Abstract
Objectives: A nationwide biomonitoring program identified the long-term trends of environmental exposures to hazardous chemicals in the general population and found geographical locations where body burdens of an exposed group significantly differed from those of the general population. The purpose of this study is to analyze the hazardous compounds associated with foods and cooking in the nationwide general population for evaluation of the environmental exposures and health risk factors and for the establishment of the reference levels at the national level. Methods: During 2009–2010, the National Institute of Food and Drug Safety Evaluation (NIFDS) conducted a nationwide human biomonitoring study, including a questionnaire survey and environmental exposure assessments for specific hazardous compounds from foods and cooking among the general population in South Korea. Results: A total of 2139 individuals voluntarily participated in 98 survey units in South Korea, including 889 (41.6%) men and 1250 women (58.4%). Bio-specimens (serum and urine) and questionnaires were collected from the study population. Acrylamides, heterocyclic amines (HCAs), phenols, and phthalates were analyzed from urine, and perfluorinated compounds (PFCs) and organic chloride pesticides (OCPs) were analyzed from serum samples. The information on exposure pathway and geographical locations for all participants was collected by questionnaire interviews, which included demographic characteristics, socioeconomic status, history of family diseases, conditions of the indoor and outdoor environment, lifestyles, occupational history, and food and dietary information. Conclusion: We describe the design of the study and sampling of human biospecimen procedures including bio-sample repository systems. The resources produced from this nationwide human biomonitoring study and survey will be valuable for use in future biomarkers studies and for the assessment of exposure to hazardous compounds associated with foods and cooking.
1. Introduction
Modern people are exposed to thousands of natural and man-made chemicals. The general population can be exposed to hazardous toxic substances through air, water, and foods. There is evidence that environmental exposure to toxic substances in the population of many countries caused various chronic diseases [1,2]. Some countries have created surveillance systems using biomarkers for measurement of exposure to a variety of pollutants including food consumption in the general population from the National Health and Nutrition Examination Surveys (NHANES) in the U.S. [3], the German Environmental Survey (GerES) in Germany [4], and the Korea National Health and Nutrition Examination Surveys in South Korea [5,6]. The potential benefits of the nationwide biomonitoring programs are to identify the long-term trends of environmental exposures to hazardous chemicals in the general population and characterize geographical locations where body burdens of the exposed group significantly differed from those of the general population [7,8]. In Korea, environmental monitoring depended mainly on air, water, food, and soil pollutants. Over the past decade, several initiatives have been launched aiming to include human data in the national environmental framework [6,9,10,11].
In this study, the National Institute of Food and Drug Safety Evaluation (NIFDS) in South Korea planned a nationwide study project of “The collection of biological samples for human biomonitoring and data analysis in 2009–2010”, which promoted biomarker-based exposure assessment of specific pollutants associated with foods and cooking in the general population. The general purpose of the NIFDS project was to establish reference levels among the general population at the national level and to evaluate their variability based on nationwide sample sizes. Second, we wish to develop an association study for research on the sources and routes of hazardous compounds associated with foods and cooking.
This paper describes the research design, rationale, human biosampling procedures, and biospecimen controls for use in the nation-wide biomonitoring program of hazardous chemicals associated with foods and cooking in Korea.
2. Study Design
The conceptual basis and design of this study have been made by an expert advisory group, the Environmental Health Study in Korea (EHKor), which included professors, epidemiologists, data analysts, statisticians, and other professionals.
The study design consists of five steps: the selection of subjects, collection of human bio-specimens and questionnaires, sample preparation and delivery, analyses of samples (serum and urine), and establishment of a database system (Figure 1). This study was performed for adults (over 18 years old) for a period of two years (2009–2011), and another similar study was planned for children for a period of two years (2010–2011).
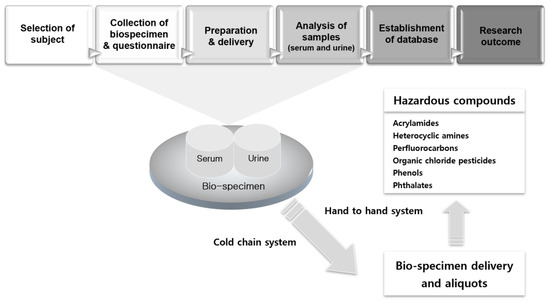
Figure 1.
Flow-chart for the human biomonitoring survey.
2.1. Selection of Subjects
The target population of this study was adults aged 18–70 years, living in households in Korea. The primary sampling unit for this survey used census enumeration districts, which are defined geographical areas containing around 60 dwellings. Based on data from the 2005 Korea Census, the country was divided into 265,350 enumeration districts. Participants in this study were chosen through a stratified two-stage random sampling. Using the probability proportional to size sampling, 98 enumeration districts were selected for this study. Basically, 10 households were selected by systematic sampling within sample enumeration districts and all adults aged 18–70 years within each household were asked to take part in this survey (Table 1).

Table 1.
Demographic characteristics of the target population participating in this study.
The stratification variables for this study were the region and enumeration district classifications, which were determined based on the main house type (residential apartment or single-family house) and urbanization level (town or rural) of the enumeration district. This stratification provided a total of 34 strata for the study. At least 2 enumeration districts were selected within each stratum.
Target subjects of this study were adults (18 to 70 years old). Subjects living in the sample household who agreed to participate in the projects completed informed consent; they were asked to answer the epidemiologic questionnaire in their homes. Biological specimens (serum and urine) were taken at the local public health center by trained phlebotomists. Completed consent participants included 2139 people in 98 sample enumeration districts (Figure 2). The study was approved by the institutional review board at ASAN Medical Center and each subject gave written informed consent (code: 2009-0369).
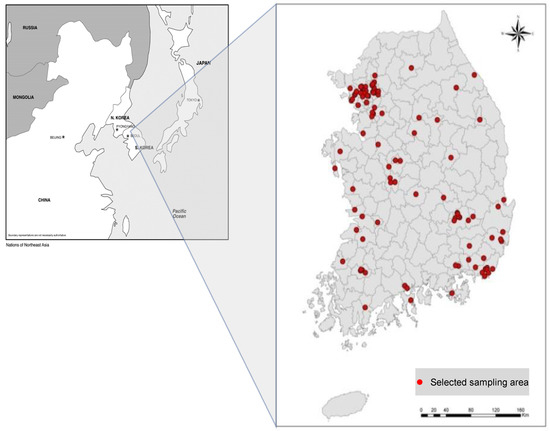
Figure 2.
A total of 2139 persons from 98 units participated in the human biomonitoring survey.
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses in this study. We calculated all estimates using a sampling weight that represented adults aged 18–70 years in Korea. Sampling weights are needed to correct for imperfections in the samples that might lead to bias and other departures between the sample and the reference population. Such imperfections include the selection of units with unequal probabilities, non-coverage of the population, and non-response [12]. The sampling weight calculations in this study account for differential selection probabilities, nonresponse, and post-stratification.
2.2. Selection of Hazardous Compounds
In this study, six hazardous chemical compounds, including acrylamides, heterocyclic amines (HCAs), perfluorinated compounds (PFCs), phenols, organic chloride pesticides (OCPs), and phthalates were selected. We analyzed a total of 69 chemicals for human biomonitoring samples collected from serum and urine, as shown in Table 2.

Table 2.
Hazardous compounds and their chemicals collected in this study.
Humans are exposed to acrylamides through food intake, smoking, beverages, cosmetics, etc. According to national research studies of the Food and Drug Administration (FDA), a high concentration of acrylamides has been reported in potato flavored snacks [13]. Although the main pathways of exposure to heterocyclic amines (HCAs) for humans might be surface waters [14], a primary route of HCAs is through food, and a study also showed that there is a positive association between HCA intake and colorectal adenoma risk [15]. In the U.S., serum perfluorooctanoate (PFOA) levels in the randomly selected residents in the water district were significantly higher than those in the general U.S. population, and the main routes of exposure to PFOA were identified as residential drinking water and work during production processes using PFOA [16]. PFCs might be accumulated in the human body [17], and PFC exposure could increase cardiovascular disease and diabetes risks [18]. Phenols are alkyl phenol compounds, which include pentylphenol, octylphenol, and nonylphenol. They are recognized as endocrine disruptors, which interfere with the body’s endocrine system, and produce adverse developmental, reproductive, and neurological problems. They are used mainly as raw materials for cosmetics and perfumes, surfactants, or propellant for pulp and paper mills, and in herbicide sprays or pesticides and toxic agents, polyvinyl chloride (PVC), polyvinyl acetate (PVA), styrene–acrylonitrile ink, anti-oxidants, or stabilizers for synthetic rubber manufacturing. In particular, we have recently made an effort to ensure that food is safe from endocrine-disrupting chemicals in order to protect human health from exposure to bisphenol A, which is a well-known endocrine disruptor found in food containers and packaging [19]. Organic chlorine pesticides (OCPs) are one type of persistent organic pollutant (POP) that are hardly decomposed by chemical, biological, and photochemical reactions in the environments. A number of hazardous chemicals among POPs have been used in pesticides, organic solvents, PVC (polyvinyl chloride), and pharmaceutical materials. They could be generated as potential by-products in the production processes. Di (2-ethylhexyl) phthalate (DEHP), one of the phthalate esters, accounted for over half of the entire usage. DEHP is a colorless, odorless, oily liquid. It is comprised of 40–50% of the weight of the final plastic product [20], and it is banned from use in toothbrushes, rubber nipples, young children’s toys, etc. However, it is allowed for use in food packaging, toys, and medical supplies (serum storage containers and intravenous tubing, etc). Its average use is known to be 4–30 μg/kg per day [21].
2.3. Collection of Bio-Specimens
This study involved collection of bio-specimens (serum and urine) from selected subjects for human biological monitoring of harmful substances. During the preparation of the sample collection, sample containers were checked for free of target chemicals. Serum separate tube (SST) was used for serum sampling. Sampling staffs were sufficiently trained using specific protocols, which included the materials necessary for sampling, procedures for taking serum and urine, and a flow diagram from welcome to the final goodbye greeting for visiting subjects in the sampling place. The consented subjects received instructions regarding the appointed place and day/time, fasting of breakfast, and holding of morning urine [22]. The volume of urine necessary from each subject was at least 45 mL for analysis of acrylamides, HCAs, phenols, and phthalates. Analysis of each chemical group required a 5 mL aliquot and backup sample. Urine collection cups were marked on the 50 mL amount of urine for reference while subjects collected urine.
Blood was collected from each subject in the amount of approximately 13–15 mL with two collection vacutainers, for which at least 5.3 mL was required as a serum for analysis of OCPs, PFCs, and total lipid. The collected sample was smoothly mixed at room temperature for 30 min for anti-coagulation using the mixture. The serum was then separated from the blood sample using a centrifuge. Serum was moved to new vacutainers, which were stored in ice boxes for stabilization. During the separation of sample serums, the erythrocytosis serums were no longer used for analysis. All clinical pathology technicians were careful not to make hemolytic serum, and not to have contact with subjects’ blood. All sampling units have an emergency system and program. In cases of pricking by the needle of the syringe during blood sampling procedures, immediately wash the wound with flowing tap water, and transfer to the hospital along with the blood donor in order to investigate whether the technicians were infected or not. Later, report the situation to the headquarters.
Two kinds of labels were used, and bar-coded. One was used for sampling procedures, and another was used for differentiation of the aliquoted samples. Labels provided information on the subject identification number and dates. The labeled sampled containers, including bio-specimens (serum and urine), were wrapped with aluminum foil to break the sunlight, and kept refrigerated at 2–8 °C.
2.4. Sample Delivery and Management
The main principles for sample delivery were the maintenance of the cold chain and hand-to-hand system (Figure 1). For cold chain delivery, we manufactured a new delivery icebox, which can maintain the inner temperature to 2–8 °C for 48 h when the outer temperature is 30–35 °C. Our specimen samples were carried from the local sampling unit to the splitting laboratory while maintaining a constant temperature using the refrigerating icebox within 48 h. The icebox was composed of compacted hard plastics; the inner temperature was maintained at 2–8 °C using frozen, portable, insulated ice packs. Upon their arrival at the laboratory, the refrigerated bio-samples were split for the analysis of the aliquots in the refrigeration room. They were then stored at −70 °C until transport to the analyzing laboratory.
A computerized inventory tracking system was developed, so that the storage location of all samples for each participant could be quickly determined. The biological sample repositories for the study are equipped with appropriate alarm systems and emergency electricity backup to prevent accidental thawing. The collected samples in local sampling units were hauled to the person in charge of transportation, in order confirm whether all of the downloaded checklists had been written or not, and the signed checklists were uploaded on cloud storage. The updated checklist was used as raw data for production of bar-coded labels, which were used at the sample splitting laboratory as soon as collected samples arrived; therefore, it was required that the updated checklist be uploaded to cloud storage on the day of delivery. In addition, statistics for the collecting ratio, sex, age, progress, and other information, depending on each professor and region, were posted on cloud storage by utilizing the uploaded data. Sampling time was filled in on the checklist because it was constantly inserted at the time of handling, dispensing, and storage of samples for use in quality management of sampling data.
2.5. Sample Analyses and Quality Control
Hazardous compounds were analyzed at six analytical laboratories in each group. Analysis of acrylamides in urine was performed at the chemical analytic laboratory of the Korea Institute of Science and Technology (KIST). Four types of chemicals of acrylamides, which included acrylamide, Glycinamide, AAMA, and GAMA, were analyzed by HPLC (Varian 212, Walnut Creek, CA, USA) with MS/MS detector (Varian, Walnut Creek, CA, USA). Analysis of Heterocyclic amines (HCAs) in urine was performed at the chemical analytic laboratory of the Korea Basic Science Institute (KBSI). Eight metabolites of HCAs, which included IQ, MeIQ, MeIQX, Glu-P-1, Glu-P-2, PhIP, AαC, and MeAαC were analyzed by HPLC (Nanispace Si-2, Shiseido, Japan) with MS detector (LCQ DECA XP, Thermo Finnigan San Jose, CA, USA). Analysis of perfluorinated compounds (PFCs) in serum was performed at the chemical analytic laboratory of Kyunghee University in South Korea. Ten types of chemicals of PFCs, including PFPA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFHxS, PFHpS, PFOS, and PFNS, were analyzed by HPLC (Agilent 1200 Series CG1367B) with MRM detector (multiple reaction monitoring) of ESI(−)-MS/MS (API 3200, MDS SCIEX, Concord, ON, Canada).
Analysis of phenols in urine was performed at the organic chemical analytic laboratory of KIST. Fifteen metabolites of phenols, which included t-BP, n-BP, n-PP, n-HX, n-HP, t-OP, n-OP, NP, bisphenol-A, triclosan, 2.4-dichlorophenol, 2,5-dichlorophenol, 2,4,6-trichlorophenol, and 2,4,6-trichlorophenol were analyzed by separation with GC (Agilent 6890 Series, Santa Clara, USA), and detection with MS (Agilent G1701DA, Santa Clara, USA). Analysis of organic chloride pesticides (OCPs) in lipids of serum was performed at the organic chemical analytic laboratory of KIST. Eighteen metabolites of OCPs, which included hexachlorobenzene, heptachlor, aldrin, oxychlorodane, heptachlor epoxide, cis-chlordane, o,p’-DDE, trans-chlordane, trans-nonachlor, p,p’-DDE, Dieldrin, o,p’-DDD, endrin, p,p’-DDD, o,p’-DDT, cis-nonachlor, p,p’-DDT, and mirex were analyzed by separation with GC (Agilent, Palo Alto, CA, USA), and detection with MS (Agilent, Palo Alto, CA, USA). Analysis of phthalates in urine was performed at the chemical analytic laboratory of Eulji University in South Korea. Fifteen metabolites of phthalates, which included MMP, MEP, MnBP, MiBP, MBzP, MCHP, MEHP, MEOHP, MEHHP, 2cx-MMHP, 5cx-MEPP, MnOP, MnPP, MiDP, and MiNP were analyzed by separation with HPLC (Nanospace SI-2, Shiseido, Japan), and detection with MS/MS (LCQ DECA XP, Thermo Finnigan San Jose, CA, USA) (Table 2).
All laboratories verified the analytical methods of all metabolites of hazardous chemicals by enforcing the International Conference on Harmonization of Technical Requirements (ICH) and FDA guidelines [23] during a period of one year (2009). The applied analytic methods were identified and developed to the standard protocols by evaluation of the specificity, linearity, precision, accuracy, sensitivity, and recovery rates during intra-day and inter-day tests. Purified standard materials were used for recovery tests; R squares of linearity rose above 0.995 in the calibration curve with 2, 1.5, 1.0, 0.5, 0.1, 0.01, and 0.001-fold diluted by a standard solution. In relation to the detection limit and the limit of quantification, valid data were applied to be 5 of the S/N ratios, below 20% of precision, and 70~120% of accuracy. During the analysis of samples, quality assurances were checked continually; coefficients of variation (CV) were required to be less than 20%, and analytical procedures were kept with the developed protocols, and so forth.
2.6. Questionnaires
The questionnaire for adults was developed as follows: investigation and statistical analysis of the presented literature, selection and structuring of items, development of the preliminary questionnaire, pre-survey with the first questionnaire, correcting the questionnaire, and development of the final questionnaire. After collection, the references/documents of six hazardous compounds (e.g., acrylamides, HCAs, PFCs, phenols, OCPs, phthalates) were investigated for their sources and exposure routes as the preliminary questionnaire items. The items were grouped for their structures according to their similar characteristics and patterns. The preliminary questionnaire was developed with these structuring items, and then used in the pre-survey. With the results of the pre-survey, the final items and structure of the questionnaire were decided, and its phrases were developed for easy comprehension as a questionnaire for general adults. Our final questionnaire was composed of seven categories, including living environment, indoor environment, diseases and medication, lifestyles, diet, day record, and general information. The questionnaire included a total of 40 questions, as shown in Table 3; 7 questions on living environment, 3 questions on disease and medication, 7 questions on lifestyle, 13 questions on diet, 3 questions on day records, and 7 questions related to general information (Table 3).

Table 3.
Exposure assessment of hazardous compounds from the questionnaire.
The phases and structures of our questionnaire have been sophisticated through extensive evaluation, consultation, and recommendation of a variety of environmental health scientists and epidemiologists in South Korea. The questionnaire was accepted by the NIFDS and approved by the Institutional Review Board (IRB) at ASAN Medical Center, and each participant agreed and signed in the written informed consent.
3. Results of Biomonitoring Data
Table 4 shows the results of biomonitoring data for six hazardous compounds and chemicals analyzed by urine and serum. The geometric mean (GM) and geometric standard error (GSE) for acrylamides in urine among the study participants were 6.77 μg/g creatinine and 0.240, respectively. As for heterocyclic amines, most of biomonitoring samples were below the limit of detection (LOD). Among perfluorocompounds (10 chemicals) in serum, total GMs were below LOD in PFPA, PFHxA, and PFHpA, and 2.825 ng/mL in PFOA, 0.900 ng/mL in PFNA, 2.159 ng/mL in PFDA, 0.432 ng/mL in PFHxS, 0.047 ng/mL in PFHpS, 10.22 ng/mL in PFOS, and 1.005 ng/mL in PFNS. Among phenols (16 chemicals) in urine, 0.111 μg/g creatinine in n-butylphenol, 0.693 μg/g creatinine in t-butylphenol, 0.284 μg/g creatinine in pentylphenol, 0.558 μg/g creatinine in hexylphenol, 0.632 μg/g creatinine in heptylphenol, 5.770 μg/g creatinine in n-octylphenol, 0.582 μg/g creatinine in t-octylphenol, 3.650 μg/g creatinine in nonylphenol, 1.880 μg/g creatinine in bisphenol-A, 0.139 μg/g creatinine in 2,4-dichlorophenol, 0.418 μg/g creatinine in 2,5-dichlorophenol, 0.081 μg/g creatinine in 2,4,5-trichlorophenol, 0.369 μg/g creatinine in 2,4,6-trichlorophenol, 1.650 μg/g creatinine in triclosan and 4.060 μg/g creatinine in benzophenone-3. Total GMs of OCPs (18 chemicals) in t-lipid of serum were below LOD. Among phthalates (10 chemicals) in urine, total GMs were 41.740 μg/g creatinine in MnHP, 16.970 μg/g creatinine in MiBP, 15.730 μg/g creatinine in MBzP, 0.188 μg/g creatinine in MCHP, 0.057 μg/g creatinine in MnOP, 8.680 μg/g creatinine in MEHP, 17.510 μg/g creatinine in MEOHP, 38.130 μg/g creatinine in MEHHP, 0.070 μg/g creatinine in MiNP, and 0.068 μg/g creatinine in MiDP. All concentrations of biomonitoring samples analyzed in serum and urine showed the reference levels among the study participants selected from the general population in Korea.

Table 4.
Results of biomonitoring data for hazardous compounds and chemicals (N = 1874).
4. Data Management System
Data obtained from this study were stored in the developed database system. Figure 3 is the entity–relationship diagram (ERD), which was composed of two systems. One was the management system for the data collected during the projects. This included the input subsets of survey data and analytical results of hazardous chemicals, window subset of statistical results of data in real-time, and merged the subset of questionnaire data and analytical results on chemicals. The other was the error checking system. The input system had the automatic function of logic error checking, for which illogical data were alarmed and held to input until the problems were resolved. All data were inputted to the database by qualified field investigators who could access the input system of the website with his/her certificated ID, and data from the questionnaire were inputted twice in order to minimize input errors. After completion of data input in local sampling units, questionnaires were transported to central headquarters, and input again with the same questionnaires to double-check for input errors. Researchers in each analytical laboratory also inputted the resulting data on hazardous chemicals at regular intervals. Local researchers, analytical researchers, and headquarters were able to see the statistical results of input data in real-time, and to effectively manage the national spread sampling units with this system. The data management system can be accessed via a website link of https://www.data.go.kr/.
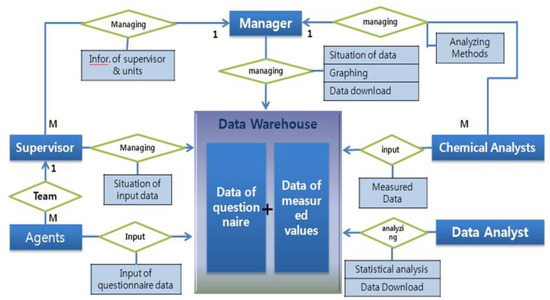
Figure 3.
A web-based entity–relationship diagram (ERD) system for the management of questionnaire data and biomonitoring data in this study.
5. Conclusions
The well-designed biomonitoring study was conducted through good sampling and handling, analysis, surveys, etc. To perform an analysis and assessment of hazardous substances, the effectiveness of biomonitoring sampling requires an appropriate strategy of implementation, exact sampling time, standardized analytical procedures, and criteria for interpretation of results [38,39].
The KiFDS project, ‘The collections of biological samples for human biomonitoring and data analysis’, is the first one of the largest nationwide systematic population-based studies on specific biomarkers for pollutants exposed from foods and cooking in South Korea. In this study, we describe the study design, rationale, and methodology, as well as baseline characteristics of the study participants. The study design was stable, reproducible and reliable in terms of management of the nationwide human biomonitoring surveys in South Korea. The human body can be exposed to chemicals analyzed in this study through the food preparation process and/or residual chemicals in foods. A number of chemicals HCA, OCPs, PFCs, phthalates, and phenols were identified at the significantly low levels; this was thought to be related to cooking, containers, environmental contamination, and food consumption.
Therefore, the study results were significantly important and reliable because we identified the national reference levels for hazardous compounds and chemicals from foods and cooking. Further studies are required to identify the significant associations between environmental exposures to food intake and cooking and adverse health outcomes among the general population in Korea in the future.
Author Contributions
Conceptualization, J.H.L., K.L., R.M.A., J.H.K. and B.-S.S.; methodology, Y.-D.H., B.-S.S., S.L., K.L.; software, Y.-D.H., B.-S.S.; validation, J.H.L., K.L., R.M.A., J.H.K., K.L. and B.-S.S.; formal analysis, S.L., K.L.; investigation, J.H.L., K.L., R.M.A., J.H.K., and B.-S.S.; resources, J.H.L., K.L., R.M.A., J.H.K. and B.-S.S.; data curation, J.H.L., K.L. and B.-S.S.; writing—original draft preparation, S.L., K.L.; writing—review and editing, S.L., K.L.; visualization, S.L., K.L.; supervision, J.H.L., R.M.A., B.-S.S.; project administration, J.H.L., R.M.A., B.-S.S.; funding acquisition, J.H.L., B.-S.S.
Funding
This study was supported by the Soonchunhyang University Research Fund and also was supported by a grant (09182KFDA607) from the Korea Food & Drug Administration in 2009.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boffetta, P. Human cancer from environmental pollutants: The epidemiological evidence. Mutat. Res. Gen. Toxicol. Enviorn. Mutagen. 2006, 608, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead Exposure and Cardiovascular Disease—A Systematic Review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Zhao, G.; Croft, J.B.; Balluz, L.S.; Mokdad, A.H. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prev. Med. 2010, 51, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Seifert, B.; Becker, K.; Hoffmann, K.; Krause, C.; Schulz, C. The German Environmental Survey 1990/1992 (GerES II): A representative population study. J. Expo. Sci. Environ. Epidemiol. 2000, 10, 103–114. [Google Scholar] [CrossRef][Green Version]
- Chung, W.; Lim, S.; Lee, S. Factors influencing gender differences in smoking and their separate contributions: Evidence from South Korea. Soc. Sci. Med. 2010, 70, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Kim, K.S.; Park, J.; Kang, M.G.; Ryu, S.Y. Association between levels of physical activity and poor self-rated health in Korean adults: The Third Korea National Health and Nutrition Examination Survey (KNHANES), 2005. Public Health 2009, 123, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Ko, A.; Kang, H.-S.; Hwang, M.-S.; Lee, H.-S. Association of urinary acrylamide concentration with lifestyle and demographic factors in a population of South Korean children and adolescents. Environ. Sci. Pollut. Res. 2019, 26, 18247–18255. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.; Werry, K.; Haines, D.; Walker, M.; Malowany, M. Human biomonitoring reference values for some non-persistent chemicals in blood and urine derived from the Canadian Health Measures Survey 2009–2013. Int. J. Hyg. Environ. Health 2018, 221, 684–696. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Lee, J.H. Urinary concentrations of trichlorophenols in the Korean adult population: Results of the National Human Biomonitoring Survey 2009. Environ. Sci. Pollut. Res. 2014, 21, 2479–2485. [Google Scholar] [CrossRef]
- Kim, S.-A.; Kwon, Y.; Kim, S.; Joung, H. Assessment of dietary mercury intake and blood mercury levels in the Korean population: Results from the Korean national environmental health survey 2012–2014. Int. J. Environ. Res. Public Health 2016, 13, 877. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.; Baek, Y.-W.; Choi, K.; Lee, K.; Kim, S.; Yu, S.D.; Choi, K. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012–2014). Int. J. Hyg. Environ. Health 2017, 220, 29–35. [Google Scholar] [CrossRef] [PubMed]
- UN. Review the Draft Handbook on Designing of Household Sample Surveys; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2005; pp. 119–120. [Google Scholar]
- Oh, S.-S.; Oh, M.-H.; Paek, S.-H.; Shin, J.-W.; Kim, A.-R.; Park, H.-N.; Kang, W.-S. Acrylamide Monitoring in Food Products and its Intake Estimation. Ann. Rep. KFDA 2007, 11, 674–675. [Google Scholar]
- Ono, Y.; Somiya, I.; Oda, Y. Identification of a carcinogenic heterocyclic amine in river water. Water Res. 2000, 34, 890–894. [Google Scholar] [CrossRef]
- Góngora, V.M.; Matthes, K.L.; Castaño, P.R.; Linseisen, J.; Rohrmann, S. Dietary heterocyclic amine intake and colorectal adenoma risk: A systematic review and meta-analysis. Cancer Epidemiol. Prev. Biomark. 2019, 28, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Emmett, E.A.; Shofer, F.S.; Zhang, H.; Freeman, D.; Desai, C.; Shaw, L.M. Community Exposure to Perfluorooctanoate: Relationships Between Serum Concentrations and Exposure Sources. J. Occup. Environ. Med. 2006, 48, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Ehresman, D.J.; Froehlich, J.W.; Olsen, G.W.; Chang, S.-C.; Butenhoff, J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Zhang, W.; Zhang, J.; Du, X.; Huang, Q.; Tian, M.; Shen, H. Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative/nitrosative stress in humans. Environ. Pollut. 2017, 229, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-O.; Jeong, M.K.; Park, C.U.; Park, M.H.; Chang, P.-S.; Lee, J.H. Effects of Riboflavin Photosensitization on the Degradation of Bisphenol A (BPA) in Model and Real-Food Systems. J. Food Sci. 2009, 74, C380–C384. [Google Scholar] [CrossRef]
- Latini, G. Potential hazards of exposure to di-(2-ethylhexyl)-phthalate in babies. a review. Boil. Neonate 2000, 78, 269–276. [Google Scholar] [CrossRef]
- David, R.M.; Moore, M.R.; Finney, D.C.; Guest, D. Reversibility of the Chronic Effects of Di(2-ethylhexyl)phthalate. Toxicol. Pathol. 2001, 29, 430–439. [Google Scholar] [CrossRef]
- Wallner-Liebmann, S.; Gralka, E.; Tenori, L.; Konrad, M.; Hofmann, P.; Dieber-Rotheneder, M.; Turano, P.; Luchinat, C.; Zatloukal, K. The impact of free or standardized lifestyle and urine sampling protocol on metabolome recognition accuracy. Genes Nutr. 2015, 10, 441. [Google Scholar] [CrossRef]
- Molzon, J.A.; Giaquinto, A.; Lindstrom, L.; Tominaga, T.; Ward, M.; Doerr, P.; Hunt, L.; Rago, L. The value and benefits of the International Conference on Harmonisation to drug regulatory authorities: advancing harmonization for better public health. Clin. Pharmacol. Ther. 2011, 89, 503–512. [Google Scholar] [CrossRef]
- Cho, C.-R. Residual Characteristics of Perfluorinated Chemicals in Main Coastal Areas and Main Stream Watersheds of Korea. Ph.D. Thesis, Cheonnam University, Cheonnam, Korea, 2008. [Google Scholar]
- Giesy, J.P.; Mabury, S.A.; Martin, J.W.; Kannan, K.; Jones, P.D.; Newsted, J.L.; Coady, K. Perfluorinated Compounds in the Great Lakes. In Persistent Organic Pollutants in the Great Lakes; Hites, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 5N, pp. 391–438. [Google Scholar]
- Kim, P.-G.; Leu, J.-H. Effects of Bisphenol A on Dams during Lactation Period in Rats. Korean J. Environ. Biol. 2003, 21, 208–215. [Google Scholar]
- Lee, M.-S.; Park, J.-Y.; Oh, S.-S. Acrylamide monitoring in home-made food products. Korean J. Food Cook. Sci. 2004, 20, 708–711. [Google Scholar]
- Colerangle, J.B.; Roy, D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J. Steroid Biochem. Mol. Boil. 1997, 60, 153–160. [Google Scholar] [CrossRef]
- Chung, R.-P.; Choi, M.-K.; Yeo, H.-G.; Chung, M.-Y. Seasonal Variations in the Concentration of Persistent Organochlorine Pesticides in Atmosphere. Korean J. Envion. Agric. 2001, 20, 79–85. [Google Scholar]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2005, 40, 32–44. [Google Scholar] [CrossRef]
- Choi, M.-K.; Chun, M.-Y. Deposition Characteristics of Atmospheric Organochlorine Pesticides on Plant (Allum tuberosum). J. Korean Soc. Environ. ANA 2008, 11, 46–54. [Google Scholar]
- Park, D.H.; Jang, H.Y.; Park, C.K.; Cheong, H.T.; Kim, C.I.; Yang, B.K. Effect of Bisphenol A Administration on Reproductive Characteristic and Blood Metabolite in Mice. J. Anim. Sci. Technol. 2004, 46, 957–966. [Google Scholar]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Lee, K.-G. Acrylamide in Cooked Foods-Detection Level, Formation Mechanism, and Toxicity of Acrylamide. Food Eng. Prog. 2003, 7, 65–72. [Google Scholar]
- Moody, C.A.; Field, J.A. Perfluorinated Surfactants and the Environmental Implications of Their Use in Fire-Fighting Foams. Environ. Sci. Technol. 2000, 34, 3864–3870. [Google Scholar] [CrossRef]
- Reynolds, T. Acrylamide and Cancer: Tunnel Leak in Sweden Prompted Studies. J. Natl. Cancer Inst. 2002, 94, 876–878. [Google Scholar] [CrossRef]
- Koh, S.B.; Park, J.H.; Yun, S.S.; Oh, S.S.; Chang, S.J.; Choi, S.H.; Cha, B.S. The Association among Exposure of Bisphenol A, Genetic Polymorphism of Metabolic Enzyme and Urinary Metabolite. Korean J. Occup. Environ. Med. 2008, 20, 112–118. [Google Scholar] [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef]
- Manno, M.; Viau, C.; Cocker, J.; Colosio, C.; Lowry, L.; Mutti, A.; Nordberg, M.; Wang, S. Biomonitoring for occupational health risk assessment (BOHRA). Toxicol. Lett. 2010, 192, 3–16. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).