Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Characteristics and Electrode Selection
2.2. Electrolysis System
2.3. Analytical Methods
3. Results
3.1. Influence of Initial pH Value
3.2. Influence of Applied Current Density
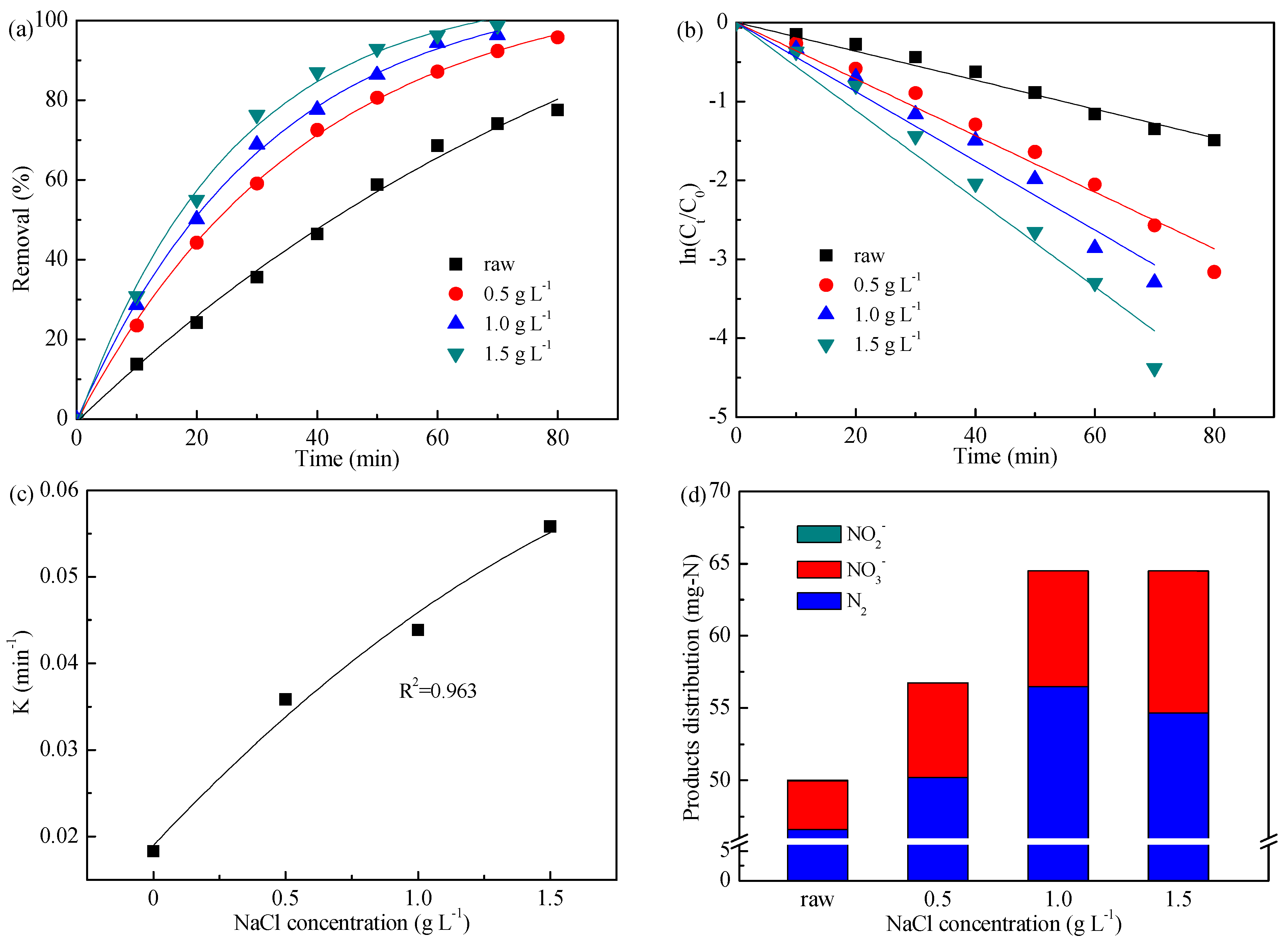
3.3. Effect of Added NaCl Concentration
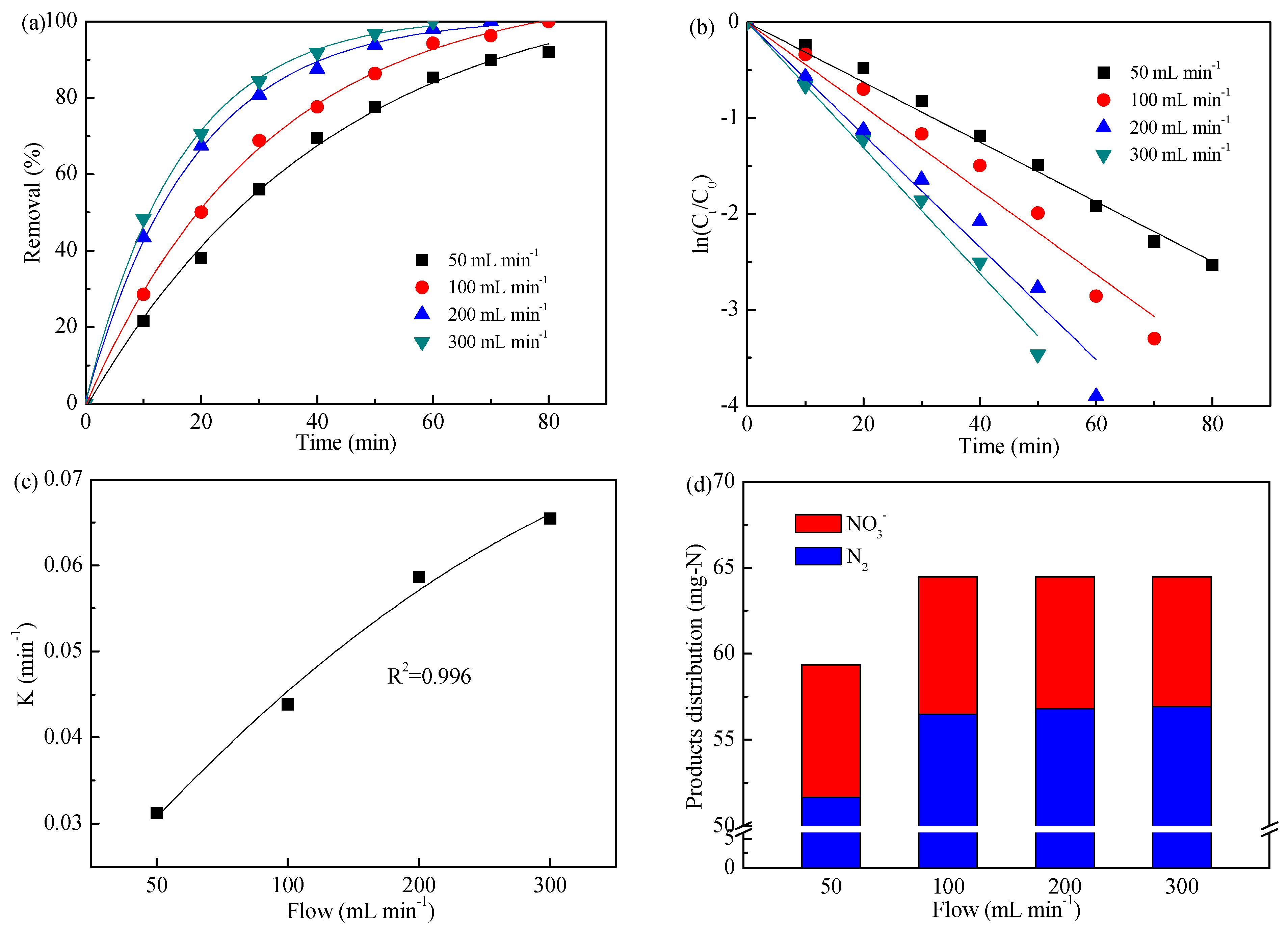
3.4. Impact of Flow
3.5. Mechanisms and Economic Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Xu, H.; Yang, S.N.; Bi, S.T.; Li, F.; Shen, C.S.; Ma, C.Y.; Tian, Q.; Liu, J.S.; Song, X.S.; et al. Treatment of industrial dyeing wastewater with a pilot-scale strengthened circulation anaerobic reactor. Bioresour. Technol. 2018, 264, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical review of abatement of ammonia from wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Falas, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomei, M.C.; Stazi, V.; Angelucci, D.M. Biological treatment of hypersaline wastewater in a continuous two-phase partitioning bioreactor: Analysis of the response to step, ramp and impulse loadings and applicability evaluation. J. Clean. Prod. 2018, 191, 67–77. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, W.N.; Wan, R.; Luo, J.Y.; Su, Y.L.; Huang, H.N.; Chen, Y.G. Increasing municipal wastewater BNR by using the preferred carbon source derived from kitchen wastewater to enhance phosphorus uptake and short-cut nitrification-denitrification. Chem. Eng. J. 2018, 344, 556–564. [Google Scholar] [CrossRef]
- Wang, M.; Payne, K.A.; Tong, S.; Ergas, S.J. Hybrid algal photosynthesis and ion exchange (HAPIX) process for high ammonium strength wastewater treatment. Water Res. 2018, 142, 65–74. [Google Scholar] [CrossRef]
- Malovanyy, A.; Sakalova, H.; Yatchyshyn, Y.; Plaza, E.; Malovanyy, M. Concentration of ammonium from municipal wastewater using ion exchange process. Desalination 2013, 329, 93–102. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Masse, D.I. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.P.; Zhang, Z.Y.; Yang, Y.N.; Chen, R.Z.; Sugiura, N. Simultaneous reduction of nitrate and oxidation of by-products using electrochemical method. J. Hazard. Mater. 2009, 171, 724–730. [Google Scholar] [CrossRef]
- Su, L.H.; Li, K.; Zhang, H.B.; Fan, M.H.; Ying, D.W.; Sun, T.H.; Wang, Y.L.; Jia, J.P. Electrochemical nitrate reduction by using a noval Co3O4/Ti cathode. Water Res. 2017, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Zhao, M.; Dong, H.; Yu, H.B.; Zhan, S.H.; Zhang, L. Energy-saving removal of methyl orange in high salinity wastewater by electrochemical oxidation via a novae Ti/SnO2-Sb anode-Air diffusion cathode system. Catal. Today 2015, 258, 156–161. [Google Scholar] [CrossRef]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Nitrogen and organic load removal from sanitary landfill leachates by anodic oxidation at Ti/Pt/PbO2, Ti/Pt/SnO2-Sb2O4 and Si/BDD. Appl. Catal. B-Environ. 2014, 148–149, 288–294. [Google Scholar] [CrossRef]
- Moreira, F.C.; Bovaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B-Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Tanyolaç, A. Electrochemical treatment of simulated industrial paint wastewater in a continuous tubular reactor. Chem. Eng. J. 2009, 148, 444–451. [Google Scholar] [CrossRef]
- Shu, J.C.; Liu, R.L.; Liu, Z.H.; Du, J.; Tao, C.Y. Manganese recovery and ammonia nitrogen removal from simulation wastewater by pulse electrolysis. Sep. Purif. Technol. 2016, 168, 107–117. [Google Scholar] [CrossRef]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächterb, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni(OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Candido, L.; Gomes, J.A.C.P. Evaluation of anode materials for the electro-oxidation of ammonia and ammonium ions. Mater. Chem. Phys. 2011, 129, 1146–1151. [Google Scholar] [CrossRef]
- Li, F.; Peng, X.; Liu, Y.B.; Mei, J.C.; Sun, L.W.; Shen, C.S.; Ma, C.Y.; Huang, M.H.; Wang, Z.W.; Sand, W.; et al. A chloride-radical-mediated electrochemical filtration system for rapid and effective transformation of ammonia to nitrogen. Chemosphere 2019, 229, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.J.; Huang, Y.H.; Huang, C.P. In-situ electrochemical formation of nickel oxyhydroxide (NiOOH) on metallic nickel foam electrode for the direct oxidation of ammonia in aqueous solution. Electrochim. Acta 2018, 281, 410–419. [Google Scholar] [CrossRef]
- Zöllig, H.; Morgenroth, E.; Udert, K.M. Inhibition of direct electrolytic ammonia oxidation due to a change in local pH. Electrochim. Acta 2015, 165, 348–355. [Google Scholar] [CrossRef]
- Zöllig, H.; Remmele, A.; Morgenroth, E.; Udert, K.M. Removal rates and energy demand of the electrochemical oxidation of ammonia and organic substances in real stored urine. Environ. Sci. Water Res. Technol. 2017, 3, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.; Suryanto, B.H.R.; MacFarlane, D.R. Electro-oxidation of ammonia on electrochemically roughened platinum electrodes. Electrochim. Acta 2019, 297, 778–783. [Google Scholar] [CrossRef]
- Bunce, N.; Bejan, D. Mechanism of electrochemical oxidation of ammonia. Electrochim. Acta 2011, 56, 8085–8093. [Google Scholar] [CrossRef]
- Ye, J.Y.; Lin, J.L.; Zhou, Z.Y.; Hong, Y.H.; Sheng, T.; Rauf, M.; Sun, S.G. Ammonia electrooxidation on dendritic Pt nanostructures in alkaline solutions investigated by in-situ FTIR spectroscopy and online electrochemical mass spectroscopy. J. Electroanal. Chem. 2018, 819, 495–501. [Google Scholar] [CrossRef]
- Shih, Y.J.; Huang, Y.H.; Huang, C.P. Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni(OH)2(s)-NiOOH(s) nanocatalysts. Electrochim. Acta 2018, 263, 261–271. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, K.; Hu, C.Z.; Liu, H.J.; Wang, Y.; Qu, J.H. Electrochemical oxidation of ammonia accompanied with electricity generation based on reverse electrodialysis. Electrochim. Acta 2018, 269, 128–135. [Google Scholar] [CrossRef]
- Zou, J.X.; Peng, X.L.; Li, M.; Xiong, Y.; Wang, B.; Dong, F.Q.; Wang, B. Electrochemical oxidation of COD from real textile wastewaters: Kinetic study and energy consumption. Chemosphere 2017, 171, 332–338. [Google Scholar] [CrossRef]
- Chen, J.M.; Xia, Y.J.; Dai, Q.Z. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Zhu, R.Y.; Yang, C.Y.; Zhou, M.M.; Wang, J.D. Industrial park wastewater deeply treated and reused by a novel electrochemical oxidation reactor. Chem. Eng. J. 2015, 260, 427–433. [Google Scholar] [CrossRef]
- Yao, J.C.; Zhou, M.M.; Wen, D.N.; Xue, Q.W.; Wang, J.D. Electrochemical conversion of ammonia to nitrogen in non-chlorinated aqueous solution by controlling pH value. J. Electroanal. Chem. 2016, 776, 53–58. [Google Scholar] [CrossRef]
- Polcaro, A.M.; Palmas, S.; Renoldi, F.; Mascia, M. On the performance of Ti/SnO2 and Ti/PbO2 anodes in electrochemical degradation of 2-chlorophenol for wastewater treatment. J. Appl. Electrochem. 1999, 29, 147–151. [Google Scholar] [CrossRef]
- Martín de Vidales, M.J.; Robles-Molina, J.; Dominguez-Romero, J.C.; Cañizares, P.; Sáez, C.; Molina-Díaz, A.; Rodrigo, M.A. Removal of sulfamethoxazole from waters and wastewaters by conductive-diamond electrochemical oxidation. J. Chem. Technol. Biotechnol. 2012, 87, 1441–1449. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.P.; Zhang, Z.Y.; Sugiura, N. Efficient electrochemical reduction of nitrate to nitrogen using Ti/IrO2–Pt anode and different cathodes. Electrochim. Acta 2009, 54, 4600–4606. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, Q.L.; Zhang, Y.S.; Wei, L.L.; Li, W.; Wang, K. The eAND process: Enabling simultaneous nitrogen-removal and disinfection for WWTP effluent. Water Res. 2015, 74, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Pan, B.J.; Shen, R.X.; Yuan, T.B.; Wang, J.D. Differential control of anode/cathode potentials of paired electrolysis for simultaneous removal of chemical oxygen demand and total nitrogen. Sci. Total Environ. 2019, 687, 198–205. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Shi, H.C.; Lu, J.H. Electrochemical treatment of ammonia in wastewater by RuO2-IrO2-TiO2/Ti electrodes. J. Appl. Electrochem. 2007, 37, 1137–1144. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, I.T.; Perk, G.I.; Lee, E.H. Electrolytic decomposition of ammonia to nitrogen in a multi-cell-stacked electrolyzer with a self-pH-adjustment function. J. Appl. Electrochem. 2006, 36, 1415–1426. [Google Scholar] [CrossRef]





| Parameters | Units | Actual Wastewater |
|---|---|---|
| pH | - | 8.3 ± 0.1 |
| Ammonia | mg-N L−1 | 161.2 ± 2.0 |
| Chemical oxygen demand (COD) | mg L−1 | 58.8 ± 3.5 |
| Chloride (Cl−) | mg L−1 | 483.3 ± 1.3 |
| Sodium (Na+) | mg L−1 | 1343.3 ± 2.6 |
| Sulfate (SO42−) | mg L−1 | 1221.6 ± 1.5 |
| Chromaticity | times | 25 |
| Conductivity | mS cm−1 | 6.61 ± 0.1 |
| Parameters | Conditions | 100 × K (min−1) | R2 |
|---|---|---|---|
| Initial pH value | 2.7 | 1.069 | 0.988 |
| 4.8 | 1.480 | 0.989 | |
| 6.9 | 0.680 | 0.986 | |
| 8.3 | 1.822 | 0.990 | |
| 12.0 | 2.135 | 0.996 | |
| Applied current density (mA cm−2) | 5 | 0.770 | 0.992 |
| 10 | 1.216 | 0.995 | |
| 15 | 1.479 | 0.991 | |
| 20 | 1.822 | 0.990 | |
| 30 | 2.239 | 0.993 | |
| NaCl concentration (g L−1) | 0 | 1.822 | 0.990 |
| 0.5 | 3.583 | 0.991 | |
| 1.0 | 4.385 | 0.988 | |
| 1.5 | 5.582 | 0.988 | |
| Flow (mL min−1) | 50 | 3.120 | 0.996 |
| 100 | 4.385 | 0.988 | |
| 200 | 5.863 | 0.990 | |
| 300 | 6.547 | 0.996 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Wang, J.; Chen, J. Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. https://doi.org/10.3390/ijerph16162931
Yao J, Mei Y, Xia G, Lu Y, Xu D, Sun N, Wang J, Chen J. Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment. International Journal of Environmental Research and Public Health. 2019; 16(16):2931. https://doi.org/10.3390/ijerph16162931
Chicago/Turabian StyleYao, Jiachao, Yu Mei, Guanghua Xia, Yin Lu, Dongmei Xu, Nabo Sun, Jiade Wang, and Jun Chen. 2019. "Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment" International Journal of Environmental Research and Public Health 16, no. 16: 2931. https://doi.org/10.3390/ijerph16162931




