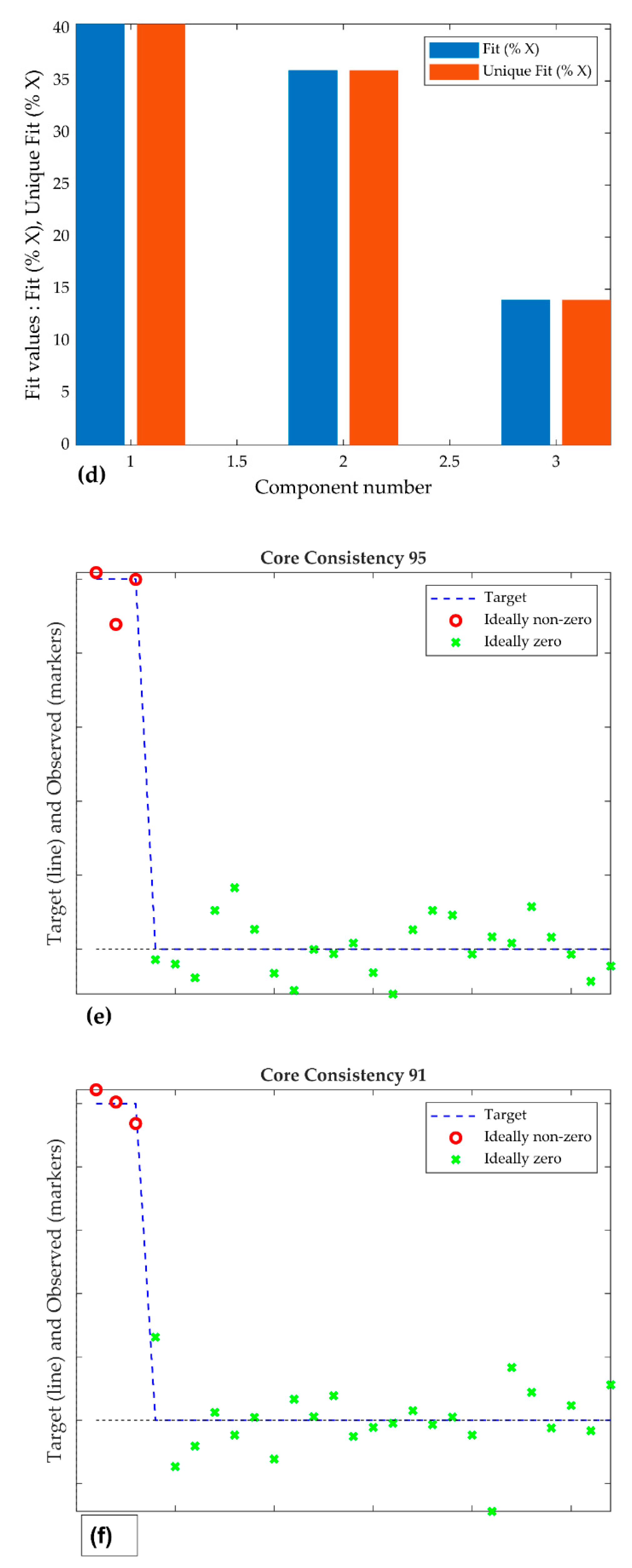
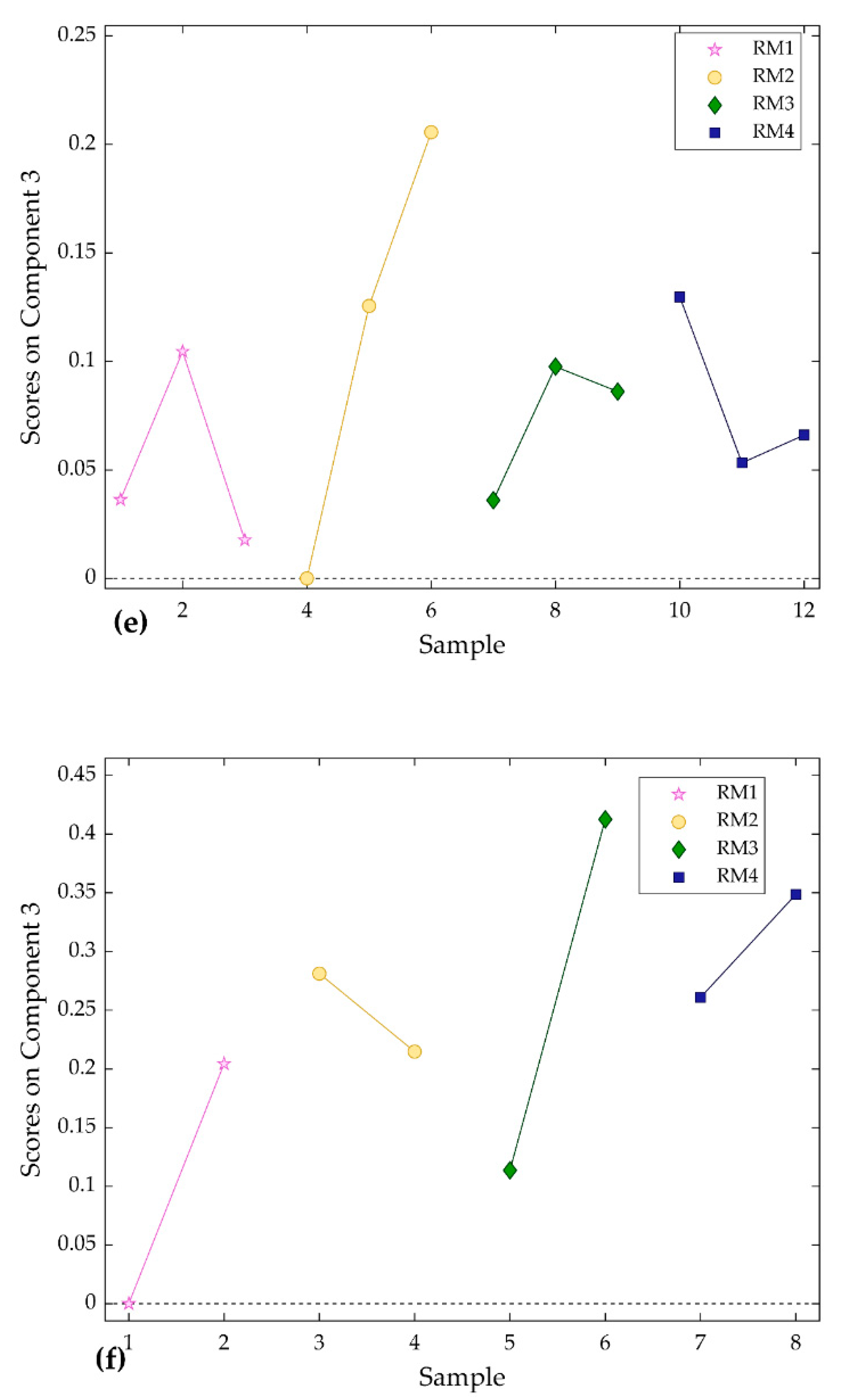
3.3. The PARAFAC Model and Results
4. Discussion
4.1. Decomposition of Overlying Components
4.2. Identification of Components
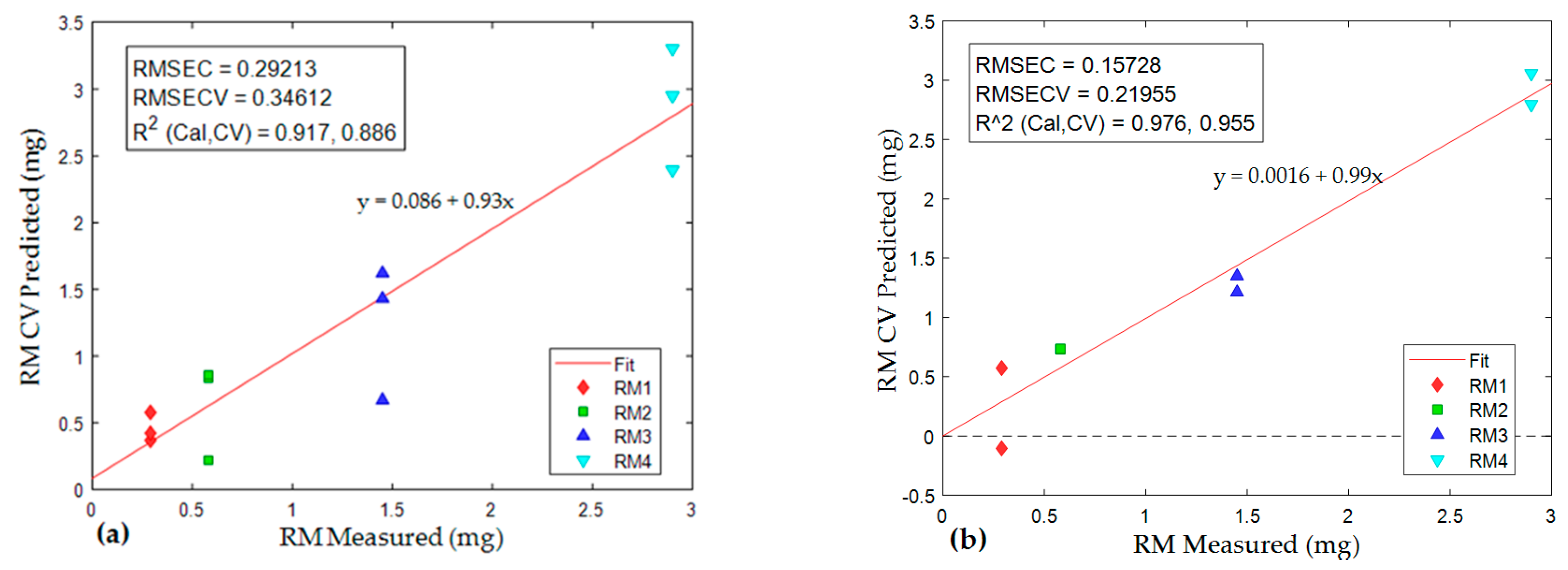
4.3. Estimation of Quantity of Components
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panko, J.M.; Chu, J.; Kreider, M.L.; Unice, K.M.; Unice, K. Measurement of airborne concentrations of tire and road wear particles in urban and rural areas of France, Japan, and the United States. Atmos. Environ. 2013, 72, 192–199. [Google Scholar] [CrossRef]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Blok, J. Environmental exposure of road borders to zinc. Sci. Total Environ. 2005, 348, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, A.J. Towards a Definition of microplastics. RIVM Lett. Rep. 2015, 116, 13–15. [Google Scholar]
- Januszewicz, K.; Klein, M.; Klugmann-Radziemska, E.; Kardas, D. Physicochemical Problems of Mineral Processing Thermogravimetric analysis/pyrolysis of used tyres and waste rubber. Physicochem. Probl. Miner. Process 2017, 53, 802–811. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- David, G.G., Jr.; Adeleye, A.S.; Sung, L.; Ho, K.T.; Robert, M.; Petersen, E.J.; Division, E.; Agency, E.P.; Division, A.E. Detection and Quantification of Graphene-Family Nanomaterials in the Environment. EPA Public Access. 2019. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Microplastics analysis in environmental samples—Recent pyrolysis-gas chromatography-mass spectrometry method improvements to increase the reliability of mass-related data. Anal. Methods 2019, 11, 2489–2497. [Google Scholar] [CrossRef]
- Litvinow, V.M.; De, P.P. Spectroscopy of Rubber and Rubbery Materials; iSmithers Rapra Publishing: Shawbury, UK, 2002. [Google Scholar]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Use of a Deuterated Internal Standard with Pyrolysis-GC/MS Dimeric Marker Analysis to Quantify Tire Tread Particles in the Environment. Int. J. Environ. Res. Public Health 2012, 9, 4033–4055. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Berridi, M.J.; González, N.; Mugica, A.; Bernicot, C. Pyrolysis-FTIR and TGA techniques as tools in the characterization of blends of natural rubber and SBR. Thermochim. Acta 2006, 444, 65–70. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.; Braun, U.; Senz, R. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Akinade, K.A.; Campbell, R.M.; Compton, D.A.C. The use of a simultaneous T G A / D S C / F T - I R system as a problem-solving tool. J. Mater. Sci. 1994, 29, 3802–3812. [Google Scholar] [CrossRef]
- Cambridge Polymer Group. Car Tire Composition Analysis by TGA-FTIR; Cambridge Polymer Group: Cambridge, UK, 2014. [Google Scholar]
- Gunasekaran, S.; Natarajan, R.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Gary, L.; Wen Jing, Z.W. Identification of Unknown Mixtures of Materials from Biopharmaceutical Manufacturing Processes by Microscopic-FTIR and Library Searching. Am. Pharm. Rev. 2011, 14, 60. [Google Scholar]
- Baum, A.; Hansen, P.; Nørgaard, L.; Sørensen, J.; Mikkelsen, J. Rapid quantification of casein in skim milk using Fourier transform infrared spectroscopy, enzymatic perturbation, and multiway partial least squares regression: Monitoring chymosin at work. J. Dairy Sci. 2016, 99, 6071–6079. [Google Scholar] [CrossRef] [PubMed]
- Melville, B.; Lucieer, A.; Aryal, J. Assessing the Impact of Spectral Resolution on Classification of Lowland Native Grassland Communities Based on Field Spectroscopy in Tasmania, Australia. Remote Sens. 2018, 10, 308. [Google Scholar] [CrossRef]
- Murphy, K.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557. [Google Scholar] [CrossRef]
- Rasmus, B. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Ragn-Sells Däckåtervinning AB. 2018. Available online: https://www.ragnsells.no/globalassets/norge/dokumenter/dekkgjenvinning/rs_pb_granulat_fin_v5-2018-01-16.pdf (accessed on 8 June 2019).
- FIFA. Handbook of Test Methods; FIFA: Zurich, Switzerland, 2015. [Google Scholar]
- Sommer, F.; Dietze, V.; Baum, A.; Sauer, J.; Gilge, S.; Maschowski, C.; Gieré, R. Tire Abrasion as a Major Source of Microplastics in the Environment. Aerosol Air Qual. Res. 2018, 18, 2014–2028. [Google Scholar] [CrossRef]
- Jusli, E.; Nor, H.M.; Jaya, R.P.; Zaiton, H.; Haron, Z. Chemical Properties of Waste Tyre Rubber Granules. Adv. Mater. Res. 2014, 911, 77–81. [Google Scholar] [CrossRef]
- Formela, K.; Cysewska, M.; Haponiuk, J. The influence of screw configuration and screw speed of co-rotating twin screw extruder on the properties of products obtained by thermomechanical reclaiming of ground tire rubber. Polim. Polym. 2014, 59, 170–177. [Google Scholar] [CrossRef]
- OECD. OECD Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; OECD: Paris, France, 2010. [Google Scholar]
- Netzsch. Thermal Analysis Methods (Part 1): TG, DSC, STA, EGA Practical Applications of Thermal Analysis Methods in Material Science; Netzsch: Selb, Germany, 2012. [Google Scholar]
- Wise, B.M.; Gallagher, N.B.; Windig, W.; Bro, R.; Shaver, J.M.; Koch, R.S. Chemometrics Tutorial for PLS _ Toolbox and Solo. Eig. Res. Inc. 2006, 3905, 102–159. [Google Scholar]
- Baum, A.; Hansen, P.W.; Meyer, A.S.; Mikkelsen, J.D. Simultaneous measurement of two enzyme activities using infrared spectroscopy: A comparative evaluation of PARAFAC, TUCKER and N-PLS modeling. Anal. Chim. Acta 2013, 790, 14–23. [Google Scholar] [CrossRef]
- Bro, R.; Kiers, H.A.L. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Engineering, M.; Republi, S. The Effect of Temperature Pyrolysis Process. Siendo 2001. [Google Scholar] [CrossRef][Green Version]
- Delva, L.; Verberckmoes, A.; Cardon, L.; Ragaert, K. The use of rubber as a compatibilizer for injection moulding of recycled post-consumer mixed polyolefines. In Proceedings of the 2nd International Conference WASTES: Solutions, Treatments and Opportunities, Braga, Portugal, 11–13 September 2013; pp. 689–694. [Google Scholar]
- Mente, P.; Motaung, T.E. Natural Rubber and Reclaimed Rubber Composites–A Systematic Review. Polym. Sci. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. National Institute of Standards and Technology, U.S. Department of Commerce, 2011. Available online: https://webbook.nist.gov/cgi/inchi?ID=C7732185&Type=IR-SPEC&Index=0 (accessed on 24 July 2019).
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, R.; Gu, S.; Zhang, H. The Overview of Thermal Decomposition of Cellulose in Lignocellulosic Biomass. In Cellulose Biomass Conversion; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Menczel, J.D.; Alcon, L.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons., Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Vogelsang, C.; Lusher, A.L.; Dadkhah, M.E.; Sundvor, I.; Umar, M.; Ranneklev, S.B.; Eidsvoll, D.; Meland, S. Microplastics in Road Dust—Characteristics, Pathways and Measures; Norwegian Environment Agency: Trondheim, Norway, 2018.



| RM | RM Quantity (mg) | Sample Type | Sample Mass (mg) | Number of Replicates | Identification Number |
|---|---|---|---|---|---|
| RM1 | 0.29 | TGIS | 50 | 3 | 1, 2, 3 |
| TGO | 0.5 | 2 | 1, 2 | ||
| RM2 | 0.58 | TGIS | 50 | 3 | 4, 5, 6 |
| TGO | 1 | 2 | 3, 4 | ||
| RM3 | 1.45 | TGIS | 50 | 3 | 7, 8, 9 |
| TGO | 2.5 | 2 | 5, 6 | ||
| RM4 | 2.90 | TGIS | 50 | 3 | 10, 11, 12 |
| TGO | 5 | 2 | 7, 8 |
| Polymer | Functional Group | Wavenumber cm−1 | Reference |
|---|---|---|---|
| NR, SBR, EPDM | Methyl | 2964 | Gunasekaran, Natarajan, and Kala, [18] |
| NR | Methylene | 2950, 2853 | Gunasekaran, Natarajan, and Kala, [18] |
| NR, SBR, EPDM | OH | 3470 | Gunasekaran, Natarajan, and Kala, [18] |
| EPDM | 1076 | V. M. LiPtvinow and P. P. De, [11] | |
| SBR | Polystyrene | 750, 700 | Fernández-Berridi et al. and Gunasekaran, Natarajan, and Kala, [14,18] |
| EPDM | Propylene | 1461, 1376 | Gunasekaran, Natarajan, and Kala, (2007) [18] |
| SBR | Vinyl | 990, 910 | Fernández-Berridi et al. and Gunasekaran, Natarajan, and Kala, [14,18] |
| SBR | Butadiene | 960 | Gunasekaran, Natarajan, and Kala, [18] |
| NR, EPDM | Ethylene | 722, 815 | Fernández-Berridi et al. [14] |
| EPDM, (NR) | Alkyl | 2929, 2856 | Fernández-Berridi et al. and Gunasekaran, Natarajan, and Kala, [14,18] |
| NBR | Alkyl | 2230 | Gunasekaran, Natarajan, and Kala, [18] |
| Polymer | Temperature (°C) | Reference |
|---|---|---|
| NR, SBR, EPDM | 376−476, 400−550 | Januszewicz, Klein, Klugmann-Radziemska, and Kardas, and M. Engineering and S. Republi, [5,34] |
| NR | 350 | Fernández-Berridi et al. [14] |
| NR, SBR, EPDM | 300−500 | Fernández-Berridi et al. [14] |
| SBR | 424 | Fernández-Berridi et al. [14] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mengistu, D.; Nilsen, V.; Heistad, A.; Kvaal, K. Detection and Quantification of Tire Particles in Sediments Using a Combination of Simultaneous Thermal Analysis, Fourier Transform Infra-Red, and Parallel Factor Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3444. https://doi.org/10.3390/ijerph16183444
Mengistu D, Nilsen V, Heistad A, Kvaal K. Detection and Quantification of Tire Particles in Sediments Using a Combination of Simultaneous Thermal Analysis, Fourier Transform Infra-Red, and Parallel Factor Analysis. International Journal of Environmental Research and Public Health. 2019; 16(18):3444. https://doi.org/10.3390/ijerph16183444
Chicago/Turabian StyleMengistu, Demmelash, Vegard Nilsen, Arve Heistad, and Knut Kvaal. 2019. "Detection and Quantification of Tire Particles in Sediments Using a Combination of Simultaneous Thermal Analysis, Fourier Transform Infra-Red, and Parallel Factor Analysis" International Journal of Environmental Research and Public Health 16, no. 18: 3444. https://doi.org/10.3390/ijerph16183444
APA StyleMengistu, D., Nilsen, V., Heistad, A., & Kvaal, K. (2019). Detection and Quantification of Tire Particles in Sediments Using a Combination of Simultaneous Thermal Analysis, Fourier Transform Infra-Red, and Parallel Factor Analysis. International Journal of Environmental Research and Public Health, 16(18), 3444. https://doi.org/10.3390/ijerph16183444





