Can Hypoxic Conditioning Improve Bone Metabolism? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Searches and Article Selection Strategy
2.2. Risk of Bias
2.3. Data Extraction
3. Results
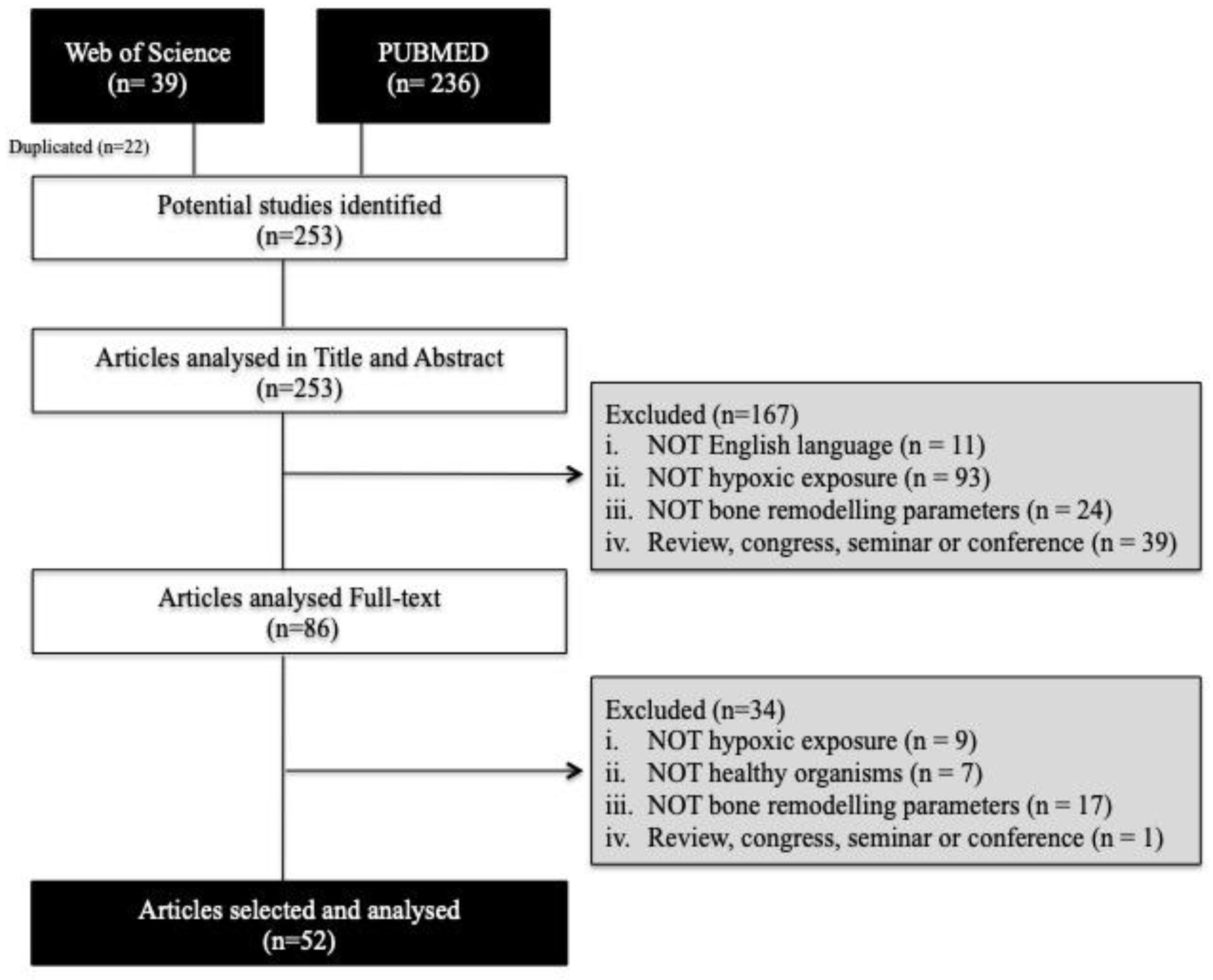
3.1. Article Selection
3.2. Risk of Bias
3.3. Data Extraction
3.3.1. In Vitro Studies
3.3.2. In Vivo Studies
4. Discussion
4.1. In Vitro Studies
4.1.1. Sustained Exposure
4.1.2. Cyclic Exposure
4.2. In Vivo Studies
4.2.1. Sustained Exposure
4.2.2. Cyclic Exposure
4.2.3. Intermittent Exposure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategies
Appendix B
| Reference | Risk of Bias Questions | Confidence Rating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Bouvard, 2014 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Binder, 2015 | - | - | NA | NA | + | - | + | + | + | ++ | + | Moderate |
| Burian, 2017 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Ciappeti, 2016 | ++ | - | NA | NA | + | - | - | + | - | ++ | + | High |
| Cicione, 2013 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| D’Ippolito, 2006 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Deschepper, 2011 | - | - | NA | NA | - | - | - | - | + | ++ | + | Moderate |
| Ding, 2014 | - | - | NA | NA | ++ | - | - | ++ | + | ++ | + | High |
| Gao, 2013 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Gu, 2016 | - | - | NA | NA | - | - | - | + | + | ++ | + | Moderate |
| Holwarth, 2010 | - | - | NA | NA | + | - | + | + | + | + | + | Low |
| Hopper, 2015 | + | - | NA | NA | + | - | - | - | + | + | + | Low |
| Hsu, 2013 | - | - | NA | NA | - | - | - | - | + | + | + | Low |
| Huang, 2011 | - | - | NA | NA | + | - | - | - | + | + | + | Low |
| Huang, 2012 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Iacono, 2018 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Inagaki, 2017 | - | - | NA | NA | + | ++ | - | + | + | ++ | + | High |
| Jin, 2010 | - | - | NA | NA | + | - | - | + | - | + | + | Low |
| Kalinina, 2015 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Lee, 2006 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Lee, 2012 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Lee, 2015 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Ma, 2014 | - | - | NA | NA | + | - | - | + | + | ++ | + | Moderate |
| Malladi, 2006 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Merceron, 2010 | - | - | NA | NA | ++ | - | - | ++ | + | + | + | High |
| Park, 2013 | - | - | NA | NA | ++ | - | - | + | + | + | + | Moderate |
| Pattapa, 2013 | ++ | - | NA | NA | ++ | - | - | + | + | + | + | High |
| Russo, 2013 | - | - | NA | NA | + | - | - | ++ | - | + | + | Moderate |
| Salamanna, 2018 | - | - | NA | NA | + | - | - | + | - | + | + | Low |
| Sengupta, 2010 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Tsang, 2013 | - | - | NA | NA | + | - | + | + | + | + | + | Low |
| Wang, 2012 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Xu, 2007 | - | - | NA | NA | ++ | - | - | + | + | + | + | Moderate |
| Xu, 2013 | - | - | NA | NA | ++ | - | - | + | + | + | + | Moderate |
| Yang, 2011 | - | - | NA | NA | + | - | - | + | + | + | + | Low |
| Yao, 2017 | + | - | NA | NA | + | - | - | + | + | + | + | Low |
| Zham, 2008 | - | - | NA | NA | + | - | - | ++ | + | ++ | + | Moderate |
| Zhang, 2017 | - | - | NA | NA | ++ | - | - | + | + | + | + | Moderate |
| Zhang, 2018 | - | - | NA | NA | - | - | - | + | - | + | + | Low |
Appendix C
| Reference | Risk of Bias Questions | Type of Study | Confidence Rating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Wang, 2016 | + | - | NA | NA | - | - | ++ | + | ++ | ++ | + | EA | High |
| Wang, 2017 | ++ | - | NA | NA | + | - | - | + | + | ++ | + | EA | High |
| Guner, 2013 | - | - | NA | NA | + | - | + | + | - | ++ | + | EA | Moderate |
| Tomiyama, 2008 | NA | NA | + | ++ | NA | NA | - | - | + | ++ | + | HCr-Se | High |
| Basu, 2013 | NA | NA | ++ | + | NA | NA | + | + | - | ++ | + | HCr-Se | High |
| Basu 2014 | NA | NA | ++ | + | NA | NA | + | + | - | ++ | + | HCo | High |
| Sforza, 2013 | NA | NA | + | ++ | NA | NA | - | + | ++ | ++ | + | HCo | High |
| Terzi, 2015 | NA | NA | -- | ++ | NA | NA | - | ++ | ++ | ++ | + | HCo | High |
| Tng, 2008 | NA | NA | -- | ++ | NA | NA | - | + | ++ | ++ | + | HCo | High |
| O’Brien, 2018 | - | - | NA | NA | NA | - | - | + | + | ++ | + | HCT | Moderate |
| Martínez-Guardado, 2019 | ++ | ++ | NA | NA | NA | ++ | + | + | ++ | ++ | + | HCT | High |
| Ramos-Campos, 2015 | + | - | NA | NA | NA | - | + | ++ | ++ | ++ | + | HCT | High |
| Rittweger, 2016 | - | - | NA | NA | NA | - | ++ | ++ | + | ++ | + | HCT | High |
Appendix D
| Domain | Animal Studies | Human Studies | In Vitro Studies | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | ++ | + | - | + | - | ++ | + | - | ||||||||||
| Performance | + | - | - | ++ | + | - | ||||||||||||
| Attrition/exclusion | ++ | - | ++ | + | - | + | - | |||||||||||
| Detection | ++ | + | ++ | + | - | ++ | + | - | ||||||||||
| Selective Reporting | ++ | + | ++ | + | ++ | + | ||||||||||||
Appendix E
| Type of Study | Level of Confidence for Health Effect Bone Remodelling |
|---|---|
| Experimental Animal | High |
| Human Controlled Trial | High |
| Human Cohort | High |
| Human Cross-Sectional | High |
| In Vitro Studies | Moderate |
References
- Dempsey, J.A.; Morgan, B.J. Humans in Hypoxia: A Conspiracy of Maladaptation? Physiology 2015, 30, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Brune, B. HIF-1 in the inflammatory microenvironment. Exp. Cell Res. 2009, 315, 1791–1797. [Google Scholar] [CrossRef]
- Goda, N.; Kanai, M. Hypoxia-inducible factors and their roles in energy metabolism. Int. J. Hematol. 2012, 95, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Basovich, S.N. Trends in the use of preconditioning to hypoxia for early prevention of future life diseases. Biosci. Trends 2013, 7, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J. Redox regulation of growth and death in cardiac myocytes. Antioxid. Redox Signal. 2006, 8, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Wang, Y.; Gozal, D. The polymorphic and contradictory aspects of intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L129–L140. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef]
- Weiss, M.; Ben-Shlomo, A.B.; Hagag, P.; Rapoport, M. Reference database for bone speed of sound measurement by a novel quantitative multi-site ultrasound device. Osteop. Int. 2000, 11, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Norling, T.L. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am. J. Phys. Med. Rehabil. 2010, 89, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Tower, R.J.; Mercken, E.M.; Vangoitsenhoven, R.; Moreau-Triby, C.; Breugelmans, T.; Nefyodova, E.; Cardoen, R.; Mathieu, C.; Van der Schueren, B.; et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J. Clin. Investig. 2018, 128, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Fan, L.; Li, J.; Yu, Z.; Dang, X.; Wang, K. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. Biomed. Res. Int. 2014, 2014, 239356. [Google Scholar] [CrossRef]
- Serebrovska, T.V.; Serebrovska, Z.O.; Egorov, E. Fitness and therapeutic potential of intermittent hypoxia training: A matter of dose. Fiziol Zh 2016, 62, 78–91. [Google Scholar] [CrossRef]
- Xi, L.; Serebrovskaya, T.V. Intermittent Hypoxia and Human Diseases; Springer: London, UK, 2012. [Google Scholar]
- Verges, S.; Chacaroun, S.; Godin-Ribuot, D.; Baillieul, S. Hypoxic Conditioning as a New Therapeutic Modality. Front. Pediatrics 2015, 3, 58. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Garvey, J.F.; Taylor, C.T.; McNicholas, W.T. Cardiovascular disease in obstructive sleep apnoea syndrome: The role of intermittent hypoxia and inflammation. Eur. Respir J. 2009, 33, 1195–1205. [Google Scholar] [CrossRef]
- Urdampilleta, A.; Gonzalez-Muniesa, P.; Portillo, M.P.; Martinez, J.A. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J. Physiol. Biochem. 2012, 68, 289–304. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- NTP. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; National Institute of Environmental Health Science: Durham, NC, USA, 2015.
- Matta, K.; Ploteau, S.; Coumoul, X.; Koual, M.; Le Bizec, B.; Antignac, J.P.; Cano-Sancho, G. Associations between exposure to organochlorine chemicals and endometriosis in experimental studies: A systematic review protocol. Environ. Int. 2019, 124, 400–407. [Google Scholar] [CrossRef]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef]
- Huang, Y.C.; Zhu, H.M.; Cai, J.Q.; Huang, Y.Z.; Xu, J.; Zhou, Y.; Chen, X.H.; Li, X.Q.; Yang, Z.M.; Deng, L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1407–1415. [Google Scholar] [CrossRef]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.S.; Ding, H.; Xie, X.T.; Zhang, C.Q. Osteogenic induction protects rat bone marrow-derived mesenchymal stem cells against hypoxia-induced apoptosis in vitro. J. Surg. Res. 2013, 184, 873–879. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Yin, J.H.; Xie, X.T.; Zhu, Z.H.; Gao, Y.S.; Zhang, C.Q. Continuous hypoxia regulates the osteogenic potential of mesenchymal stem cells in a time-dependent manner. Mol. Med. Rep. 2014, 10, 2184–2190. [Google Scholar] [CrossRef]
- Lee, S.K.; Gardner, A.E.; Kalinowski, J.F.; Jastrzebski, S.L.; Lorenzo, J.A. RANKL-stimulated osteoclast-like cell formation in vitro is partially dependent on endogenous interleukin-1 production. Bone 2006, 38, 678–685. [Google Scholar] [CrossRef]
- Burian, E.; Probst, F.; Palla, B.; Riedel, C.; Saller, M.M.; Cornelsen, M.; Konig, F.; Schieker, M.; Otto, S. Effect of hypoxia on the proliferation of porcine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells in 2- and 3-dimensional culture. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2017, 45, 414–419. [Google Scholar] [CrossRef]
- Bouvard, B.; Abed, E.; Yelehe-Okouma, M.; Bianchi, A.; Mainard, D.; Netter, P.; Jouzeau, J.Y.; Lajeunesse, D.; Reboul, P. Hypoxia and vitamin D differently contribute to leptin and dickkopf-related protein 2 production in human osteoarthritic subchondral bone osteoblasts. Arthritis Res. Ther. 2014, 16, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Ha, N.; Dai, Q.; Zhou, S.; Yu, C.; Jiang, L. Hypoxia suppresses osteogenesis of bone mesenchymal stem cells via the extracellular signalregulated 1/2 and p38mitogen activated protein kinase signaling pathways. Mol. Med. Rep. 2017, 16, 5515–5522. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Cepollaro, S.; Contartese, D.; Giavaresi, G.; Brodano, G.B.; Griffoni, C.; Gasbarrini, A.; Fini, M. Biological Rationale for the Use of Vertebral Whole Bone Marrow in Spinal Surgery. Spine 2018, 43, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G.; Granchi, D.; Fotia, C.; Savarino, L.; Dallari, D.; Del Piccolo, N.; Donati, D.M.; Baldini, N. Effects of hypoxia on osteogenic differentiation of mesenchymal stromal cells used as a cell therapy for avascular necrosis of the femoral head. Cytotherapy 2016, 18, 1087–1099. [Google Scholar] [CrossRef]
- Tsang, W.P.; Shu, Y.; Kwok, P.L.; Zhang, F.; Lee, K.K.; Tang, M.K.; Li, G.; Chan, K.M.; Chan, W.Y.; Wan, C. CD146+ human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS ONE 2013, 8, e76153. [Google Scholar] [CrossRef]
- Gu, Q.; Gu, Y.; Shi, Q.; Yang, H. Hypoxia Promotes Osteogenesis of Human Placental-Derived Mesenchymal Stem Cells. Tohoku J. Exp. Med. 2016, 239, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Park, S.H.; Patel, A.; Carn, J.; Lee, K.; Kaplan, D.L. Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Eng. Part A 2010, 16, 3623–3634. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, T.; Furu, M.; Nasu, A.; Kajita, Y.; Mitsui, H.; Ueda, M.; Aoyama, T.; Nakayama, T.; Nakamura, T.; et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem. Biophys. Res. Commun. 2010, 391, 1471–1476. [Google Scholar] [CrossRef]
- Binder, B.Y.; Sagun, J.E.; Leach, J.K. Reduced serum and hypoxic culture conditions enhance the osteogenic potential of human mesenchymal stem cells. Stem Cell Rev. 2015, 11, 387–393. [Google Scholar] [CrossRef]
- Xu, Y.; Malladi, P.; Chiou, M.; Bekerman, E.; Giaccia, A.J.; Longaker, M.T. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007, 13, 2981–2993. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lui, P.P.; Rui, Y.F. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng. Part A 2012, 18, 484–498. [Google Scholar] [CrossRef]
- Holzwarth, C.; Vaegler, M.; Gieseke, F.; Pfister, S.M.; Handgretinger, R.; Kerst, G.; Muller, I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010, 11, 11. [Google Scholar] [CrossRef]
- Russo, V.; Yu, C.; Belliveau, P.; Hamilton, A.; Flynn, L.E. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl. Med. 2014, 3, 206–217. [Google Scholar] [CrossRef]
- Iacono, E.; Pascucci, L.; Bazzucchi, C.; Cunto, M.; Ricci, F.; Rossi, B.; Merlo, B. Could hypoxia influence basic biological properties and ultrastructural features of adult canine mesenchymal stem/stromal cells? Vet. Res. Commun. 2018, 42, 297–308. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.C.; Kim, T.W.; Jung, B.J.; Lee, Y.; Shim, E.K.; Park, S.; Choi, E.Y.; Cho, K.S.; Kim, C.S. Human bone marrow stem cells cultured under hypoxic conditions present altered characteristics and enhanced in vivo tissue regeneration. Bone 2015, 78, 34–45. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, C.T.; Wei, Y.H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2013, 31, 2779–2788. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, K.H.; Choi, H.K.; Shim, J.S.; Whang, S.Y.; Hahn, S.J.; Kwon, O.J.; Oh, I.H. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp. Mol. Med. 2013, 45, e44. [Google Scholar] [CrossRef]
- Ma, H.P.; Ma, X.N.; Ge, B.F.; Zhen, P.; Zhou, J.; Gao, Y.H.; Xian, C.J.; Chen, K.M. Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif. 2014, 47, 527–539. [Google Scholar] [CrossRef]
- Yao, Y.; Deng, Q.; Sun, C.; Song, W.; Liu, H.; Zhou, Y. A genome-wide analysis of the gene expression profiles and alternative splicing events during the hypoxia-regulated osteogenic differentiation of human cartilage endplate-derived stem cells. Mol. Med. Rep. 2017, 16, 1991–2001. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Liu, H.; Qu, F.; Fan, J.; Mao, K.; Yin, Y.; Liu, J.; Geng, Z.; Wang, Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp. Mol. Pathol. 2013, 94, 33–39. [Google Scholar] [CrossRef]
- Yang, D.C.; Yang, M.H.; Tsai, C.C.; Huang, T.F.; Chen, Y.H.; Hung, S.C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef]
- Cicione, C.; Muinos-Lopez, E.; Hermida-Gomez, T.; Fuentes-Boquete, I.; Diaz-Prado, S.; Blanco, F.J. Effects of severe hypoxia on bone marrow mesenchymal stem cells differentiation potential. Stem Cells Int. 2013, 2013, 232896. [Google Scholar] [CrossRef]
- Pattappa, G.; Thorpe, S.D.; Jegard, N.C.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng. Part Cmethods 2013, 19, 68–79. [Google Scholar] [CrossRef]
- Zahm, A.M.; Bucaro, M.A.; Srinivas, V.; Shapiro, I.M.; Adams, C.S. Oxygen tension regulates preosteocyte maturation and mineralization. Bone 2008, 43, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1139–C1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, Y.; Akahane, M.; Shimizu, T.; Inoue, K.; Egawa, T.; Kira, T.; Ogawa, M.; Kawate, K.; Tanaka, Y. Modifying oxygen tension affects bone marrow stromal cell osteogenesis for regenerative medicine. World J. Stem Cells 2017, 9, 98–106. [Google Scholar] [CrossRef]
- Hopper, N.; Wardale, J.; Brooks, R.; Power, J.; Rushton, N.; Henson, F. Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in in vivo Osteochondral Defect Model. PLoS ONE 2015, 10, e0133937. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Cherel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yun, Z.; Peng, H.; Yan, S.; Zhang, H.; Qiu, X.; Wen, Y.; Long, H.; Ma, B. The hypobaric hypoxia environment impairs bone strength and quality in rats. Int J. Clin. Exp. Med. 2017, 10, 9397–9406. [Google Scholar]
- O’Brien, K.A.; Pollock, R.D.; Stroud, M.; Lambert, R.J.; Kumar, A.; Atkinson, R.A.; Green, D.A.; Anton-Solanas, A.; Edwards, L.M.; Harridge, S.D.R. Human physiological and metabolic responses to an attempted winter crossing of Antarctica: The effects of prolonged hypobaric hypoxia. Physiol. Rep. 2018, 6, e13613. [Google Scholar] [CrossRef]
- Basu, M.; Malhotra, A.S.; Pal, K.; Kumar, R.; Bajaj, R.; Verma, S.K.; Ghosh, D.; Sharma, Y.K.; Sawhney, R.C. Alterations in Different Indices of Skeletal Health after Prolonged Residency at High Altitude. High. Alt. Med. Biol. 2014, 15, 170–175. [Google Scholar] [CrossRef]
- Basu, M.; Malhotra, A.S.; Pal, K.; Chatterjee, T.; Ghosh, D.; Haldar, K.; Verma, S.K.; Kumar, S.; Sharma, Y.K.; Sawhney, R.C. Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: A longitudinal study. High. Alt. Med. Biol 2013, 14, 150–154. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Sun, D.; Xin, J.; Wang, L.; Huang, D.; Wu, W.; Xian, C.J. Short-Term Hypoxia Accelerates Bone Loss in Ovariectomized Rats by Suppressing Osteoblastogenesis but Enhancing Osteoclastogenesis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2962–2971. [Google Scholar] [CrossRef] [Green Version]
- Guner, I.; Uzun, D.D.; Yaman, M.O.; Genc, H.; Gelisgen, R.; Korkmaz, G.G.; Hallac, M.; Yelmen, N.; Sahin, G.; Karter, Y.; et al. The effect of chronic long-term intermittent hypobaric hypoxia on bone mineral density in rats: Role of nitric oxide. Biol. Trace Elem. Res. 2013, 154, 262–267. [Google Scholar] [CrossRef]
- Martinez-Guardado, I.; Ramos-Campo, D.J.; Olcina, G.J.; Rubio-Arias, J.A.; Chung, L.H.; Marin-Cascales, E.; Alcaraz, P.E.; Timon, R. Effects of high-intensity resistance circuit-based training in hypoxia on body composition and strength performance. Eur. J. Sport Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Rubio-Arias, J.A.; Jimenez-Diaz, J.F. Effects in body composition and bone mineral density of simulate altitude program in triathletes. Nutr. Hosp. 2015, 32, 1252–1260. [Google Scholar]
- Rittweger, J.; Debevec, T.; Frings-Meuthen, P.; Lau, P.; Mittag, U.; Ganse, B.; Ferstl, P.G.; Simpson, E.J.; Macdonald, I.A.; Eiken, O.; et al. On the combined effects of normobaric hypoxia and bed rest upon bone and mineral metabolism: Results from the PlanHab study. Bone 2016, 91, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Tng, H.Y.; Thu, W.P.P.; Logan, S.; Aris, I.M.; Cauley, J.; Yong, E.L. Sleep apnea and femoral neck BMD among Singaporean mid-life women. Arch. Osteoporos. 2018, 13, 19. [Google Scholar] [CrossRef]
- Terzi, R.; Yilmaz, Z. Bone mineral density and changes in bone metabolism in patients with obstructive sleep apnea syndrome. J. Bone Miner. Metab. 2016, 34, 475–481. [Google Scholar] [CrossRef]
- Sforza, E.; Thomas, T.; Barthelemy, J.C.; Collet, P.; Roche, F. Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects. Sleep 2013, 36, 1509–1515. [Google Scholar]
- Tomiyama, H.; Okazaki, R.; Inoue, D.; Ochiai, H.; Shiina, K.; Takata, Y.; Hashimoto, H.; Yamashina, A. Link between obstructive sleep apnea and increased bone resorption in men. Osteoporos. Int. A J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2008, 19, 1185–1192. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, Y.; Lei, L.; Jiang, C.; An, S.; Zhan, Y.; Cheng, Q.; Zhao, Z.; Wang, J.; et al. Effects of hypoxia on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mol. Cell. Biochem. 2012, 362, 25–33. [Google Scholar] [CrossRef]
- Mateika, J.H.; El-Chami, M.; Shaheen, D.; Ivers, B. Intermittent hypoxia: A low-risk research tool with therapeutic value in humans. J. Appl. Physiol. 2015, 118, 520–532. [Google Scholar] [CrossRef]
- Barcelo, A.; Pierola, J.; de la Pena, M.; Esquinas, C.; Fuster, A.; Sanchez-de-la-Torre, M.; Carrera, M.; Alonso-Fernandez, A.; Ladaria, A.; Bosch, M.; et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur. Respir. J. 2011, 37, 1418–1423. [Google Scholar] [CrossRef]
- Destors, M.; Tamisier, R.; Baguet, J.P.; Levy, P.; Pepin, J.L. Cardiovascular morbidity associated with obstructive sleep apnea syndrome. Rev. Mal. Respir. 2014, 31, 375–385. [Google Scholar] [CrossRef]
- Huang, J.; Deng, F.; Wang, L.; Xiang, X.R.; Zhou, W.W.; Hu, N.; Xu, L. Hypoxia induces osteogenesis-related activities and expression of core binding factor alpha1 in mesenchymal stem cells. Tohoku J. Exp. Med. 2011, 224, 7–12. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Z.; Wei, J.; Yu, Y.; Luo, J.; Zhou, J.; Li, Y.; Zheng, X.; Tang, W.; Liu, L.; et al. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-regulation of Hif-1alpha. Int. J. Biol. Sci. 2018, 14, 449–460. [Google Scholar] [CrossRef]
- Lee, J.H.; Kemp, D.M. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem. Biophys. Res. Commun. 2006, 341, 882–888. [Google Scholar] [CrossRef]
- Hung, S.P.; Ho, J.H.; Shih, Y.R.; Lo, T.; Lee, O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 260–266. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef]
- Ishijima, M.; Rittling, S.R.; Yamashita, T.; Tsuji, K.; Kurosawa, H.; Nifuji, A.; Denhardt, D.T.; Noda, M. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J. Exp. Med. 2001, 193, 399–404. [Google Scholar] [CrossRef]
- Dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell Physiol. 2010, 223, 27–35. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhang, Z.Q.; Fang, S.L.; Liu, Y.R.; Jiang, G.; Li, K.F. Effects of hypoxia on proliferation and osteogenic differentiation of periodontal ligament stem cells: An in vitro and in vivo study. Genet. Mol. Res. 2014, 13, 10204–10214. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Lowe, E.T.; Hardingham, T.E. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 834–840. [Google Scholar] [CrossRef]
- Martin-Rendon, E.; Hale, S.J.; Ryan, D.; Baban, D.; Forde, S.P.; Roubelakis, M.; Sweeney, D.; Moukayed, M.; Harris, A.L.; Davies, K.; et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells 2007, 25, 1003–1012. [Google Scholar] [CrossRef]
- Gordillo, G.M.; Sen, C.K. Revisiting the essential role of oxygen in wound healing. Am. J. Surg. 2003, 186, 259–263. [Google Scholar] [CrossRef]
- Utting, J.C.; Flanagan, A.M.; Brandao-Burch, A.; Orriss, I.R.; Arnett, T.R. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem. Funct. 2010, 28, 374–380. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Raggatt, L.J.; Partridge, N.C. Parathyroid hormone: A double-edged sword for bone metabolism. Trends Endocrinol. Metab. Tem. 2004, 15, 60–65. [Google Scholar] [CrossRef]
- Bingham, P.J.; Brazell, I.A.; Owen, M. The effect of parathyroid extract on cellular activity and plasma calcium levels in vivo. J. Endocrinol. 1969, 45, 387–400. [Google Scholar] [CrossRef]
- Bozzini, C.E.; Lezon, C.E.; Norese, M.F.; Conti, M.I.; Martinez, M.P.; Olivera, M.I.; Alippi, R.M. Evidence from catch-up growth and hoarding behavior of rats that exposure to hypobaric air lowers the body-mass set point. Growth Dev. Aging 2005, 69, 81–88. [Google Scholar]
- Vuori, I.M. Dose-response of physical activity and low back pain, osteoarthritis, and osteoporosis. Med. Sci. Sports Exerc. 2001, 33 (Suppl. 6), S551–S586. [Google Scholar] [CrossRef] [Green Version]
- Kelly, L.P.; Basset, F.A. Acute Normobaric Hypoxia Increases Post-exercise Lipid Oxidation in Healthy Males. Front. Physiol. 2017, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Dawson-Hughes, B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am. J. Med. 2004, 116, 634–639. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chron Obs. Pulmon Dis. 2011, 6, 199–208. [Google Scholar]
- Knapp, K.M.; Blake, G.M.; Spector, T.D.; Fogelman, I. Multisite quantitative ultrasound: Precision, age- and menopause-related changes, fracture discrimination, and T-score equivalence with dual-energy X-ray absorptiometry. Osteoporos. Int. A J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2001, 12, 456–464. [Google Scholar] [CrossRef]
- Litovka, I.H. Alimentary and oxygen deprivation as the modulator of the bone tissue physiological remodelling rate in young rats. Fiziol. Zh. 2008, 54, 85–93. [Google Scholar]
- Swanson, C.M.; Shea, S.A.; Stone, K.L.; Cauley, J.A.; Rosen, C.J.; Redline, S.; Karsenty, G.; Orwoll, E.S. Obstructive sleep apnea and metabolic bone disease: Insights into the relationship between bone and sleep. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2015, 30, 199–211. [Google Scholar] [CrossRef]
- Orriss, I.R.; Knight, G.E.; Utting, J.C.; Taylor, S.E.; Burnstock, G.; Arnett, T.R. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell Physiol. 2009, 220, 155–162. [Google Scholar] [CrossRef]
| Hypoxia Effects on Outcomes | Hypoxia Level (% PiO2) | Duration, Frequency | References | Confidence Rating |
|---|---|---|---|---|
| Sustained Exposure | ||||
| ↑RUNX2-g ↑ALP-g ↑ALP ↑ALP-activity ↑Col1A1-g ↑Col1A1 ↑Osteocalcin-g ↑Osteocalcin ↑Calcium deposit | 0.1% | 1 days | Huang, 2012 [23] | Moderate |
| 1% | 2 days | Kalinina, 2015 [24] | Moderate | |
| 12 days | Deschepper, 2011 [25] | Moderate | ||
| 21 days | Gao, 2013 [26] Ding, 2014 [27] | Moderate High | ||
| 2% | NR | Lee, 2006 [28] Burin, 2017 [29] | Moderate Low | |
| 1 day | Bouvard, 2014 [30] | Moderate | ||
| 2 days | Zhang, 2018 [31] | Low | ||
| 3 days | Salamanna, 2018 [32] | Low | ||
| 12 days | Ciapetti, 2016 [33] | High | ||
| 14 days | Tsang, 2013 [34] | Low | ||
| 21 days | Tsang, 2013 [34] | Low | ||
| 5% | 3 days | Gu, 2016 [35] | Moderate | |
| 14 days | Ding, 2014 [27] | High | ||
| 49 days | Sengupta, 2010 [36] | Low | ||
| ➔ RUNX2-g ➔ ALP-g ➔ ALP ➔ ALP-activity ➔ Col1A1-g ➔ Col1A1 ➔ Osteocalcin ➔ Osteopontin-g ➔ Osteopontin ➔ Calcium deposit | 1% | 14 days | Jin, 2010 [37] | Low |
| 21 days | Binder, 2015 [38] Ding, 2014 [27] | Moderate High | ||
| 2% | 5 days | Xu, 2007 [39] | Moderate | |
| 8 days | Xu, 2007 [39] | Moderate | ||
| 12 days | Ciapetti, 2016 [33] | High | ||
| 14 days | Tsang, 2013 [34] Zhang, 2017 [31] | Low Moderate | ||
| 21 days | Tsang, 2013 [34] Lee, 2012 [40] | Low Moderate | ||
| 3% | 14 days | Holzwarth, 2010 [41] | Low | |
| 5% | NR | Russo, 2014 [42] | Moderate | |
| 3 days | Gu, 2016 [35] | Moderate | ||
| 21 days | Binder, 2015 [38] | Moderate | ||
| 49 days | Sengupta, 2010 [36] | Low | ||
| 7% | NR | Iacono, 2018 [43] | Low | |
| ↓ RUNX2-g ↓ RUNX22 ↓ ALP-g ↓ ALP ↓ ALP-activity ↓ Col1A1-g ↓ Col1A1 ↓ Osteocalcin-g ↓ Osteocalcin ↓ Osteopontin-g ↓ Osteopontin ↓ Calcium deposit | 1% | NR | Lee, 2015 [44] Hsu, 2013 [45] Park, 2013 [46] | Moderate Low Moderate |
| 2 days | Ma, 2014 [47] | Moderate | ||
| 21 days | Ding, 2014 [27] Yao, 2017 [48] Xu, 2013 [49] Yang, 2011 [50] Cicione, 2013 [51] | High Low Moderate Low Moderate | ||
| 2% | NR | Burian, 2017 [29] | Low | |
| 3 days | Salamanna, 2018 [32] | Low | ||
| 5 days | Xu, 2007 [39] Huang, 2012 [23] | Moderate High | ||
| 6 days | Pattappa, 2013 [52] | Moderate | ||
| 7 days | Zham, 2008 [53] | Moderate | ||
| 8 days | Xu, 2007 [39] | High | ||
| 14 days | Zhang, 2017 [31] | Moderate | ||
| 21 days | Huang, 2012 [23] Tsang, 2013 [34] Malladi, 2006 [54] Lee, 2012 [40] | Moderate Low Low Moderate | ||
| 3% | 14 days | Holzwarth, 2010 [41] | Low | |
| 5% | NR | Russo, 2014 [42] | Moderate | |
| 6 days | Pattappa, 2013 [52] | High | ||
| 14 days | Inagaki, 2017 [55] | High | ||
| 21 days | Hopper, 2015 [56] | Low | ||
| 28 days | Merceron, 2010 [57] | High | ||
| Cyclic Exposure | ||||
| ➔ ALP activity ➔ Calcium deposit ↓ ALP ↓ RUNX2 ↓ Osteocalcin | 1% 3% 5% 10% | 15 days 2 × 3 min/day | Dìppolito, 2006 [58] | Moderate |
| Hypoxia Effects on Outcomes | Sample | Intervention | References | Confidence Rating | |||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Age | Size | Conditions (n) | Hypoxia Level (Meters; % PiO2) | Duration, Frequency | Exposure Type | |||
| Sustained Exposure | |||||||||
| ↓BV/TV ↓Tb.N ↓BMD-total | Sprague-Dawley rats | 12 week-old | Hypoxia (n = 4) Normoxia (n = 4) | 6000 m | 3 weeks | Normobaric | Wang, 2017 [59] | High | |
| ↓BMD-spine | Healthy adults | 24–58 years | 5 | NR | 2500 m | 24 weeks | Hypobaric | O´Brien, 2019 [60] | Moderate |
| ↓SOS-R ➔SOS-T ↓SOS-M ↓SOS-P ↓T-score-R ↓T-score-P ↑ALP ↓BAP ↑Calcium deposit ↓25-Vit D ↓i-PTH ↓CICP ↓NTX ↓DPD/Cr | Healthy adults | 21–47 years | 2600 | Normoxia (n = 1300) Hypoxia (n = 1300) | 3450 m | 16 weeks | Hypobaric | Basu, 2014 [61] | High |
| ➔SOS-R ➔SOS-T ↓SOS-M ↓SOS-P ➔Z-score-R ➔Z-score-T ↓Z-score-M ↓Z-score-P ➔Calcium ↑Phosphorous ↓ALP ↓BAP ↓25-Vit D ↓Calcitonin ↑i-PTH ➔DPD/Cr | Healthy adults | 21–47 years | 221 | Hypoxia (n = 221) | 3000–3754 m (24 weeks) + 5400–6700 m (16 weeks) | 40 weeks | Hypobaric | Basu, 2013 [62] | High |
| Cyclic Exposure | |||||||||
| ➔BV/TV ➔Tb.N ➔BMD-total ➔BMC-total ↓ BV/TV ↓Tb.N ↓BMD-total ↓BMC-total | Sprague-Dawley rats | 12 week-old | 37 | Hypoxia (n = 7) Normoxia (n = 6) Ovariectomized Hypoxia (n = 12) Ovariectomized Normoxia (n = 12) | 3000–5000 m | 2 weeks, 4 h/day | Normobaric | Wang, 2016 [63] | High |
| ↑BMD-spine | Wistar albino rats | 6 months-old | 20 | Hypoxia (n = 10) Normoxia (n = 10) | 4500 m | 5 weeks 5 days/week 5 h/day | Hypobaric | Guner, 2013 [64] | Moderate |
| ↑BMD-total | Healthy adults | 24.6 ± 2.8 years | 28 | Hypoxia (n = 15) Normoxia (n = 13) | 15% PiO2 | 8 weeks 2days/week | Normobaric | Martínez-Guardado, 2019 [65] | High |
| ➔BMD-total | Trained triathletes | 27 years | 18 | Hypoxia Training (n = 9) Control (n = 9) | 15% PiO2 | 7 weeks, 2days/week 60 min/day | Normobaric | Ramos-Campos, 2015 [66] | High |
| ↓BMC-total ↑BMC-total | Healthy young | 26.4 years | 14 | Hypoxia Bed Rest (n = 14) Hypoxia Ambulatory (n = 14) Normoxia Bed Rest (n = 14) | 4000 m | 21 days | Normobaric | Rittweger, 2016 [67] | High |
| Intermittent Exposure | |||||||||
| ➔BMD-spine | Menopausal Women with OSAS | 56.3 ± 6.2 years | 1201 | NR | NR | NR | NR | Tng, 2018 [68] | High |
| ↑CTX | Adults with OSAS | 51 years | 50 | OSA (n = 30) Control (n = 20) | NR | NR | NR | Terzi, 2016 [69] | High |
| ↑BMD-total | Adults with OSAS | 68.6 ± 0.8 years | 833 | OSA (n = 459) Control (n = 373) | NR | NR | NR | Sforza, 2013 [70] | High |
| ➔CTX ➔RANKL ➔OPG ➔CTX ➔RANKL ➔OPG ↑CTX ↓RANKL ➔OPG | Adults with OSAS | 51.0 ± 13 years | 65 | Mild OSAS (n = 10) Moderate OSAS (n = 12) Severe OSAS (n = 28) Control (n = 15) | NR | NR | NR | Tomiyama, 2008 [71] | High |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Cardenosa, M.; Camacho-Cardenosa, A.; Timón, R.; Olcina, G.; Tomas-Carus, P.; Brazo-Sayavera, J. Can Hypoxic Conditioning Improve Bone Metabolism? A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1799. https://doi.org/10.3390/ijerph16101799
Camacho-Cardenosa M, Camacho-Cardenosa A, Timón R, Olcina G, Tomas-Carus P, Brazo-Sayavera J. Can Hypoxic Conditioning Improve Bone Metabolism? A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(10):1799. https://doi.org/10.3390/ijerph16101799
Chicago/Turabian StyleCamacho-Cardenosa, Marta, Alba Camacho-Cardenosa, Rafael Timón, Guillermo Olcina, Pablo Tomas-Carus, and Javier Brazo-Sayavera. 2019. "Can Hypoxic Conditioning Improve Bone Metabolism? A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 10: 1799. https://doi.org/10.3390/ijerph16101799





