Chemical Composition and Deposition Fluxes of Water-Soluble Inorganic Ions on Dry and Wet Deposition Samples in Wuhan, China
Abstract
:1. Introduction
2. Methodology
2.1. Data and Sampling Site
2.2. Sample Analysis
- C: Determined ion concentration (mg/L) in volume of a sample
- V1: Volume (L) after dilution of the sample solution taken at the time of measurement
- V2: Sample volume of air in standard state (m3)
- M1: Mass (g) of the filter before sampling
- M2: Mass (g) of the filter after sampling
- F: Deposition flux (mg·m−2·month−1)
- Qt: Total settlement (mg·month−1)
- S: Sampling area (m2)
- S = 3.14 × D2/4/104
- D: Diameter of the sampler (cm)
2.3. HYSPLIT Model and Meteorological Data
3. Results and Discussion
3.1. Analysis of PM2.5 Concentration
3.2. Concentration of Water-Soluble Inorganic Ions
3.2.1. Equivalent Ratio of / in PM2.5
3.2.2. Analysis of Water-Soluble Inorganic Ions in Wet Deposition
3.2.3. Analysis of Water-Soluble Inorganic Ions in Dry Deposition
3.3. Estimation of Deposition Flux of Water-Soluble Inorganic ions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, Z.; Zhang, L.; Cao, J.; Tian, J.; Liu, L.; Wang, G.; Zhao, Z.; Wang, X.; Zhang, R.; Liu, S. Chemical composition, sources, and deposition fluxes of water-soluble inorganic ions obtained from precipitation chemistry measurements collected at an urban site in northwest China. J. Environ. Monit. 2012, 14, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Y.; Kong, S.; Wen, B.; Chen, K.; Yuan, L.; Li, Q. Altitudinal effect to the size distribution of water soluble inorganic ions in PM at Huangshan, China. Atmos. Environ. 2014, 98, 242–252. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, Z.; Fang, M.; Rahn, K.A.; Kester, D.R. Dry and wet deposition of elements in Hong Kong. Mar. Chem. 2005, 97, 124–139. [Google Scholar] [CrossRef]
- Budhavant, K.B.; Safai, P.D.; Gawhane, R.D.; Raju, M.P.; Mahajan, C.M.; Satsangi, P.G. Atmospheric Wet and Dry Depositions of Ions over an Urban Location in South-West India. Aerosol Air Qual. Res. 2012, 12, 561–570. [Google Scholar] [CrossRef]
- Slinn, S.A.; Slinn, W.G.N. Predictions for particle deposition on natural waters. Atmos. Environ. 1980, 14, 1013–1016. [Google Scholar] [CrossRef]
- Sehmel, G.A. Particle and gas dry deposition: A review. Atmos. Environ. 1980, 14, 983–1011. [Google Scholar] [CrossRef]
- Kulshrestha, U.C.; Sarkar, A.K.; Srivastava, S.S.; Parashar, D.C. Wet-only and bulk deposition studies at New Delhi (India). Water Air Soil Pollut. 1995, 85, 2137–2142. [Google Scholar] [CrossRef]
- Gambaro, A.; Radaelli, M.; Piazza, R.; Maria, A.; Contini, D.; Belosi, F.; Zangrando, R.; Cescon, P. Organic micropollutants in wet and dry depositions in the Venice Lagoon. Chemosphere 2009, 76, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Castillo, S.; Alastuey, A.; Cuevas, E.; Querol, X.; Avila, A. Quantifying dry and wet deposition fluxes in two regions of contrasting African influence: The NE Iberian Peninsula and the Canary Islands. Atmosphere 2017, 8, 86. [Google Scholar] [CrossRef]
- Chu, C.C.; Fang, G.C.; Chen, J.C.; Yang, I.L. Dry deposition study by using dry deposition plate and water surface sampler in Shalu, central Taiwan. Environ. Monit. Assess. 2008, 146, 441–451. [Google Scholar] [CrossRef]
- Inomata, Y.; Igarashi, Y.; Chiba, M.; Shinoda, Y.; Takahashi, H. Dry and wet deposition of water-insoluble dust and water-soluble chemical species during spring 2007 in Tsukuba, Japan. Atmos. Environ. 2009, 43, 4503–4512. [Google Scholar] [CrossRef]
- Saxena, A.; Kulshrestha, U.C.; Kumar, N.; Kumari, K.M.; Prakash, S.; Srivastava, S.S. Dry deposition of sulphate and nitrate to polypropylene surfaces in a semi-arid area of India. Atmos. Environ. 1997, 31, 2361–2366. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, Y.; Sun, J.; Zhang, L.; Shen, X.; Lin, W.; Yang, Y. Variability, formation and acidity of water-soluble ions in PM2.5 in Beijing based on the semi-continuous observations. Atmos. Res. 2014, 145–146, 1–11. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Cheng, H.; Lv, X.; Gong, W.; Wang, X.; Zhang, G. Seasonal variations and chemical characteristics of PM2.5 in Wuhan, Central China. Sci. Total Environ. 2015, 518–519, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, J.; Zhao, W.; Cheng, J.; Cheng, S. Seasonal Variations and Correlation Analysis of Water-Soluble Inorganic Ions in PM2.5 in Wuhan, 2013. Atmosphere 2016, 7, 49. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Zhao, Q.; Du, Z.; He, K.; Ma, Y.; Duan, F.; Chen, G.; Zhao, Q. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos. Chem. Phys. 2011, 11, 5207–5219. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Gao, J.; Zhang, L.; Zhang, R.; Che, H.; Zhang, Z.; Lin, Z.; Jing, J.; Cao, J.; Hsu, S. PM2.5 pollution in a megacity of southwest China: Source apportionment and implication. Atmos. Chem. Phys. 2014, 14, 8679–8699. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Z.; Qiao, T.; Zhang, Y.; Xiu, G.; Yu, J. Chemical characterization, the transport pathways and potential sources of PM 2.5 in Shanghai: Seasonal variations. Atmos. Res. 2015, 158–159, 66–78. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Brimblecombe, P.; Fat, Y.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef]
- Lyu, X.P.; Chen, N.; Guo, H.; Zeng, L.; Zhang, W.; Shen, F.; Quan, J.; Wang, N. Chemical characteristics and causes of airborne particulate pollution in warm seasons in Wuhan, central China. Atmos. Chem. Phys. 2016, 16, 10671–10687. [Google Scholar] [CrossRef] [Green Version]
- Mbululo, Y.; Qin, J.; Yuan, Z. Evolution of atmospheric boundary layer structure and its relationship with air quality in Wuhan, China. Arab. J. Geosci. 2017, 10, 477. [Google Scholar] [CrossRef]
- Weiwei, L.; Yanxin, W.; Xavier, Q.; Xinguo, Z.; Alastuey, A.; Angel, L.; Mar, V. Geochemical and statistical analysis of trace metals in atmospheric particulates in Wuhan, central China. Environ. Geol. 2006, 51, 121–132. [Google Scholar]
- Lyu, X.P.; Wang, Z.W.; Cheng, H.R.; Zhang, F.; Zhang, G.; Wang, X.M.; Ling, Z.H.; Wang, N. Chemical characteristics of submicron particulates (PM1.0) in Wuhan, Central China. Atmos. Res. 2015, 161, 169–178. [Google Scholar] [CrossRef]
- Zhangxiong, Z.; Ke, H.; Zhen, H.; Ting, Y.; Longjiao, S. Characteristic Analysis of OC and EC in PM2.5 of Typical Haze Weather in Wuhan City. Meteorol. Environ. Res. 2014, 5, 10–13. [Google Scholar]
- Song, J.; Guang, W.; Li, L.; Xiang, R. Assessment of air quality status in Wuhan, China. Atmosphere 2016, 7, 56. [Google Scholar] [CrossRef]
- Mbululo, Y.; Qin, J.; Hong, J.; Yuan, Z. Characteristics of Atmospheric Boundary Layer Structure during PM2.5 and Ozone Pollution Events in Wuhan, China. Atmosphere 2018, 9, 359. [Google Scholar] [CrossRef]
- Pui, D.Y.H.; Chen, S.C.; Zuo, Z. PM2.5 in China: Measurements, sources, visibility and health effects, and mitigation. Particuology 2014, 13, 1–26. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Voorhees, A.S.; Zhao, B.; Jang, C.; Jiang, J.; Fu, J.S.; Ding, D.; Zhu, Y.; Hao, J. Assessment of short-term PM2.5-related mortality due to different emission sources in the Yangtze River Delta, China. Atmos. Environ. 2015, 123, 440–448. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Mwangi, J.K.; Wang, L.-C.; Wu, Y.-L.; Tseng, C.-Y.; Chang, K.-H. Atmospheric PM2.5 characteristics and long-term trends in Tainan city, Southern Taiwan. Aerosol Air Qual. Res. 2016, 16, 2488–2511. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. GB 13580.2-92 Collection and Preservation of the Wet Precipitation Sample; China Environmental Science Press: Beijing, China, 1992; Volume 3.
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Rolph, G.D.; Stein, A.; Stunder, B. Real-Time Environmental Applications and Display sYstem: READY; NOAA Air Resources Laboratory: College Park, MD, USA, 2017.
- Ministry of Environmental Protection of the People’s Republic of China. GB 3095-2012 People’s Republic of China National Ambient Air Quality Standard; China Environmental Science Press: Beijing, China, 2012; p. 6.
- WHO. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; WHO/SDE/PHE/OEH/06.02; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Ianniello, A.; Spataro, F.; Esposito, G.; Allegrini, I.; Hu, M.; Zhu, T. Chemical characteristics of inorganic ammonium salts in PM2.5 in the atmosphere of Beijing (China). Atmos. Chem. Phys. 2011, 11, 10803–10822. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Chan, C.K.; Fang, M.; Cadle, S.; Chan, T.; Mulawa, P.; He, K.; Ye, B. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 2002, 36, 4223–4234. [Google Scholar] [CrossRef]
- Zhao, M.; Qiao, T.; Huang, Z.; Zhu, M.; Xu, W.; Xiu, G. Comparison of ionic and carbonaceous compositions of PM2.5 in 2009 and 2012 in Shanghai, China. Sci. Total Environ. 2015, 536, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawei, Z.; Larssen, T.; Dongbao, Z.; Shidong, G.; Vogt, R.; Seip, H.; Lund, O. Acid deposition and acidification of soil and water in the Tie Shan Ping area, Chongqing, China. Water Air Soil Pollut. 2001, 130, 1733–1738. [Google Scholar] [CrossRef]
- Pan, Y.-P.; Zhu, X.-Y.; Tian, S.-L.; Wang, L.-L.; Zhang, G.-Z.; Zhou, Y.-B.; Xu, P.; Hu, B.; Wang, Y.-S. Wet deposition and scavenging ratio of air pollutants during an extreme rainstorm in the North China Plain. Atmos. Ocean. Sci. Lett. 2017, 10, 348–353. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. GB 3838-2002 Environmental Quality Standard for Surface Water; China Environmental Science Press: Beijing, China, 2002.
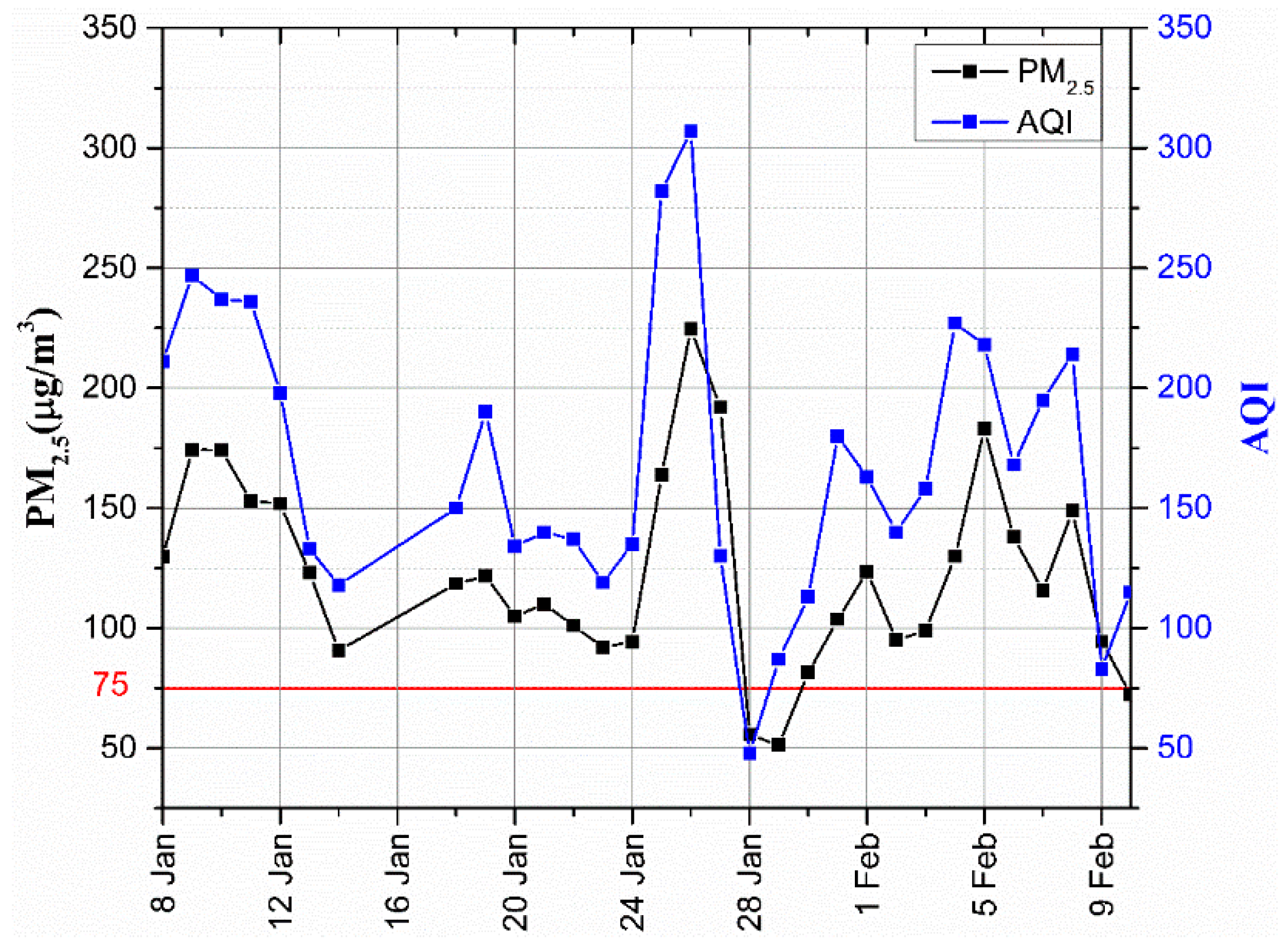

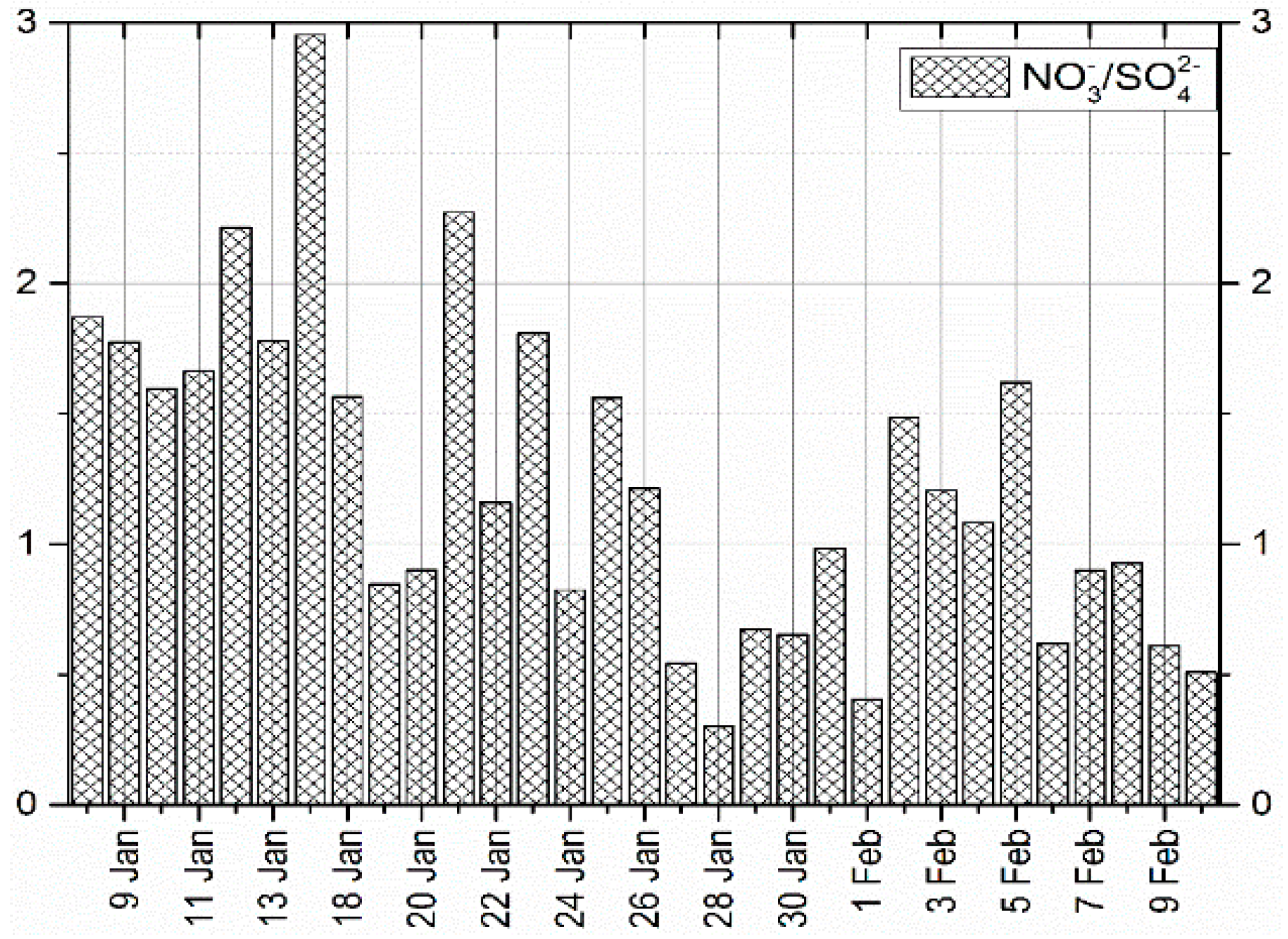
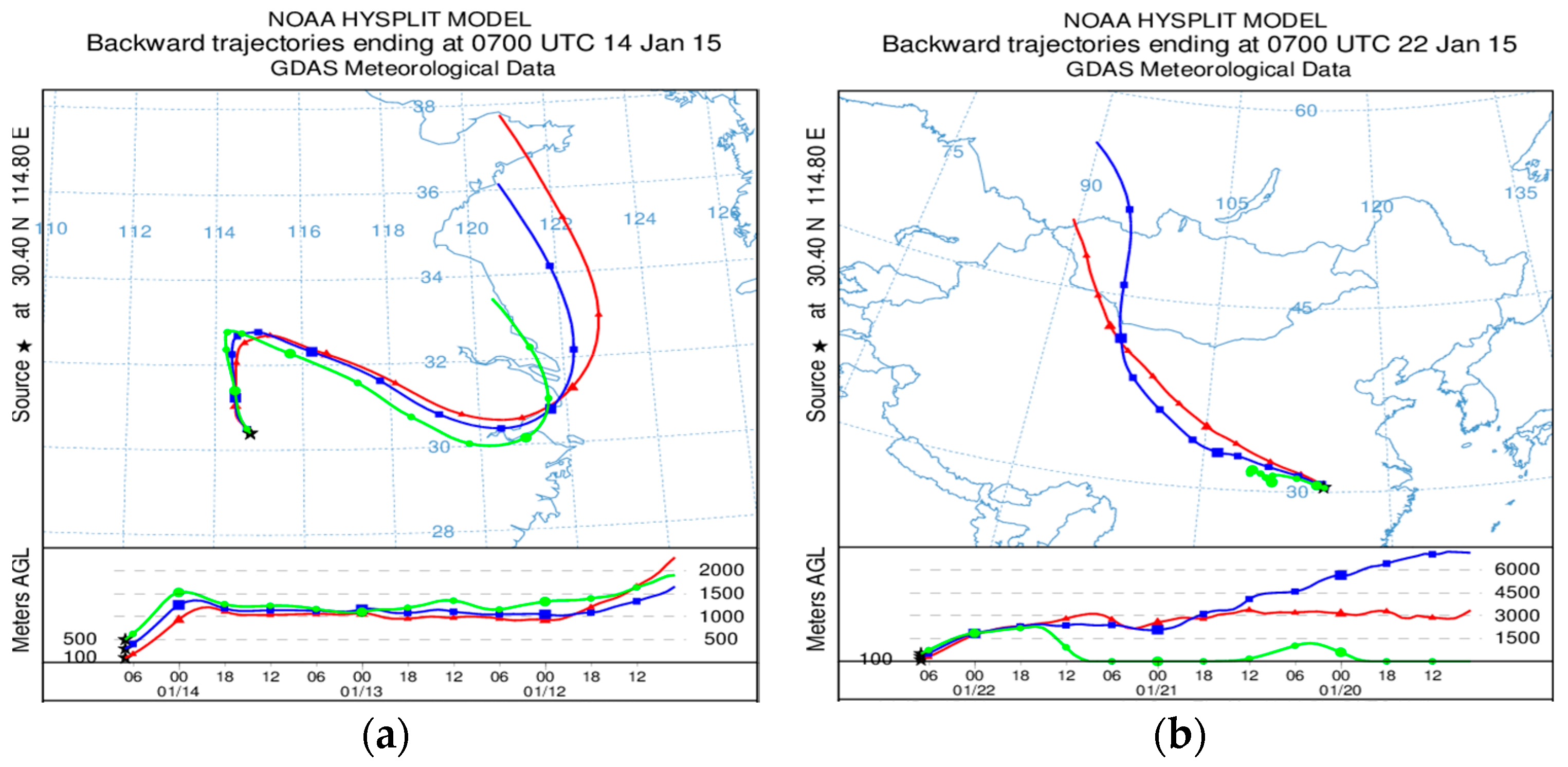


| Air Quality Index (AQI) | Air Quality Index Level | Air Quality Index Category |
|---|---|---|
| 0–50 | Level 1 | Excellent |
| 51–100 | Level 2 | Good |
| 101–150 | Level 3 | Slightly polluted |
| 151–200 | Level 4 | Moderate polluted |
| 201–300 | Level 5 | Heavy pollution |
| >300 | Level 6 | Severe pollution |
| Components | PM2.5 | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | 1 | 0.33 | 0.47 | 0.39 | 0.26 | 0.30 | 0.18 | 0.04 | 0.40 |
| Cl− | 1 | 0.69 | 0.49 | 0.04 | 0.03 | 0.51 | 0.12 | 0.76 | |
| NO3− | 1 | 0.71 | 0.21 | 0.05 | 0.53 | 0.09 | 0.71 | ||
| SO42− | 1 | 0.08 | 0.10 | 0.26 | 0.13 | 0.40 | |||
| Ca2+ | 1 | 0.22 | 0.11 | 0.21 | 0.17 | ||||
| Mg2+ | 1 | 0.18 | 0.14 | 0.07 | |||||
| K+ | 1 | 0.18 | 0.55 | ||||||
| Na+ | 1 | 0.37 | |||||||
| NH4+ | 1 |
| Precipitation | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ |
|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| 24 January | 4.07 | 10.21 | 10.39 | 3.69 | 0.63 | 1.13 | 1.21 | 0.81 |
| 26 January | 4.75 | 22.16 | 28.41 | 2.30 | 0.32 | 1.05 | 0.74 | 3.29 |
| 28 January | 4.57 | 12.22 | 14.10 | 0.99 | 0.9 | 0.02 | 0.49 | 1.63 |
| 1 February | 3.22 | 3.00 | 4.56 | 0.79 | 0.04 | BDL | 0.74 | 1.39 |
| 2 February | 3.32 | 2.34 | 4.07 | 3.54 | 0.38 | 0.36 | 1.10 | 0.30 |
| Date | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ | PM2.5 Concentration |
|---|---|---|---|---|---|---|---|---|---|
| 23 January 2015–28 January 2015 | 0.8 | 23.44 | 12.96 | 6.66 | 0.16 | 1.66 | 0.77 | 3.63 | 91.95 |
| 2.08 | 5.45 | 10.05 | 5.36 | 0.05 | 0.21 | 0.18 | 3.10 | 55.61 | |
| Difference | −1.28 | 17.99 | 2.91 | 1.30 | 0.11 | 1.45 | 0.59 | 0.53 | 36.34 |
| Removal efficiency | - | 77% | 22% | 19% | 70% | 87% | 77% | 14% | 40% |
| 31 January 2015–2 February 2015 | 2.37 | 17.58 | 17.89 | 0.73 | 0.05 | 0.83 | 0.29 | 4.90 | 103.67 |
| 0.45 | 7.57 | 18.74 | 0.72 | 0.05 | 0.67 | 0.45 | 3.94 | 95.04 | |
| Difference | 1.92 | 10.01 | −0.85 | 0.01 | 0.00 | 0.16 | −0.16 | 0.96 | 8.63 |
| Removal efficiency | 82% | 57% | - | 2% | - | 18% | - | 20% | 8% |
| Components | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ |
|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| Mean | 3.41 | 5.14 | 6.37 | 3.10 | 0.23 | 0.34 | 1.36 | 2.05 |
| Standard deviation | 0.16 | 3.04 | 1.66 | 1.06 | 0.09 | 0.35 | 0.86 | 0.68 |
| Monthly Deposition Flux (mg·m−2·month−1) | |||||||
|---|---|---|---|---|---|---|---|
| Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ |
| 804.17 | 1549.82 | 1912.17 | 585.28 | 55.03 | 84.81 | 422.20 | 422.93 |
| Date | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ | Sum of Cations/Anions |
|---|---|---|---|---|---|---|---|---|---|
| meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | ||
| 24 January | 0.12 | 0.17 | 0.22 | 0.18 | 0.05 | 0.03 | 0.05 | 0.05 | 0.71 |
| 26 January | 0.13 | 0.36 | 0.59 | 0.12 | 0.03 | 0.03 | 0.03 | 0.18 | 0.36 |
| 28 January | 0.13 | 0.20 | 0.29 | 0.05 | 0.01 | 0.01 | 0.02 | 0.09 | 0.29 |
| 1 February | 0.09 | 0.05 | 0.10 | 0.04 | 0.01 | - | 0.03 | 0.08 | 0.67 |
| 2 February | 0.09 | 0.04 | 0.09 | 0.18 | 0.03 | 0.01 | 0.05 | 0.02 | 1.32 |
| Date | Cl− | NO3− | SO42− | Ca2+ | Mg2+ | K+ | Na+ | NH4+ | Sum of Cations/Anions |
|---|---|---|---|---|---|---|---|---|---|
| meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | ||
| 8–12 January | 0.09 | 0.04 | 0.10 | 0.15 | 0.02 | 0.01 | 0.04 | 0.07 | 1.26 |
| 12–16 January | 0.10 | 0.07 | 0.14 | 0.16 | 0.02 | 0.01 | 0.03 | 0.15 | 1.19 |
| 16–20 January | 0.09 | 0.06 | 0.14 | 0.22 | 0.02 | 0.03 | 0.05 | 0.15 | 1.62 |
| 20–24 January | 0.09 | 0.08 | 0.18 | 0.16 | 0.01 | 0.01 | 0.13 | 0.06 | 1.05 |
| 28 January–1 February | 0.09 | 0.03 | 0.09 | 0.08 | 0.01 | 0.01 | 0.03 | 0.12 | 1.19 |
| 2–6 February | 0.10 | 0.17 | 0.15 | 0.10 | 0.01 | 0.00 | 0.04 | 0.11 | 0.62 |
| 6–10 February | 0.10 | 0.13 | 0.19 | 0.22 | 0.03 | 0.01 | 0.08 | 0.14 | 1.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Mbululo, Y.; Yang, M.; Yuan, Z.; Nyihirani, F.; Zheng, X. Chemical Composition and Deposition Fluxes of Water-Soluble Inorganic Ions on Dry and Wet Deposition Samples in Wuhan, China. Int. J. Environ. Res. Public Health 2019, 16, 132. https://doi.org/10.3390/ijerph16010132
Qin J, Mbululo Y, Yang M, Yuan Z, Nyihirani F, Zheng X. Chemical Composition and Deposition Fluxes of Water-Soluble Inorganic Ions on Dry and Wet Deposition Samples in Wuhan, China. International Journal of Environmental Research and Public Health. 2019; 16(1):132. https://doi.org/10.3390/ijerph16010132
Chicago/Turabian StyleQin, Jun, Yassin Mbululo, Muyi Yang, Zhengxuan Yuan, Fatuma Nyihirani, and Xiang Zheng. 2019. "Chemical Composition and Deposition Fluxes of Water-Soluble Inorganic Ions on Dry and Wet Deposition Samples in Wuhan, China" International Journal of Environmental Research and Public Health 16, no. 1: 132. https://doi.org/10.3390/ijerph16010132





