Colonization of Dental Unit Waterlines by Helicobacter pylori: Risk of Exposure in Dental Practices
Abstract
:1. Introduction
2. Materials and Methods
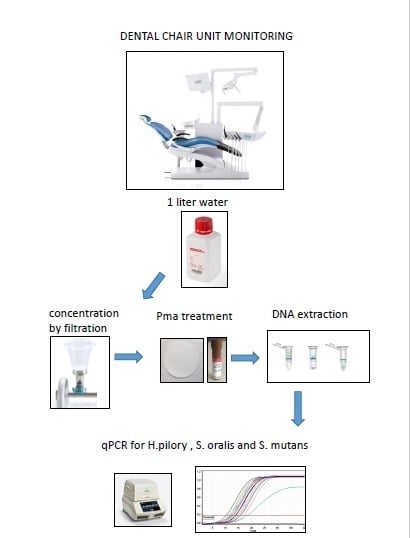
2.1. Sampling Water from DUWLs
2.2. Sampling Water from the Tap
2.3. DNA Extraction and PMA Treatment
2.4. Quantification of Viable H. pylori and Oral Streptococci Using PMA-qPCR
2.5. Risk Assessment Questionnaire and Survey of General Dental Practice Attitudes
3. Results
3.1. Detection of H. pylori and Oral Streptococci by PMA-qPCR
3.2. Questionnaire Answers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Brown, L.M. Helicobacter pylori: Epidemiology and Routes of Transmission. Epidemiol. Rev. 2000, 22, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, M.; Schulz, H.J.; Rüden, H.; Vogt, K. Do conventional cleaning and disinfection techniques avoid the risk of endoscopic Helicobacter pylori transmission? Endoscopy 2003, 35, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.N.; Queiroz, D.M.M.; Bezerra, F.J.G.; Pontes, L.K.; Rodrigues, R.T.; Braga, L.L.B.C. Prevalence of Helicobacter pylori infection in children from an urban community in north-east Brazil and risk factors for infection. Eur. J. Gastroenterol. Hepatol. 2004, 16, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Sanchez, M.L.; Yang, S.; Haggerty, T.D.; Hurst, P.; Perez, P.G.; Julie, P. Gastroenteritis and Transmission of Helicobacter pylori Infection in Households. Emerg. Infect. Dis. 2006, 12, 1701–1708. [Google Scholar]
- Hopkins, R.J.; Vial, P.A.; Ferreccio, C.; Ovalle, J.; Prado, P.; Sotomayor, V.; Russell, R.G.; Wasserman, S.S.; Morris, J.G. Seroprevalence of Helicobacter pylori in Chile: Vegetables may serve as one route of transmission. J. Infect. Dis. 1993, 168, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.L.; Owen, R.J.; Said, B.; Lai, S.; Lee, J.V.; Surman, L.S.; Nichols, G. Detection of Helicobacter pylori by PCR but not culture in water and biofilm samples from drinking water distribution systems in England. J. Appl. Microbiol. 2004, 97, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Safaei, H.G.; Rahimi, E.; Zandi, A.; Rashidipour, A. Helicobacter pylori as a zoonotic infection: the detection of H. pylori antigens in the milk and faeces of cows. J. Res. Med. Sci. 2011, 16, 184–187. [Google Scholar]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef]
- Bellack, N.R.; Koehoorn, M.W.; MacNAB., Y.C.; Morshed, M.G. A conceptual model of water’s role as a reservoir in Helicobacter pylori transmission: a review of the evidence. Epidemiol. Infect. 2006, 134, 439. [Google Scholar] [CrossRef]
- Gião, M.S.; Azevedo, N.F.; Wilks, S.A.; Vieira, M.J.; Keevil, C.W. Persistence of Helicobacter pylori in Heterotrophic Drinking-Water Biofilms. Appl. Environ. Microbiol. 2008, 74, 5898–5904. [Google Scholar] [CrossRef]
- Nurgalieva, Z.Z.; Malaty, H.M.; Graham, D.Y.; Almuchambetova, R.; Machmudova, A.; Kapsultanova, D.; Osato, M.S.; Hollinger, F.B.; Zhangabyl, A. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am. J. Trop. Med. Hyg. 2002, 67, 201–206. [Google Scholar] [CrossRef]
- Fujimura, S.; Kato, S.; Watanabe, A. Water source as a Helicobacter pylori transmission route: a 3-year follow-up study of Japanese children living in a unique district. J. Med. Microbiol. 2008, 57, 909–910. [Google Scholar] [CrossRef]
- Khan, A.; Farooqui, A.; Kazmi, S.U. Presence of Helicobacter pylori in drinking water of Karachi, Pakistan. J. Infect. Dev. Ctries. 2012, 6, 251–255. [Google Scholar] [CrossRef]
- Moreno, Y.; Ferrús, M.A. Specific detection of cultivable Helicobacter pylori cells from wastewater treatment plants. Helicobacter 2012, 17, 327–332. [Google Scholar] [CrossRef]
- Fayle, S.A.; Pollard, M.A. Decontamination of dental unit water systems: A review of current recommendations. Br. Dent. J. 1996, 181, 369–372. [Google Scholar] [CrossRef]
- Bahrami, A.R.; Rahimi, E.; Ghasemian, S.H. Detection of Helicobacter pylori in city water, dental units’ water, and bottled mineral water in Isfahan, Iran. Sci. World J. 2013, 2013, 280510. [Google Scholar] [CrossRef]
- Sajadi, D.A.; Noles, D.G. Prevalence of Helicobacter pylori in Dental Unit Waterlines. In Proceedings of the 89th General Session & Exhibition of the IADR Conference, San Diego, CA, USA, 17 March 2011. [Google Scholar]
- Hardo, P.G.; Tugnait, A.; Hassan, F.; Lynch, D.A.; West, A.P.; Mapstone, N.P.; Quirke, P.; Chalmers, D.M.; Kowolik, M.J.; Axon, A.T. Helicobacter pylori infection and dental care. Gut 1995, 37, 44–46. [Google Scholar] [CrossRef]
- Gebara, E.C.E.; Pannuti, C.; Faria, C.M.; Chehter, L.; Mayer, M.P.A.; Lima, P.A. Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol. Immunol. 2004, 19, 277–280. [Google Scholar] [CrossRef]
- Al, A.M.; Al Hamoudi, N.; Anil, S.; Al, J.A.; Hamoudi, W.K. Is the presence of Helicobacter pylori in dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can. J. Gastroenterol. J. Can. Gastroenterol. 2009, 23, 177–179. [Google Scholar]
- Berroteran, A.; Perrone, M.; Correnti, M.; Cavazza, M.E.; Tombazzi, C.; Goncalvez, R.; Lecuna, V. Detection of Helicobacter pylori DNA in the oral cavity and gastroduodenal system of a Venezuelan population. J. Med. Microbiol. 2002, 51, 764–770. [Google Scholar] [CrossRef]
- Vale, F.F.; Vítor, J.M.B. Transmission pathway of Helicobacter pylori: does food play a role in rural and urban areas? Int. J. Food Microbiol. 2010, 138, 1–12. [Google Scholar] [CrossRef]
- Benaissa, M.; Babin, P.; Quellard, N.; Pezennec, L.; Cenatiempo, Y.; Fauchère, J.L. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect. Immun. 1996, 64, 2331–2335. [Google Scholar]
- Hua, J.; Ho, B. Is the coccoid form of Helicobacter pylori viable? Microbios 1996, 87, 103–112. [Google Scholar]
- Nocker, A.; Sossa, F.P.; Burr, M.D.; Camper, A.K. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef]
- Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco, R.S.R.; Zotti, C.M. Legionella in water samples: how can you interpret the results obtained by quantitative PCR? Mol. Cell Probes 2015, 29, 7–12. [Google Scholar]
- Pankhurst, C.L.; Johnson, N.W.; Woods, R.G. Microbial contamination of dental unit waterlines: the scientific argument. Int. Dent. J. 1998, 48, 359–368. [Google Scholar] [CrossRef]
- Walker, J.T.; Bradshaw, D.J.; Bennett, A.M.; Fulford, M.R.; Martin, M.V.; Marsh, P.D. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 2000, 66, 3363–3367. [Google Scholar] [CrossRef]
- Kohn, W.G.; Harte, J.A.; Malvitz, D.M.; Collins, A.S.; Cleveland, J.L.; Eklund, K.J.; Dolores, M.M. Guidelines for infection control in dental health care settings-2003. J. Am. Dent. Assoc. 2004, 135, 33–47. [Google Scholar] [CrossRef]
- European Union. Council Directive 98/83/EC of on the quality of water intended for human consumption. J. Eur. Communities 1998, 41, 32–54. [Google Scholar]
- Johnson, C.H.; Rice, E.W.; Reasoner, D.J. Inactivation of Helicobacter pylori by chlorination. Appl. Environ. Microbiol. 1997, 63, 4969–4970. [Google Scholar]
- Aziz, R.K.; Khalifa, M.M.; Sharaf, R.R. Contaminated water as a source of Helicobacter pylori infection: A review. J. Adv. Res. 2015, 6, 539–547. [Google Scholar] [CrossRef]
- Fitzgibbon, E.J.; Bartzokas, C.A.; Martin, M.V.; Gibson, M.F.; Graham, R. The source, frequency and extent of bacterial contamination of dental unit water systems. Br. Dent. J. 1984, 157, 98–101. [Google Scholar] [CrossRef]
- Barbeau, J.; Tanguay, R.; Faucher, E.; Avezard, C.; Trudel, L.; Côté, L.; Prévost, A.P. Multiparametric analysis of waterline contamination in dental units. Appl. Environ. Microbiol. 1996, 62, 3954–3959. [Google Scholar] [Green Version]
- Petti, S.; Tarsitani, G. Detection and quantification of dental unit water line contamination by oral streptococci. Infect. Control Hosp. Epidemiol. 2006, 27, 504–509. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomuzzi, M.; Zotti, C.M.; Ditommaso, S. Colonization of Dental Unit Waterlines by Helicobacter pylori: Risk of Exposure in Dental Practices. Int. J. Environ. Res. Public Health 2019, 16, 2981. https://doi.org/10.3390/ijerph16162981
Giacomuzzi M, Zotti CM, Ditommaso S. Colonization of Dental Unit Waterlines by Helicobacter pylori: Risk of Exposure in Dental Practices. International Journal of Environmental Research and Public Health. 2019; 16(16):2981. https://doi.org/10.3390/ijerph16162981
Chicago/Turabian StyleGiacomuzzi, Monica, Carla M. Zotti, and Savina Ditommaso. 2019. "Colonization of Dental Unit Waterlines by Helicobacter pylori: Risk of Exposure in Dental Practices" International Journal of Environmental Research and Public Health 16, no. 16: 2981. https://doi.org/10.3390/ijerph16162981