The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. Animal Experimentation
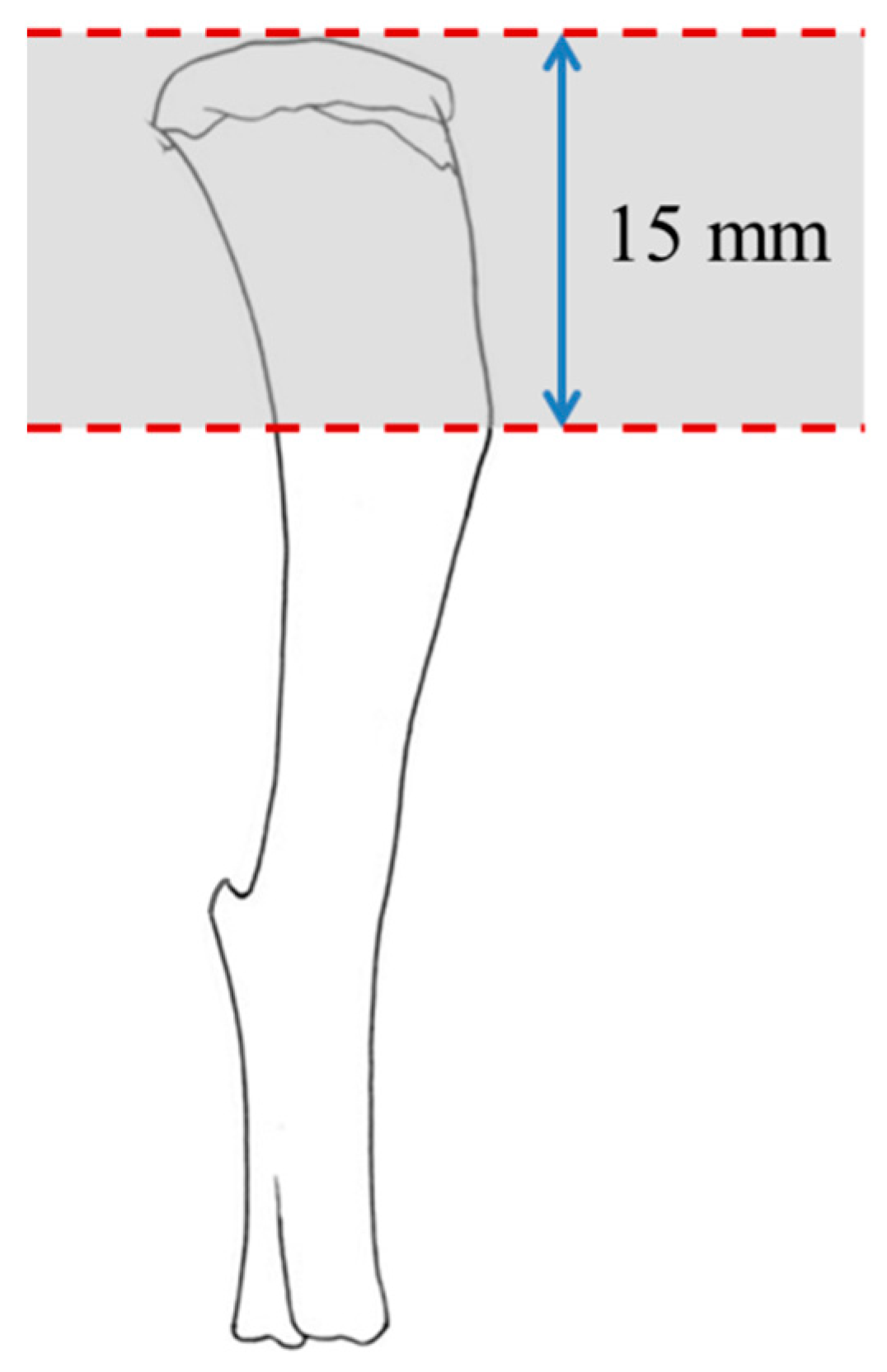
2.3. Protein Extraction and Quantification
2.4. Assessment of Bone-Related Peptides
2.5. Statistical Analysis
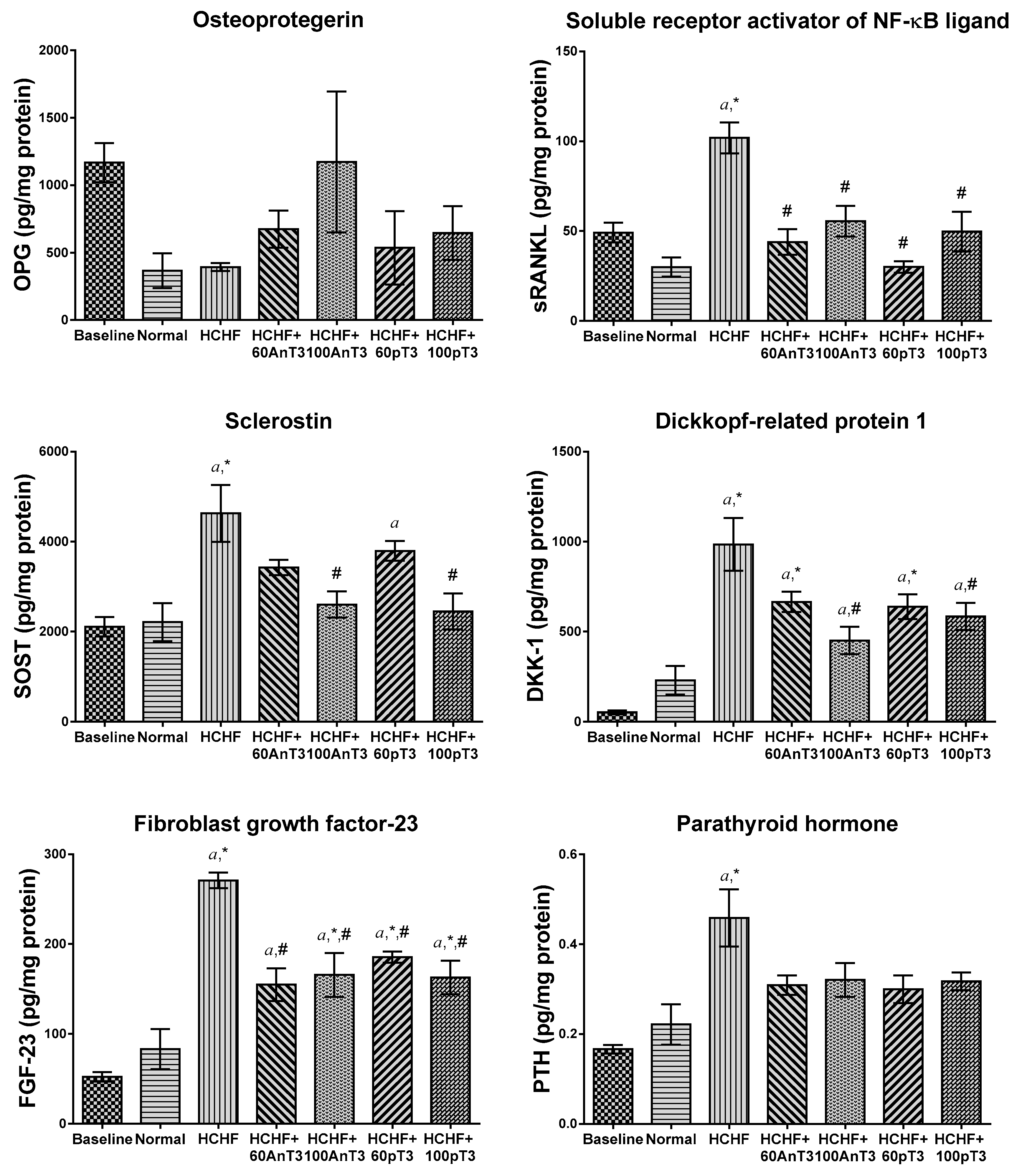
3. Results
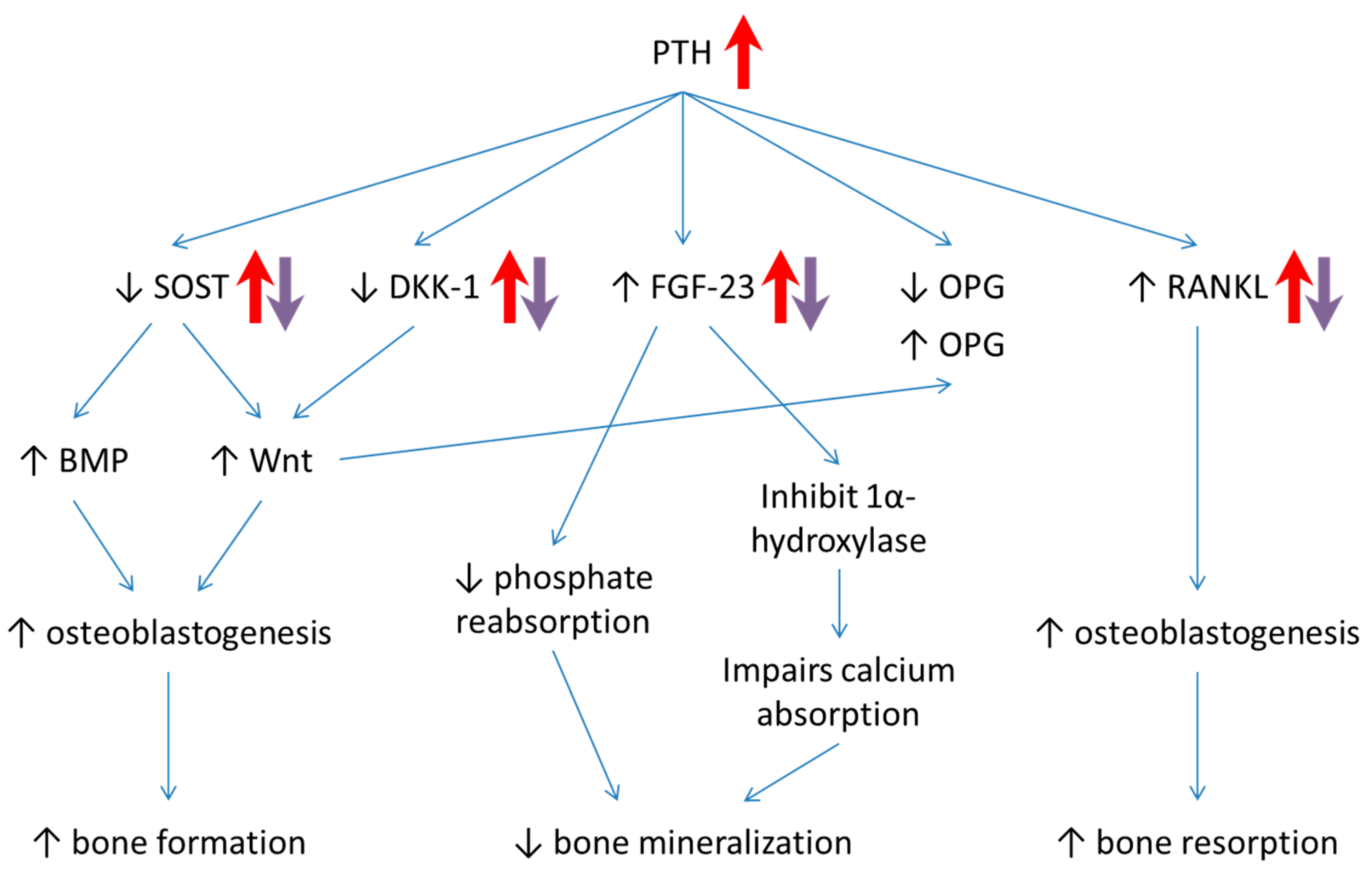
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Effects of a Modified High-carbohydrate High-fat Diet on Metabolic Syndrome Parameters in Male Rats. Exp. Clin. Endocrinol. Diabetes 2018, 126, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS ONE 2018, 13, e0192416. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Jamil, N.A.; Ima-Nirwana, S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed. Pharmacother. 2018, 98, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Bellido, T. Osteocytes and Skeletal Pathophysiology. Curr. Mol. Boil. Rep. 2015, 1, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK System. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Gensure, R.C.; Gardella, T.J.; Jüppner, H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem. Biophys. Res. Commun. 2005, 328, 666–678. [Google Scholar] [CrossRef]
- Jüppner, H. Phosphate and FGF-23. Kidney Int. 2011, 79, S24–S27. [Google Scholar] [CrossRef] [Green Version]
- Perwad, F.; Zhang, M.Y.; Tenenhouse, H.S.; Portale, A.A. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am. J. Physiol. Renal. Physiol. 2007, 293, F1577–F1583. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Mohamad, N.V.; Ibrahim, N.; Chin, K.Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the Vitamin E of the 21st Century: It’s Potential Against Cancer and Other Chronic Diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S.; Vitamin, E. As a Potential Interventional Treatment for Metabolic Syndrome: Evidence from Animal and Human Studies. Front. Pharmacol. 2017, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence. Int. J. Mol. Sci. 2019, 20, 1355. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. 2015, 39, 438–446. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFkB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Burdeos, G.C.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol Attenuates Triglyceride Accumulation in HepG2 Cells and F344 Rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef]
- Shibata, A.; Kawakami, Y.; Kimura, T.; Miyazawa, T.; Nakagawa, K. Alpha-Tocopherol Attenuates the Triglyceride- and Cholesterol-Lowering Effects of Rice Bran Tocotrienol in Rats Fed a Western Diet. J. Agric. Food Chem. 2016, 64, 5361–5366. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Effects of Vitamin E from Elaeis guineensis (Oil Palm) in a Rat Model of Bone Loss Due to Metabolic Syndrome. Int. J. Environ. Res. Public Heal. 2018, 15, 1828. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Jia, Y.-B.; Jiang, D.-M.; Ren, Y.-Z.; Liang, Z.-H.; Zhao, Z.-Q.; Wang, Y.-X. Inhibitory effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at early stage of steroid-induced femoral head necrosis. Mol. Med. Rep. 2017, 15, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.L.; Bahraini, A.; Pierce, J.L.; Bass, S.M.; Yu, K.; Elsayed, R.; Elsalanty, M.; Johnson, M.H.; McNeil, A.; McNeil, P.L.; et al. Inhibition of Osteocyte Membrane Repair Activity via Dietary Vitamin E Deprivation Impairs Osteocyte Survival. Calcif. Tissue Int. 2019, 104, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of delta-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2019, 1–13. [Google Scholar]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, C.A.; Plotkin, L.I.; Galli, C.; Goellner, J.J.; Gortázar, A.R.; Allen, M.R.; Robling, A.G.; Bouxsein, M.; Schipani, E.; Turner, C.H.; et al. Control of Bone Mass and Remodeling by PTH Receptor Signaling in Osteocytes. PLoS ONE 2008, 3, e2942. [Google Scholar] [CrossRef]
- Tu, X.; Edwards, R.; Olivos, N.; Benson, J.; Galli, C.; Pellegrini, G.; Bivi, N.; Plotkin, L.; Bellido, T. Conditional deletion of the parathyroid hormone (PTH) receptor 1 from osteocytes results in decreased bone resorption and a progressive increase in cancellous bone mass. J. Bone Miner. Res. 2011, 26, S16. [Google Scholar]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Uehara, S.; Koide, M.; Takahashi, N. The regulation of osteoclast differentiation by Wnt signals. BoneKEy Rep. 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Jilka, R.L.; Manolagas, S.C.; O’Brien, C.A. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J. Biol. Chem. 2002, 277, 48868–48875. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Liu, M.; Yang, D.; Bouxsein, M.L.; Saito, H.; Galvin, R.S.; Kuhstoss, S.A.; Thomas, C.C.; Schipani, E.; Baron, R.; et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010, 11, 161–171. [Google Scholar] [CrossRef]
- Yao, G.-Q.; Wu, J.-J.; Troiano, N.; Insogna, K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J. Bone Mine.r Metab. 2011, 29, 141–148. [Google Scholar] [CrossRef]
- Kramer, I.; Loots, G.G.; Studer, A.; Keller, H.; Kneissel, M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2010, 25, 178–189. [Google Scholar] [CrossRef]
- Li, J.; Sarosi, I.; Cattley, R.C.; Pretorius, J.; Asuncion, F.; Grisanti, M.; Morony, S.; Adamu, S.; Geng, Z.; Qiu, W.; et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 2006, 39, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Niu, Q.-T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Morvan, F.; Boulukos, K.; Clément-Lacroix, P.; Roman, S.R.; Suc-Royer, I.; Vayssière, B.; Ammann, P.; Martin, P.; Pinho, S.; Pognonec, P.; et al. Deletion of a Single Allele of the Dkk1 Gene Leads to an Increase in Bone Formation and Bone Mass. J. Bone Miner. Res. 2006, 21, 934–945. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Joiner, D.M.; Oyserman, S.M.; Sharma, P.; Goldstein, S.A.; He, X.; Hauschka, P.V. Bone mass is inversely proportional to Dkk1 levels in mice. Bone 2007, 41, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Srinivasan, B.; Mödder, U.I.; Peterson, J.M.; McCready, L.K.; Riggs, B.L.; Dwyer, D.; Stolina, M.; Kostenuik, P.; Khosla, S. Effects of Parathyroid Hormone Treatment on Circulating Sclerostin Levels in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 5056–5062. [Google Scholar] [CrossRef] [PubMed]
- Holguin, N.; Brodt, M.D.; Silva, M.J. Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice. J. Bone Miner. Res. 2016, 31, 2215–2226. [Google Scholar] [CrossRef]
- Guo, Y.-C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. Alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef]
- Shalhoub, V.; Ward, S.C.; Sun, B.; Stevens, J.; Renshaw, L.; Hawkins, N.; Richards, W.G. Fibroblast Growth Factor 23 (FGF23) and Alpha-Klotho Stimulate Osteoblastic MC3T3.E1 Cell Proliferation and Inhibit Mineralization. Calcif. Tissue Int. 2011, 89, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on osteocyte function. Bone 2013, 54, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N. Parathyroid Hormone Signaling in Osteocytes. JBMR Plus 2017, 2, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Pajevic, P.D.; Selig, M.; Barry, K.J.; Yang, J.Y.; Shin, C.S.; Baek, W.Y.; Kim, J.E.; Kronenberg, H.M. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. 2012, 27, 2075–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Pirih, F.; Lu, J.; Ye, F.; Bezouglaia, O.; Atti, E.; Ascenzi, M.; Tetradis, S.; Demer, L.; Aghaloo, T.; Tintut, Y. Adverse Effects of Hyperlipidemia on Bone Regeneration and Strength. J. Bone Miner. Res. 2012, 27, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E.; Von Mühlen, D. Vitamin D, Parathyroid Hormone Levels, and the Prevalence of Metabolic Syndrome in Community-Dwelling Older Adults. Diabetes Care 2007, 30, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Lee, J.H.; Kim, H.N.; Lee, Z.H. alpha-Tocotrienol inhibits osteoclastic bone resorption by suppressing RANKL expression and signaling and bone resorbing activity. Biochem. Biophys. Res. Commun. 2011, 406, 546–551. [Google Scholar] [CrossRef]
- Lee, Z.; Lee, J.; Kim, H.; Yang, D.; Jung, K.; Ha, H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. Bone 2009, 44, S140–S141. [Google Scholar] [CrossRef]
- Ibrahim, N.; Mohamed, N.; Soelaiman, I.N.; Shuid, A.N. The Effects of Targeted Deliveries of Lovastatin and Tocotrienol on Ossification-Related Gene Expressions in Fracture Healing in an Osteoporosis Rat Model. Int. J. Environ. Res. Public Heal. 2015, 12, 12958–12976. [Google Scholar] [CrossRef] [Green Version]
- Norazlina, M.; Maizatul-Neza, J.; Azarina, A.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Effects of vitamin E on receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) in rats treated with nicotine. Med J. Malays. 2010, 65, 14–17. [Google Scholar]
- Norazlina, M.; Chua, C.W.; Ima-Nirwana, S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med J. Malays. 2004, 59, 623–630. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. https://doi.org/10.3390/ijerph16183313
Wong SK, Chin K-Y, Ima-Nirwana S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. International Journal of Environmental Research and Public Health. 2019; 16(18):3313. https://doi.org/10.3390/ijerph16183313
Chicago/Turabian StyleWong, Sok Kuan, Kok-Yong Chin, and Soelaiman Ima-Nirwana. 2019. "The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells" International Journal of Environmental Research and Public Health 16, no. 18: 3313. https://doi.org/10.3390/ijerph16183313





