Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
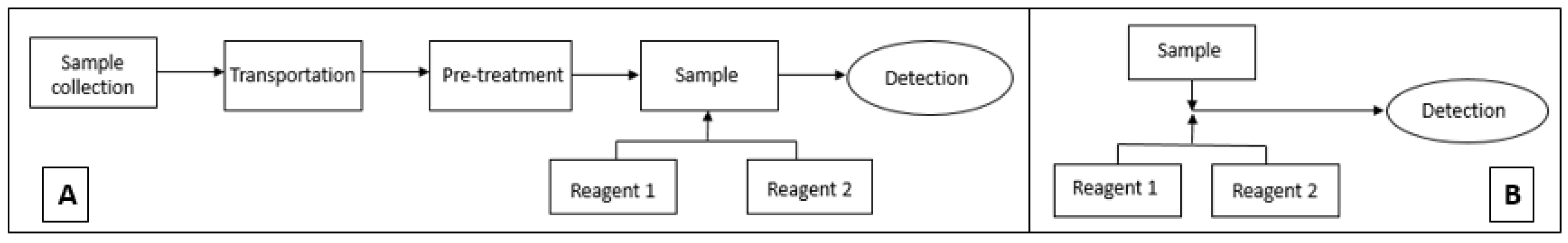
2.3. Sample Preparation
2.4. Path Length
2.5. Sample Cell Cleaning Validation
2.6. Optimisation of Parameters
2.6.1. pH
2.6.2. Sample/Reagent Ratio
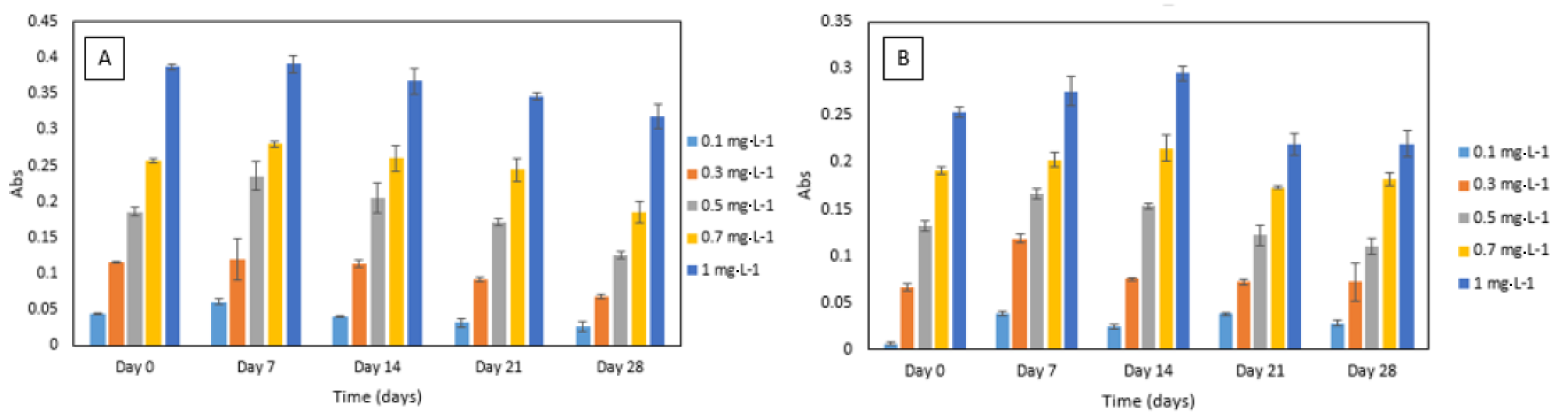
2.6.3. Reagent Stability
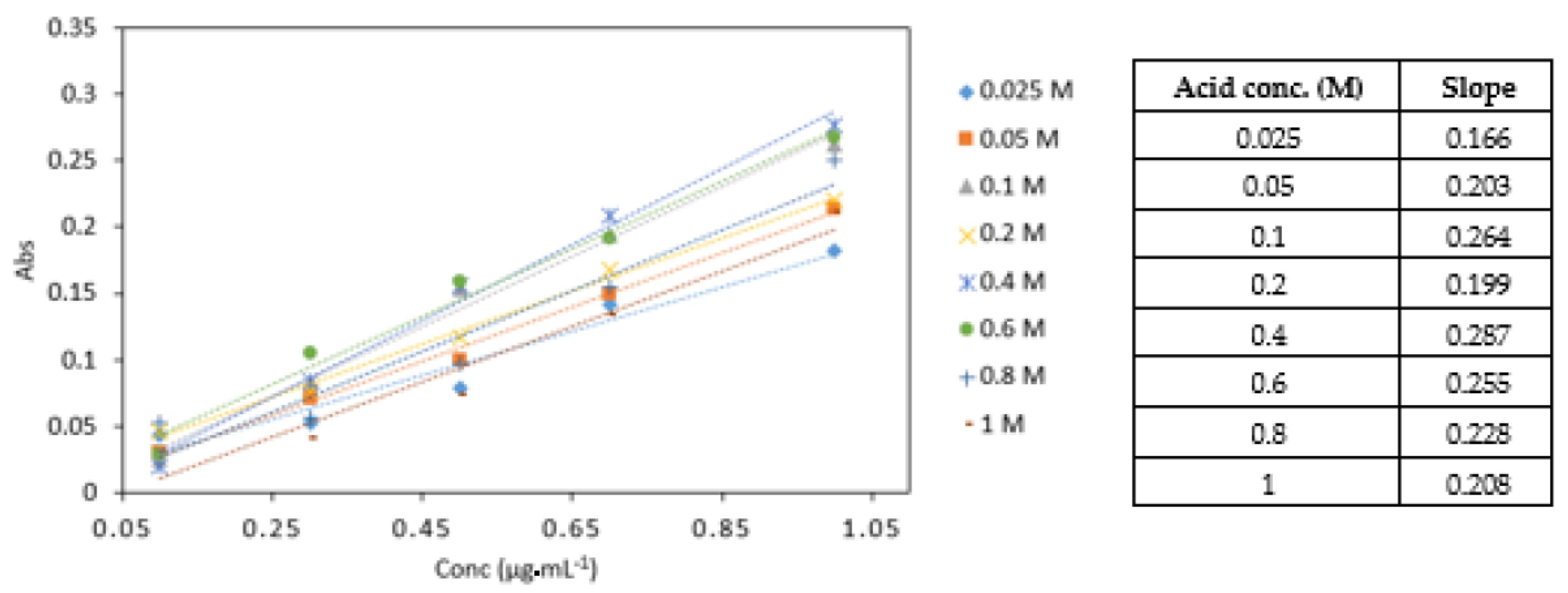
2.6.4. Effect of Different Acid Concentrations
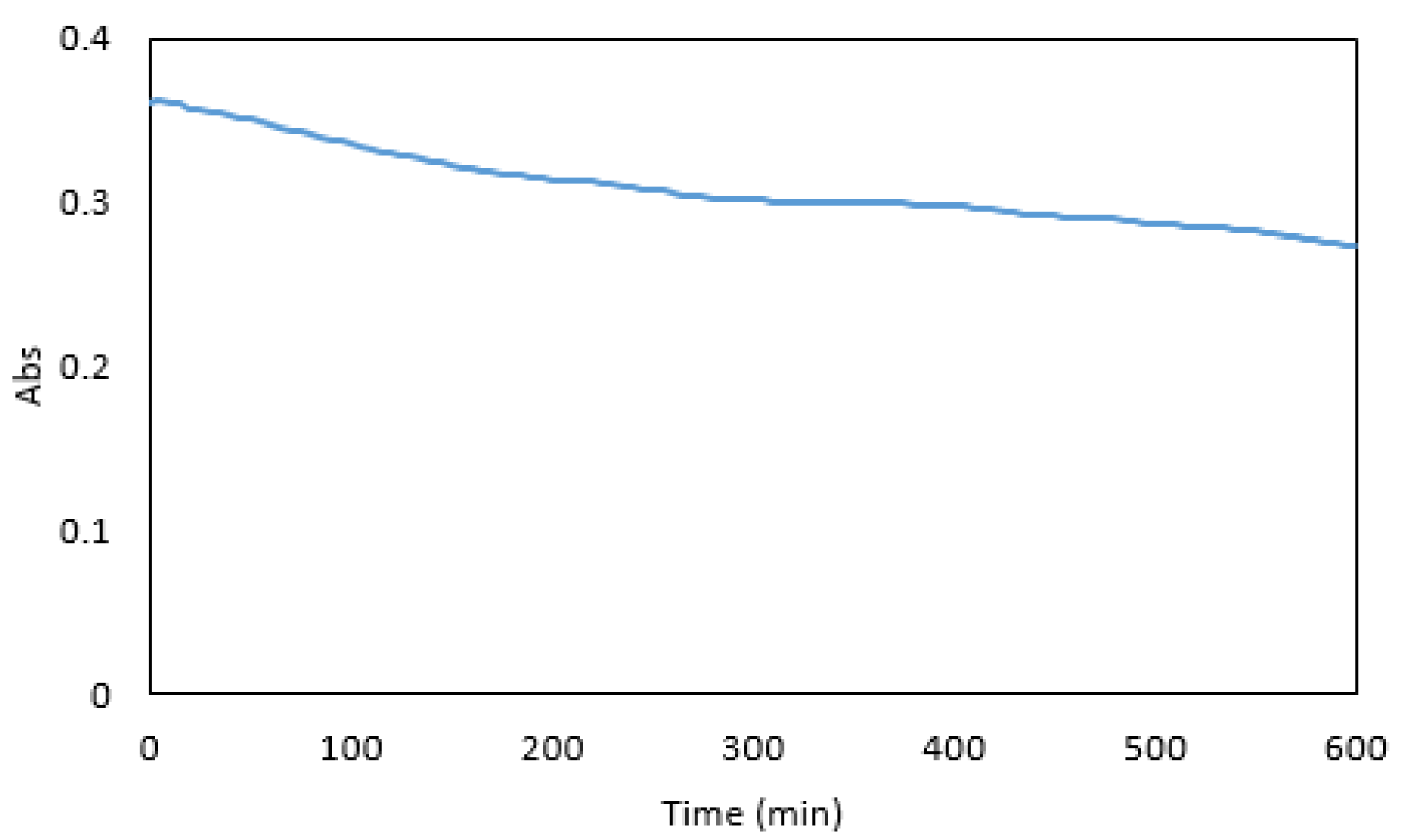
2.7. Colour Stability
2.8. Interference
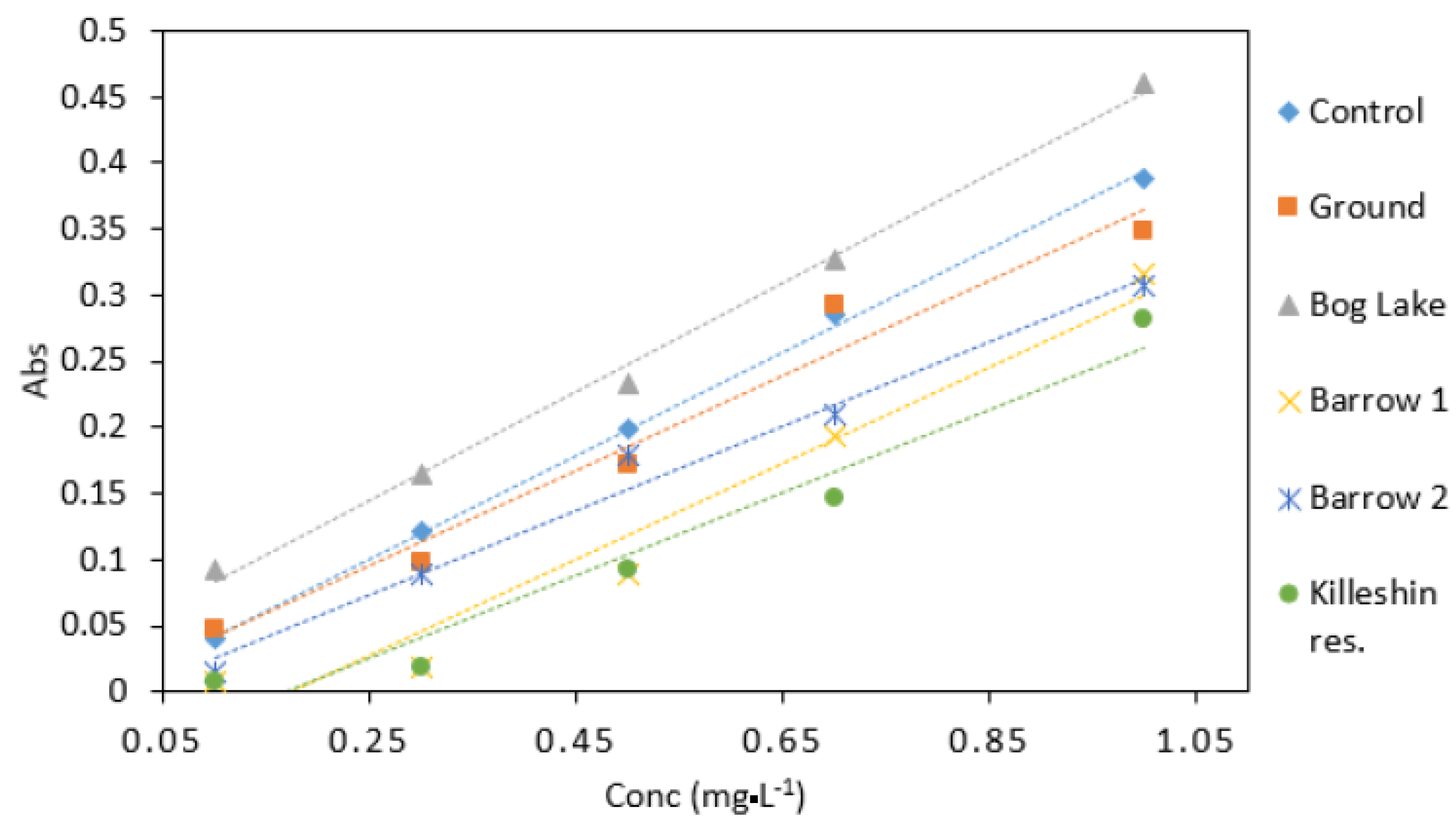
2.9. Environmental Samples
2.10. Comparison between Optimised DPC Method and ICP-MS
3. Results
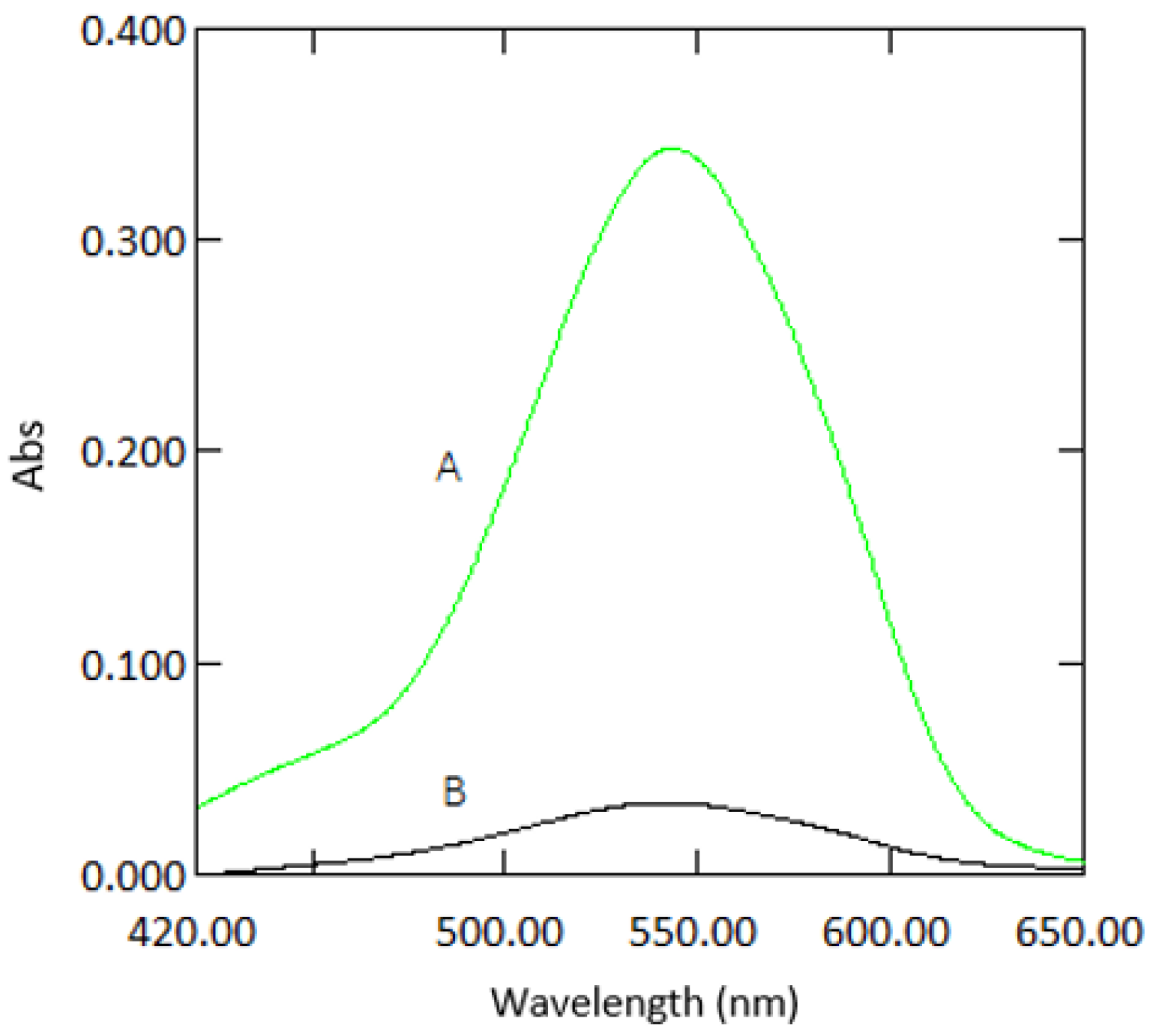
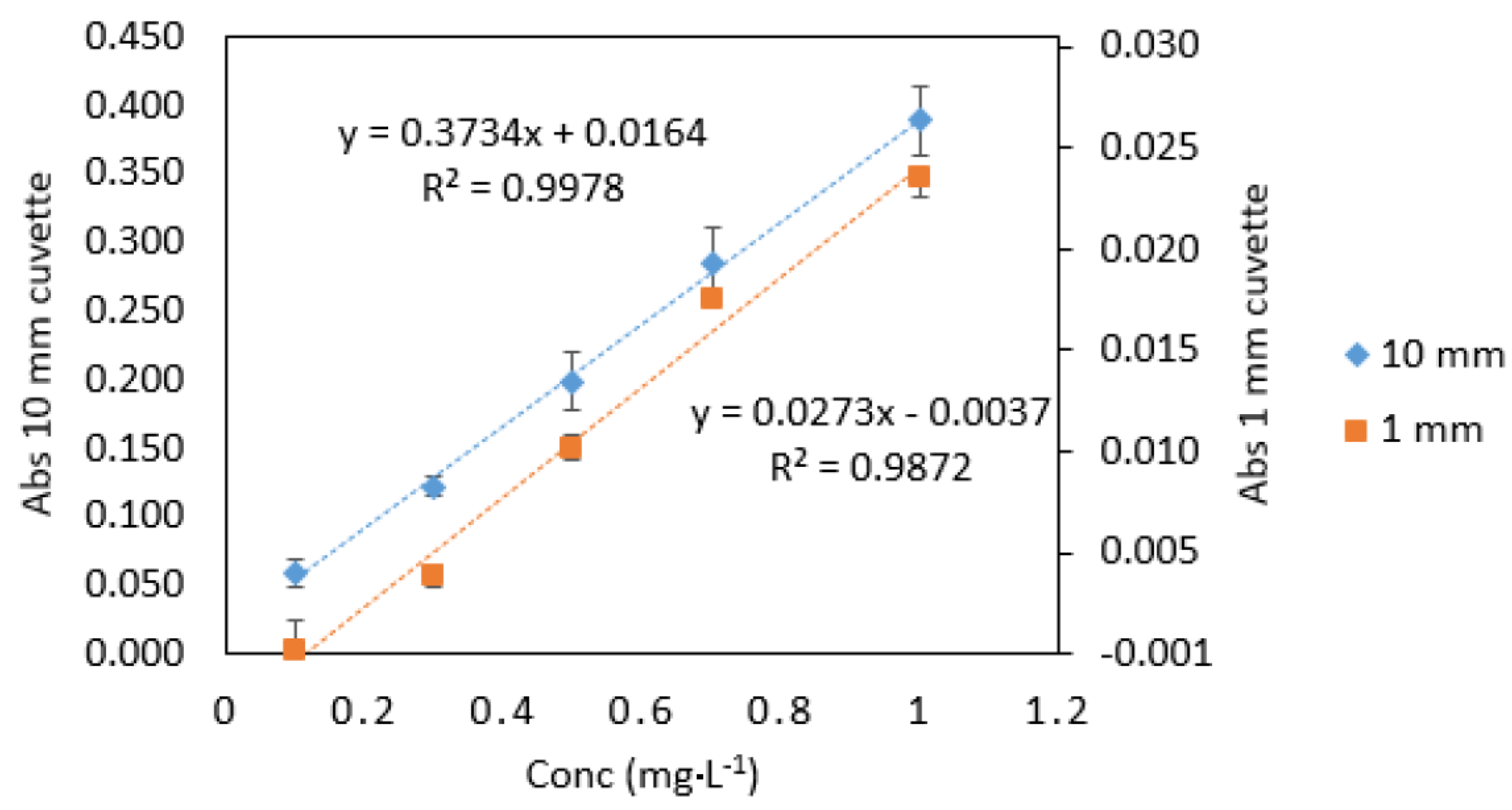
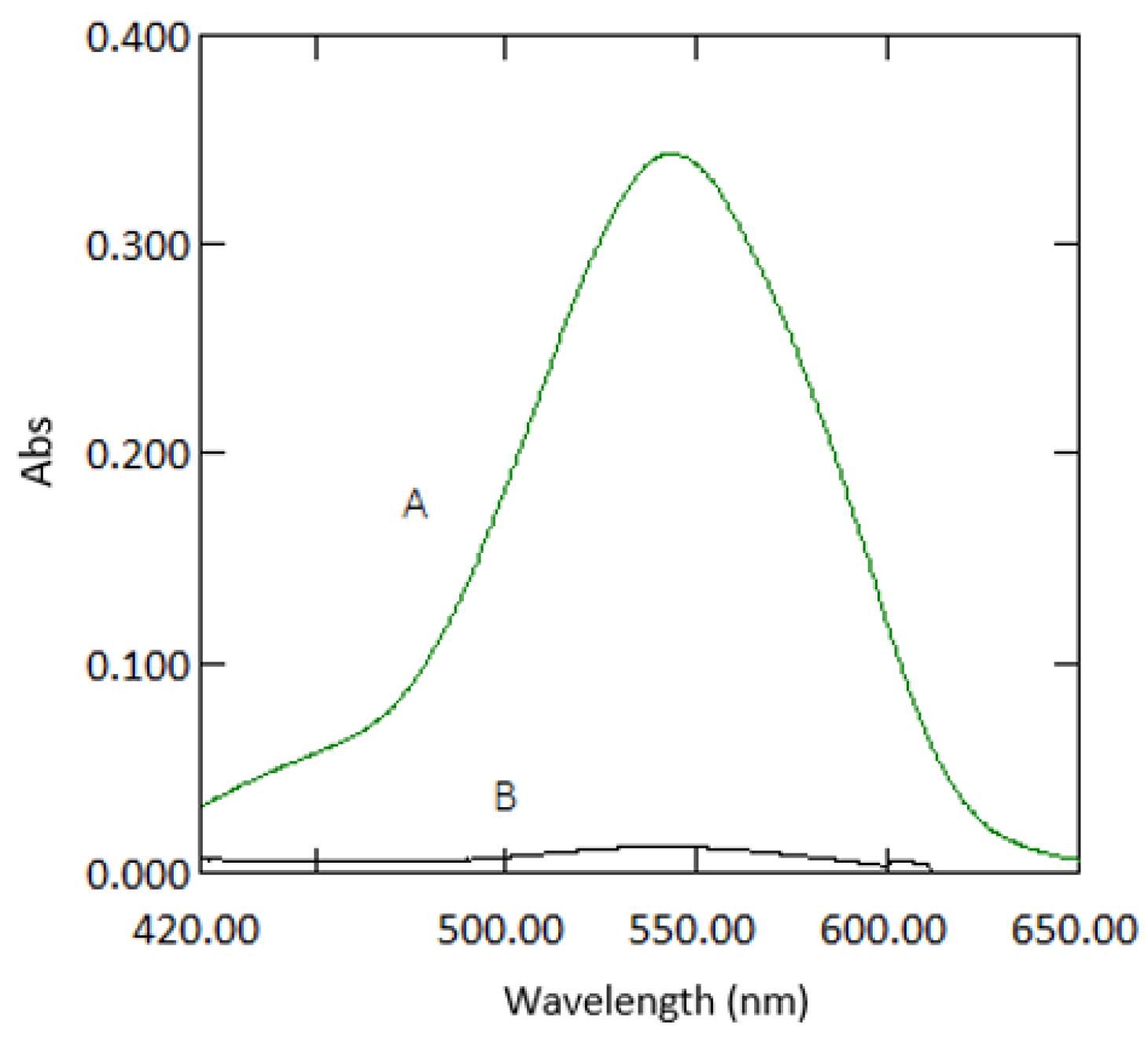
3.1. Path Length
3.2. Sample Cell Cleaning Validation
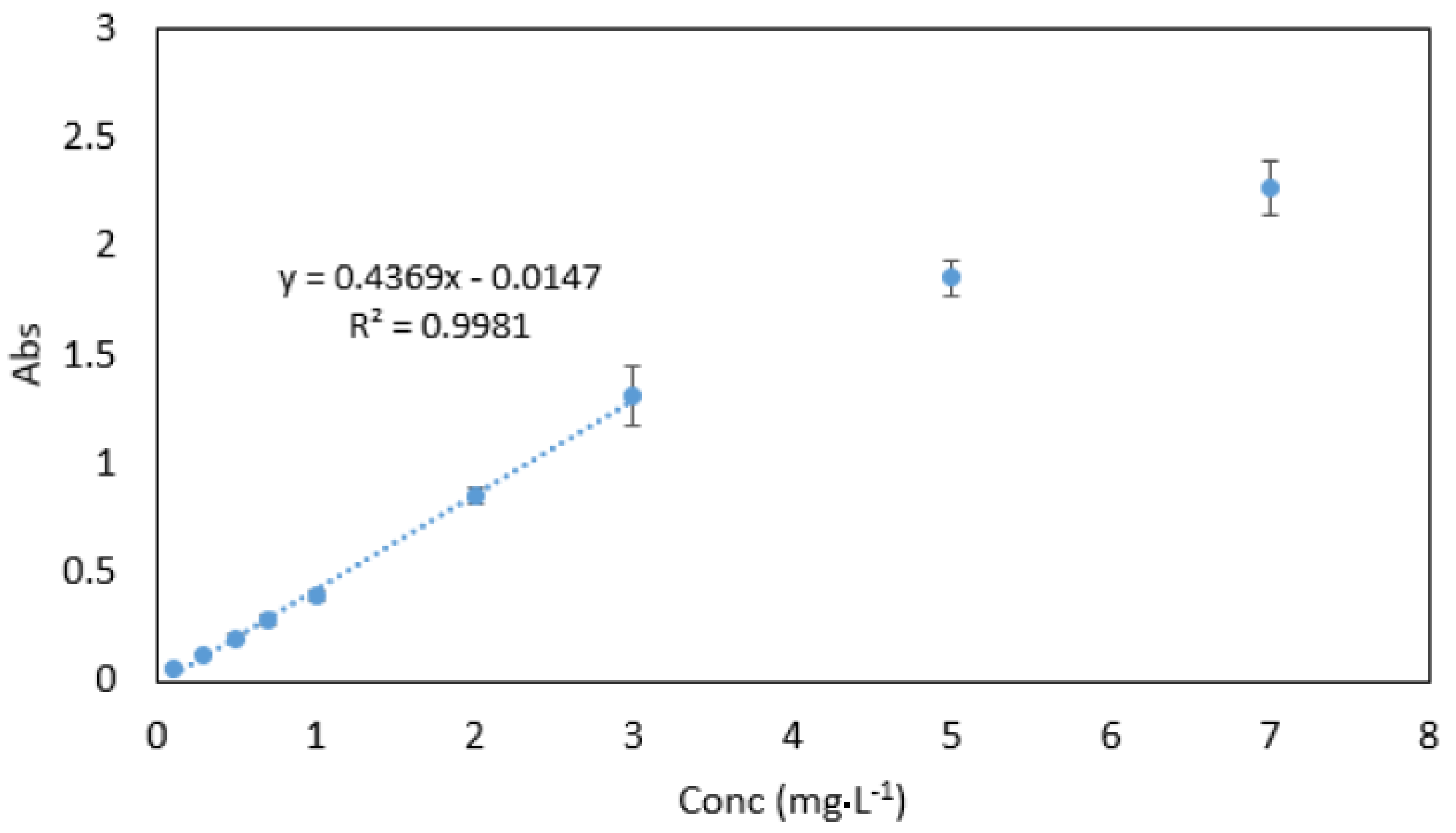
3.3. Optimisation of Parameters
3.3.1. pH
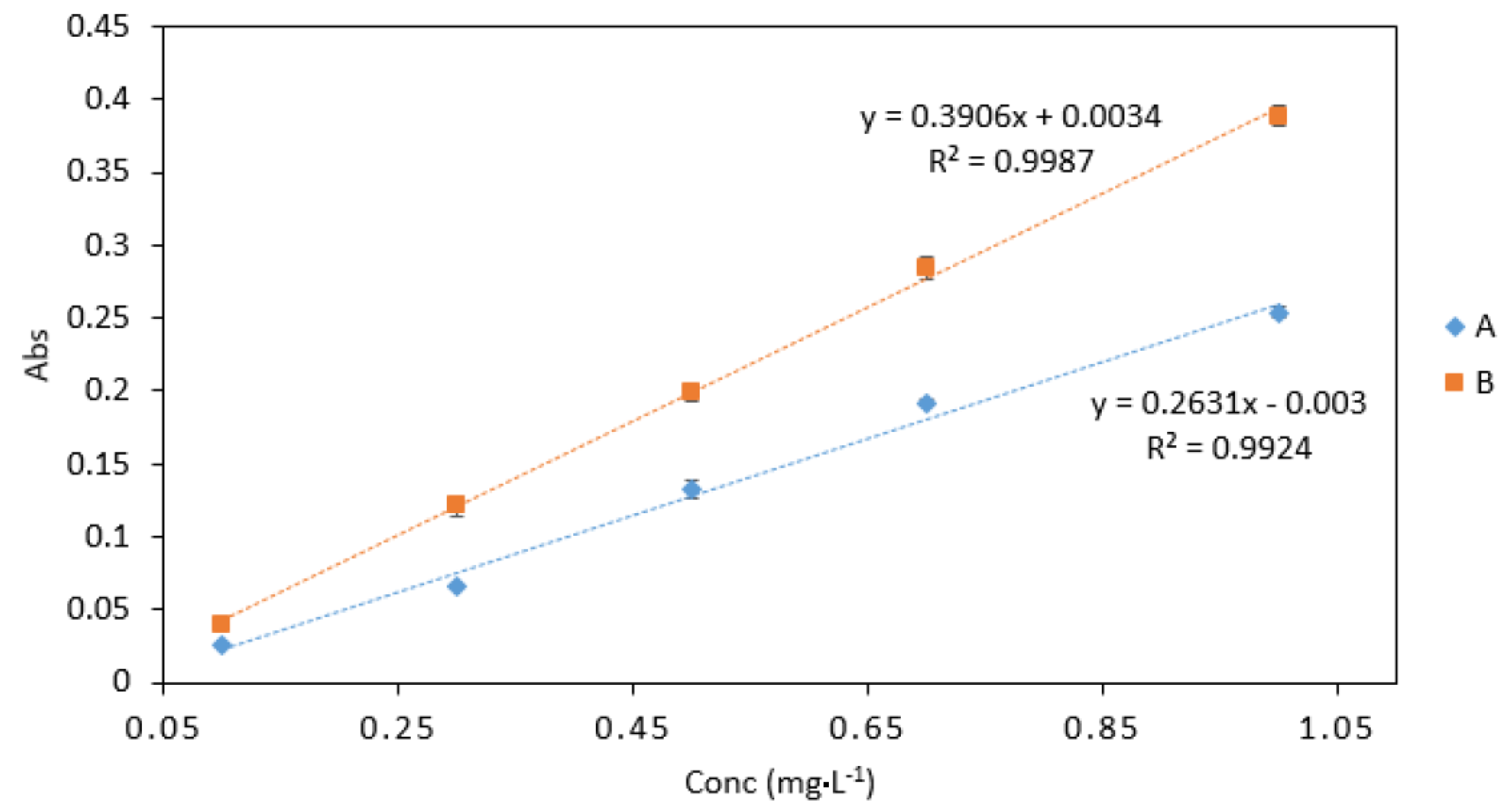
3.3.2. Sample/Reagent Ratio
3.3.3. Reagent Stability
3.3.4. Effect of Different Acid Concentrations
3.4. Colour Stability
3.5. Interference
3.6. Environmental Water Samples
3.7. Comparison between Optimised DPC Method and ICP-MS
3.8. Analytical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quantin, C.; Ettler, V.; Garnier, J.; Šebek, O. Sources and extractibility of chromium and nickel in soil profiles developed on Czech serpentinites. C. R. Geosci. 2008, 340, 872–882. [Google Scholar] [CrossRef]
- Ashraf, A.; Bibi, I.; Niazi, N.K.; Ok, Y.S.; Murtaza, G.; Shahid, M.; Kunhikrishnan, A.; Li, D.; Mahmood, T. Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int. J. Phytoremediat. 2017, 19, 605–613. [Google Scholar] [CrossRef]
- Sloof, R. Towards Healthier Water Resources Management. Waterlines 1990, 9, 2. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C.M. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Butera, S.; Trapp, S.; Astrup, T.F.; Christensen, T.H. Soil retention of hexavalent chromium released from construction and demolition waste in a road-base-application scenario. J. Hazard. Mater. 2015, 298, 361–367. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Xu, Z.; Pan, Y.; Liu, J.; Du, L. An effective PDMS microfluidic chip for chemiluminescence detection of cobalt (II) in water. Microsyst. Technol. 2012, 19, 99–103. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J. Chromium Contamination Accident in China: Viewing Environment Policy of China. Environ. Sci. Technol. 2011, 45, 8605–8606. [Google Scholar] [CrossRef]
- WHO. Chromium in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Sharma, D.C.; Forster, C.F. Removal of hexavalent chromium using sphagnum moss peat. Water Res. 1993, 27, 1201–1208. [Google Scholar] [CrossRef]
- Jin, W.; Wu, G.; Chen, A. Sensitive and selective electrochemical detection of chromium (vi) based on gold nanoparticle-decorated Titania nanotube arrays. Analyst 2014, 139, 235–241. [Google Scholar] [CrossRef]
- Guertin, J.; Jacobs, J.A.; Avakian, C.P. Chromium (VI) Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 784. [Google Scholar]
- McNeill, L.S.; McLean, J.E.; Parks, J.L.; Edwards, M.A. Hexavalent chromium review, part 2: Chemistry, occurrence, and treatment. J. Am. Water Works Assoc. 2012, 104, E395–E405. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2008, 200, 59–77. [Google Scholar] [CrossRef]
- Cespón-Romero, R.M.; Yebra-Biurrun, M.C.; Bermejo-Barrera, M.P. Preconcentration and speciation of chromium by the determination of total chromium and chromium (III) in natural waters by flame atomic absorption spectrometry with a chelating ion-exchange flow injection system. Anal. Chim. Acta 1996, 327, 37–45. [Google Scholar] [CrossRef]
- Martone, N.; Rahman, G.M.M.; Pamuku, M.; Kingston, H.M.S. Determination of Chromium Species in Dietary Supplements Using Speciated Isotope Dilution Mass Spectrometry with Mass Balance. J. Agric. Food Chem. 2013, 61, 9966–9976. [Google Scholar] [CrossRef]
- Kim, J.; Seo, S.; Kim, Y.; Kim, D.H. Review of carcinogenicity of hexavalent chrome and proposal of revising approval standards for an occupational cancers in Korea. Ann. Occup. Environ. Med. 2018, 30, 7. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, S.I.; Niazi, S.N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Achmad, R.; Budiawan, B.; Auerkari, E. Effects of Chromium on Human Body. Annu. Res. Rev. Biol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Gray, D.J. Naturally occurring Cr6+in shallow groundwaters of the Yilgarn Craton, Western Australia. Geochem. Explor. Environ. Anal. 2003, 3, 359–368. [Google Scholar] [CrossRef]
- Becquer, T.; Quantin, C.; Sicot, M.; Boudot, J. Chromium availability in ultramafic soils from New Caledonia. Sci. Total Environ. 2003, 301, 251–261. [Google Scholar] [CrossRef]
- Beaumont, J.J.; Sedman, R.M.; Reynolds, S.D.; Sherman, C.D.; Li, L.H.; Howd, R.A.; Sandy, M.S.; Zeise, L.; Alexeeff, G.V. Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology 2008, 19, 12–23. [Google Scholar] [CrossRef]
- Fantoni, D.; Brozzo, G.; Canepa, M.; Cipolli, F.; Marini, L.; Ottonello, G.; Zuccolini, M. Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environ. Geol. 2002, 42, 871–882. [Google Scholar] [CrossRef]
- Pellerin, C.; Booker, S.M. Reflections on hexavalent chromium: Health hazards of an industrial heavyweight. Environ. Health Perspect. 2000, 108, 402–407. [Google Scholar] [CrossRef]
- Singh, R.K.; Sengupta, B.; Bali, R.; Shukla, B.P.; Gurunadharao, V.V.S.; Srivatstava, R. Identification and mapping of chromium (VI) plume in groundwater for remediation: A case study at Kanpur, Uttar Pradesh. J. Geol. Soc. India 2009, 74, 49–57. [Google Scholar] [CrossRef]
- Armienta, M.A.; Morton, O.; Rodríguez, R.; Cruz, O.; Aguayo, A.; Ceniceros, N. Chromium in a Tannery Wastewater Irrigated Area, León Valley, Mexico. Bull. Environ. Contam. Toxicol. 2001, 66, 189–195. [Google Scholar] [CrossRef]
- Ressalan, S.; Chauhan, R.S.; Goswami, A.K.; Purohit, D.N. Review of Spectrophotometric Methods for Determination of Chromium. Rev. Anal. Chem. 1997, 16, 69–171. [Google Scholar] [CrossRef]
- Parks, J.L.; McNeill, L.; Frey, M.; Eaton, A.D.; Haghani, A.; Ramirez, L.; Edwards, M. Determination of total chromium in environmental water samples. Water Res. 2004, 38, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, J.A.; Ball, J.W.; Bullen, T.D.; Sutley, S.J. Chromium, chromium isotopes and selected trace elements, western Mojave Desert, USA. Appl. Geochem. 2008, 23, 1325–1352. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Wang, Z.; Ren, W.; Zhang, Y.; Shen, Z.; Li, T.; Wu, A. Selective colorimetric detection of Cr(iii) and Cr(vi) using gallic acid capped gold nanoparticles. Dalton Trans. 2016, 45, 8347–8354. [Google Scholar] [CrossRef]
- Li, H.-F.; Lin, J.-M. Applications of microfluidic systems in environmental analysis. Anal. Bioanal. Chem. 2008, 393, 555–567. [Google Scholar] [CrossRef]
- Cogan, D.; Fay, C.; Boyle, D.; Osborne, C.; Kent, N.; Cleary, J.; Diamond, D. Development of a low cost microfluidic sensor for the direct determination of nitrate using chromotropic acid in natural waters. Anal. Methods 2015, 7, 5396–5405. [Google Scholar] [CrossRef]
- Milani, A.; Statham, P.J.; Mowlem, M.C.; Connelly, D.P. Development and application of a microfluidic in-situ analyzer for dissolved Fe and Mn in natural waters. Talanta 2015, 136, 15–22. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X. Colorimetric speciation of Cr (III) and Cr (VI) with a gold nanoparticle probe. Anal. Methods 2013, 5, 1442. [Google Scholar] [CrossRef]
- Provin, C.; Fukuba, T.; Okamura, K.; Fujii, T. An integrated microfluidic system for manganese anomaly detection based on chemiluminescence: Description and practical use to discover hydrothermal plumes near the Okinawa Trough. IEEE J. Ocean. Eng. 2013, 38, 178–185. [Google Scholar] [CrossRef]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab Chip 2010, 10, 477–483. [Google Scholar] [CrossRef]
- Nguyen, T.; Zoëga Andreasen, S.; Wolff, A.; Duong Bang, D. From Lab on a Chip to Point of Care Devices: The Role of Open Source Microcontrollers. Micromachines 2018, 9, 403. [Google Scholar] [CrossRef]
- Cleary, J.; Maher, D.; Diamond, D. Development and Deployment of a Microfluidic Platform for Water Quality Monitoring. Smart Sen. Real-Time Water Qual. Monit. 2013, 4, 125–148. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, T.N.; Muralikrishna, S.; Suresh kumar, K.; Nagaraju, G.; Ramakrishnappa, T. Electrochemical detection and photochemical detoxification of hexavalent chromium (Cr(vi)) by Ag doped TiO2 nanoparticles. Anal. Methods 2015, 7, 3493–3499. [Google Scholar] [CrossRef]
- Tu, J.; Gan, Y.; Liang, T.; Wan, H.; Wang, P. A miniaturized electrochemical system for high sensitive determination of chromium(VI) by screen-printed carbon electrode with gold nanoparticles modification. Sens. Actuators B Chem. 2018, 272, 582–588. [Google Scholar] [CrossRef]
- Sari, T.K.; Takahashi, F.; Jin, J.; Zein, R.; Munaf, E. Electrochemical Determination of Chromium(VI) in River Water with Gold Nanoparticles–Graphene Nanocomposites Modified Electrodes. Anal. Sci. 2018, 34, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.; Xu, H.; Chen, J.; Shi, G.; Jin, L. Nafion stabilized silver nanoparticles modified electrode and its application to Cr(VI) detection. J. Electroanal. Chem. 2011, 652, 60–65. [Google Scholar] [CrossRef]
- Khanfar, M.; Al-Faqheri, W.; Al-Halhouli, A. Low Cost Lab on Chip for the Colorimetric Detection of Nitrate in Mineral Water Products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef]
- Li, Y.; Xue, H. Determination of Cr(III) and Cr(VI) species in natural waters by catalytic cathodic stripping voltammetry. Anal. Chim. Acta 2001, 448, 121–134. [Google Scholar] [CrossRef]
- Chailapakul, O.; Korsrisakul, S.; Siangproh, W.; Grudpan, K. Fast and simultaneous detection of heavy metals using a simple and reliable microchip-electrochemistry route: An alternative approach to food analysis. Talanta 2008, 74, 683–689. [Google Scholar] [CrossRef]
- Baker, C.A.; Duong, C.T.; Grimley, A.; Roper, M.G. Recent advances in microfluidic detection systems. Bioanalysis 2009, 1, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Chang, Q.; Liu, J.; Clevers, J.G.P.W.; Kooistra, L. Identification of soil heavy metal sources and improvement in spatial mapping based on soil spectral information: A case study in northwest China. Sci. Total Environ. 2016, 565, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Abi Kaed Bey, S.K.; Connelly, D.P.; Legiret, F.-E.; Harris, A.J.K.; Mowlem, M.C. A high-resolution analyser for the measurement of ammonium in oligotrophic seawater. Ocean Dyn. 2011, 61, 1555–1565. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, L.-R.; Yang, K.-B.; Zhong, X.; Huang, R.-B.; Zheng, L.-S. p-Dimethylaminobenzaldehyde thiosemicarbazone: A simple novel selective and sensitive fluorescent sensor for Mercury(II) in aqueous solution. Talanta 2006, 69, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Water Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Idros, N.; Chu, D. Triple-Indicator-Based Multidimensional Colorimetric Sensing Platform for Heavy Metal Ion Detections. ACS Sens. 2018, 3, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, B.; Qi, A.; Tian, C.; Han, J.; Shi, Y.; Chen, L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta 2018, 178, 426–431. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Guo, S.; Wang, E. Selective, peroxidase substrate based “signal-on” colorimetric assay for the detection of chromium (VI). Anal. Chim. Acta 2008, 630, 181–185. [Google Scholar] [CrossRef]
- Sunil, A.; Rao, S.J. Photometric and fluorimetric determination of chromium(VI) using metal-oxo mediated reaction of 1-(2-hydroxyphenyl)thiourea in micellar medium. J. Anal. Chem. 2015, 70, 159–165. [Google Scholar] [CrossRef]
- Revanasiddappa, H.D.; Kumar, T.N.K. Spectrophotometric determination of trace amounts of chromium with citrazinic acid. J. of Anal. Chem. 2001, 56, 1084–1088. [Google Scholar] [CrossRef]
- Sreevani, I.; Reddy, P.R.; Reddy, V.K. A rapid and simple spectrophotometric determination of traces of chromium (VI) in waste water samples and in soil samples by using 2-hydroxy, 3-methoxy benzaldehyde thiosemicarbazone (HMBATSC). J. Appl. Phys. 2013, 3, 40–45. [Google Scholar] [CrossRef]
- Onchoke, K.K.; Sasu, S.A. Determination of Hexavalent Chromium (Cr(VI)) Concentrations via Ion Chromatography and UV-Vis Spectrophotometry in Samples Collected from Nacogdoches Wastewater Treatment Plant, East Texas (USA). Adv. Environ. Chem. 2016, 2016, 3468635. [Google Scholar] [CrossRef]
- Wurster, S.; Kratz, E.; Lachenmeier, D.W.; Mildau, G. Spectrophotometric Quantification of Toxicologically Relevant Concentrations of Chromium(VI) in Cosmetic Pigments and Eyeshadow Using Synthetic Lachrymal Fluid Extraction. Int. J. Spectr. 2012, 2012, 985131. [Google Scholar] [CrossRef]
- Amin, A.S.; El-Sheikh, R.; Shaltout, M.I. Utilization of 2-Amino-6-(1,3-thiazol-2yldiazenyl)phenol for Chromium Speciation in Environmental Samples Spectrophotometrically. Can. Chem. Trans. 2015, 3, 171–183. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Wang, S.; Chen, L. Red-to-blue colorimetric detection of chromium via Cr (III)-citrate chelating based on Tween 20-stabilized gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]

| Instrumental Parameters | Scanning Parameters |
|---|---|
| Plasma flow: 15 L·min−1 | Scanning mode: Peak hopping |
| Auxiliary flow: 1.55 L·min−1 | Number of replicates: 3 |
| Nebuliser flow: 0.9 L·min−1 | Pump rate: 9 rpm |
| Sheath gas flow: 0.2 L·min−1 | Rinse time: 40 s |
| Sampling depth: 6.5·mm | Sample uptake delay: 50 s |
| Power: 1.4 kW | Internal standards: Li6, Sc45, Y89, Tb159, Ho165, Th232 |
| Solvent | Abs | Abs | Abs | Average | SD | % RSD |
|---|---|---|---|---|---|---|
| Water | 0.012 | 0.018 | 0.014 | 0.015 | 0.003 | 20.830 |
| 1% HCl | 0.041 | 0.017 | 0.081 | 0.046 | 0.032 | 69.780 |
| 1% HNO3 | 0.004 | 0.003 | 0.001 | 0.003 | 0.002 | 57.282 |
| Methanol | 0.088 | 0.101 | 0.105 | 0.098 | 0.009 | 9.070 |
| Acetonitrile | 0.021 | 0.022 | 0.033 | 0.025 | 0.007 | 26.283 |
| Acetone | 0.085 | 0.081 | 0.086 | 0.084 | 0.003 | 3.150 |
| Tween 20 | 0.013 | 0.012 | 0.007 | 0.011 | 0.003 | 30.136 |
| 1% H2SO4 | 0.006 | 0.005 | 0.006 | 0.006 | 0.001 | 10.189 |
| Control | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 34.641 |
| Interferents | Tolerance Limit (mg·L−1) |
|---|---|
| Fe (III) | 1 |
| Cr (III), Mn, Mg, NO3 | 10 |
| PO4 | 100 |
| Sample | ICP-MS Unspiked Sample (mg·L−1) | ICP-MS (mg·L−1) | DPC Method (mg·L−1) | Percentage Difference (%) |
|---|---|---|---|---|
| Control | 0.000 | 0.883 | 1.012 | 14.607 |
| Ground | 0.002 | 0.930 | 1.000 | 7.544 |
| Killeshin res. | 0.002 | 0.959 | 1.063 | 10.805 |
| St Mullins | 0.001 | 0.987 | 1.101 | 11.595 |
| Bog Lake | 0.001 | 1.060 | 0.960 | 9.467 |
| Barrow | 0.002 | 0.907 | 1.024 | 13.249 |
| Detection Principle | ʎmax (nm) | LOD (mg·L−1) | Linear Range (mg·L−1) | Reference |
|---|---|---|---|---|
| µPDAs | 453 | 0.041 | 0.041–0.072 | 54 |
| µPDAs | 530 | 30.000 | 40.000–400.000 | 53 |
| Rotational µPDAs | 445 | 0.180 | 0.500–10.000 | 55 |
| Gold nanoparticles | 520 | 0.001 | 0.010–0.130 | 63 |
| Spectrophotometric | 503 | 0.030 | 0.010–0.400 | 62 |
| Spectrophotometric | 385 | 0.014 | 0.260–26.000 | 59 |
| Spectrophotometric | 543 | 0.023 | 0.030–3.000 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method. Int. J. Environ. Res. Public Health 2019, 16, 1803. https://doi.org/10.3390/ijerph16101803
Lace A, Ryan D, Bowkett M, Cleary J. Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method. International Journal of Environmental Research and Public Health. 2019; 16(10):1803. https://doi.org/10.3390/ijerph16101803
Chicago/Turabian StyleLace, Annija, David Ryan, Mark Bowkett, and John Cleary. 2019. "Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method" International Journal of Environmental Research and Public Health 16, no. 10: 1803. https://doi.org/10.3390/ijerph16101803





