In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
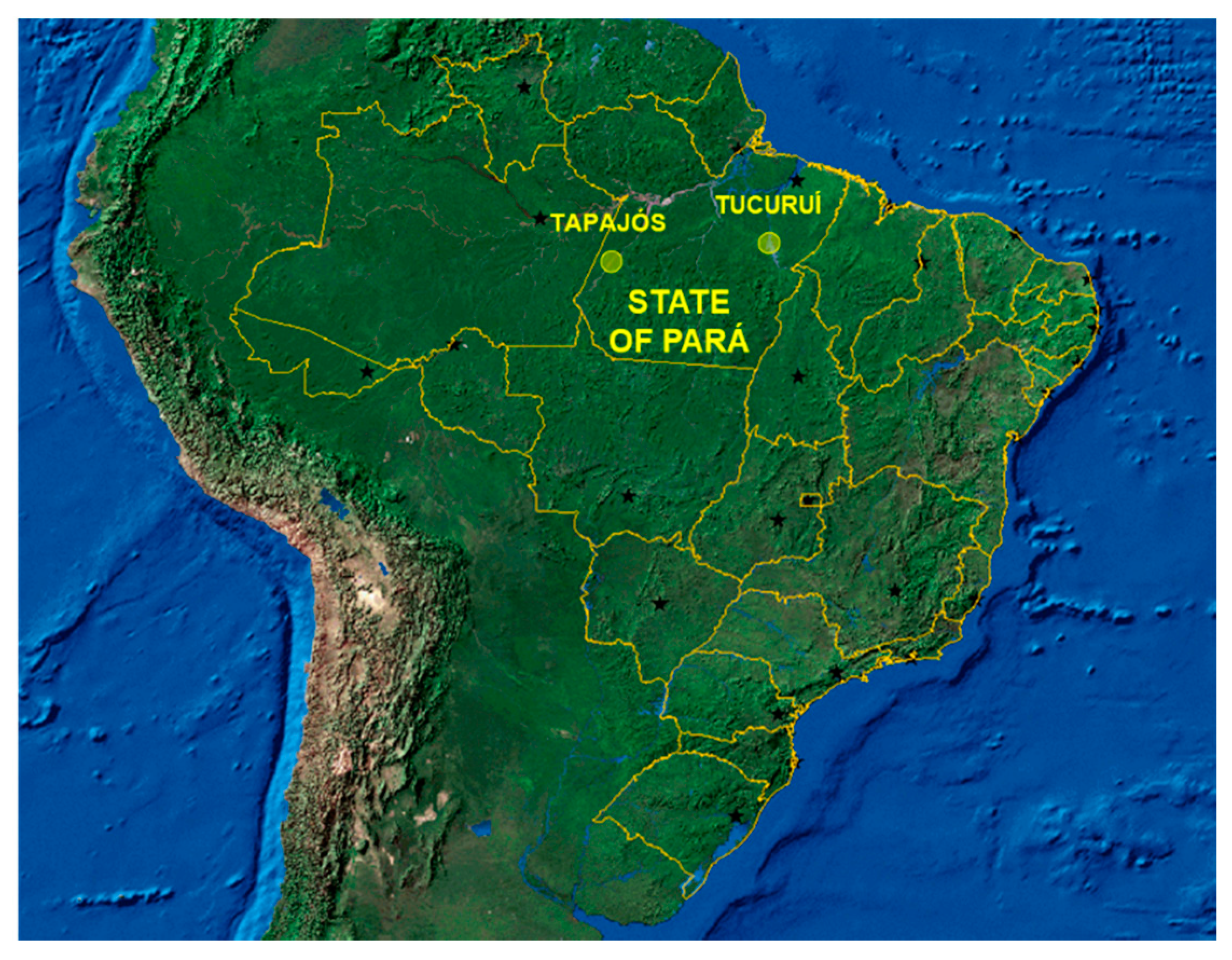
2.2. Study Population and Inclusion/Exclusion Criteria
2.3. Sample and Data Collection
2.4. Detection of the Apolipoprotein E4 Genotype (APOE4)
2.5. Statistical Analysis
3. Results
3.1. Analysis 1—NCDs in the Total Population
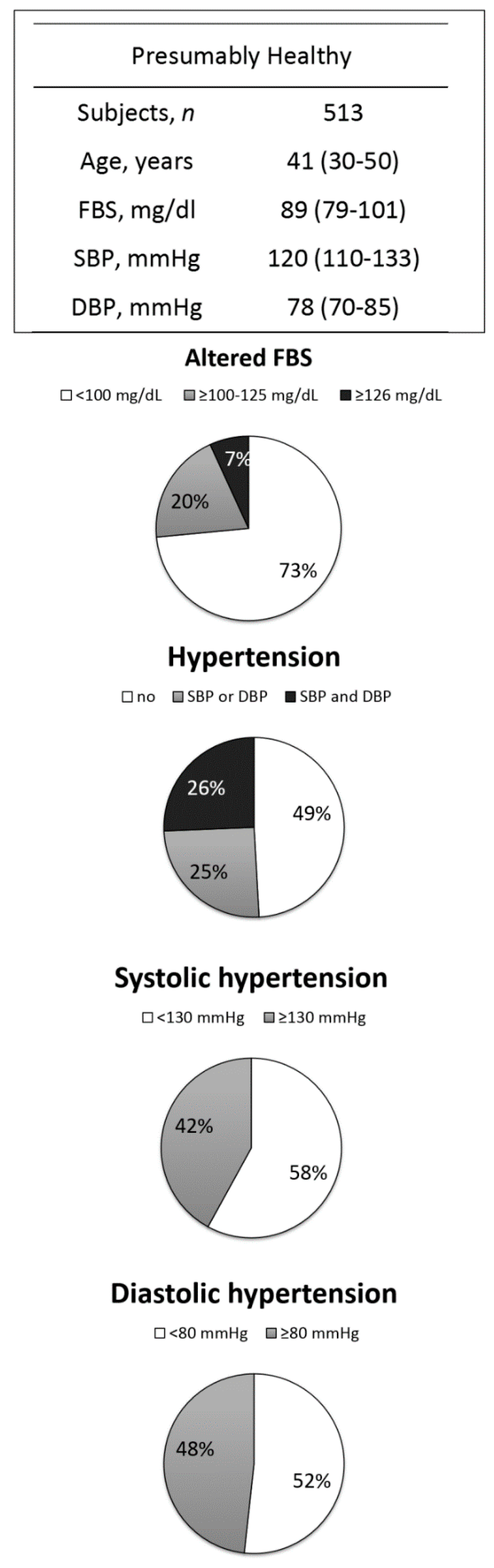
3.2. Analysis 2—NCDs in the ‘Presumably Healthy’ Sub-Group
3.3. Analysis 3—Genetic Risk Factor (APOE4) for NCDs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IBGE. 2016. Available online: http://www.ibge.gov.br/home/estatistica/populacao/estimativa2016/default.shtm (accessed on 25 July 2018).
- Muzaka, V. Lessons from Brazil: On the difficulties of building a universal health care system. J. Glob. Health 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Human Development Atlas in Brazil. Brazil 2013. Available online: http://atlasbrasil.org.br/2013/en/home/ (accessed on 25 July 2018).
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Fernandez-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Alvarez-Leite, J.; Do Nascimento, J.L.M.; Amador, M.; et al. Genetic susceptibility to neurodegeneration in Amazon: Apolipoprotein E genotyping in vulnerable populations exposed to mercury. Front. Genet. 2018. [Google Scholar] [CrossRef]
- Piperata, B.A.; Spence, J.E.; Da-Gloria, P.; Hubbe, M. The nutrition transition in Amazonia: Rapid economic change and its impact on growth and development in Ribeirinhos. Am. J. Phys. Anthropol. 2011, 146, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Richtel, M. How Big Business Got Brazil hooked on Junk Food. New York Times, 16 September 2017. Available online: https://www.nytimes.com/interactive/2017/09/16/health/brazil-obesity-nestle.html (accessed on 25 July 2018).
- World Health Organization. Noncommunicable Diseases. Fact Sheet; WHO: Geneva, Switzerland, 2017; Available online: http://www.who.int/mediacentre/factsheets/fs355/en/ (accessed on 25 July 2018).
- Freitas, R.G.; Campana, E.M.; Pozzan, R.; Brandao, A.A.; Brandao, A.P.; Magalhaes, M.E.; Silva, D.A. Apoe and ldlr gene polymorphisms and dyslipidemia tracking. Rio de Janeiro study. Arq. Bras. Cardiol. 2015, 104, 468–474. [Google Scholar] [CrossRef] [PubMed]
- El-Lebedy, D.; Raslan, H.M.; Mohammed, A.M. Apolipoprotein e gene polymorphism and risk of type 2 diabetes and cardiovascular disease. Cardiovasc. Diabetol. 2016, 15. [Google Scholar] [CrossRef]
- Midorikawa, K.; Soukaloun, D.; Akkhavong, K.; Southivong, B.; Rattanavong, O.; Sengkhygnavong, V.; Pyaluanglath, A.; Sayasithsena, S.; Nakamura, S.; Midorikawa, Y.; et al. Apoe genotype in the ethnic majority and minority groups of laos and the implications for non-communicable diseases. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; de Oliveira, M.A.; Souza-Monteiro, J.R.; Paraense, R.O.; Ribeiro-Dos-Santos, A.; Vieira, J.; Silva, A.; Macchi, B.M.; do Nascimento, J.L.M.; Burbano, R.M.R.; et al. Role for apolipoprotein e in neurodegeneration and mercury intoxication. Front. Biosci. 2018, 10, 229–241. [Google Scholar]
- Mahley, R.W. Apolipoprotein e: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.K.; Khan, I.A.; Syed, R. Association of apolipoprotein e polymorphism with type 2 diabetes mellitus in a saudi population. DNA Cell Biol. 2014, 33, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Z.; Wu, X.; Shu, Y.; Lu, D. Apolipoprotein e polymorphism is associated with lower extremity deep venous thrombosis: Color-flow doppler ultrasound evaluation. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dhanasekaran, P.; Alexander, E.T.; Rader, D.J.; Phillips, M.C.; Lund-Katz, S. Molecular mechanisms responsible for the differential effects of apoe3 and apoe4 on plasma lipoprotein-cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Liu, C.C.; Van Ingelgom, A.J.; Martens, Y.A.; Linares, C.; Knight, J.A.; Painter, M.M.; Sullivan, P.M.; Bu, G. Apolipoprotein e4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 2017, 96, 115–129. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Noncommunicable Diseases (NCD) Country Profiles; WHO: Geneva, Switzerland, 2014; Available online: http://apps.who.int/iris/bitstream/10665/128038/1/9789241507509_eng.pdf (accessed on 25 July 2018).
- Vigitel. Vigitel Brasil 2016 Vigilância de Fatores de Risco e Proteção Para Doenças Crônicas Por Inquérito Telefônico. Brazil: Ministério da Saúde; 2016. Available online: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/leia-mais-o-ministerio/673-secretaria-svs/vigilancia-de-a-a-z/doencas-cronicas-nao-transmissiveis/l2-doencas-cronicas-nao-transmissiveis/14128-vigitel-2006-a-2013 (accessed on 25 July 2018).
- BRASIL. A Vigilância, o Controle e a Prevenção das Doenças Crônicas Não-Transmissíveis: Dcnt No Contexto do Sistema Único de Saúde Brasileiro; Ministério Da Saúde/Opas/Oms: Brasilia, Brazil, 2005. [Google Scholar]
- Coutinho, W.F.; Silva Junior, W.S. Diabetes care in brazil. Ann. Glob. Health 2015, 81, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. 2. Classification and diagnosis of diabetes. Diabetes Care 2016, 39 (Suppl. 1), S13–S22. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.J.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Azevedo e Silva, G.; Menezes, A.M.; Monteiro, C.A.; Barreto, S.M.; Chor, D.; Menezes, P.R. Chronic non-communicable diseases in Brazil: Burden and current challenges. Lancet 2011, 377, 1949–1961. [Google Scholar] [CrossRef]
- Baena, C.P. Doença Cardiovascular: Tendência de Mortalidade No Brasil e Prevenção Global; Pontifical Catholic University of Paraná (PUCPR): Curitiba, Brazil, 2013. [Google Scholar]
- Leino, T.; Lodenius, M. Human hair mercury levels in Tucurui area, State of Para, Brazil. Sci. Total Environ. 1995, 175, 119–125. [Google Scholar] [CrossRef]
- Fillion, M.; Mergler, D.; Sousa Passos, C.J.; Larribe, F.; Lemire, M.; Guimaraes, J.R. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ. Health Glob. Access Sci. Source 2006, 5. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.C.; Crespo-Lopez, M.E.; Vieira, J.L.; Oikawa, T.; Guimaraes, G.A.; Araujo, C.C.; Amoras, W.W.; Ribeiro, D.R.; Herculano, A.M.; do Nascimento, J.L.; et al. Mercury pollution and childhood in Amazon riverside villages. Environ. Int. 2007, 33, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.C.; Macchi, B.M.; Vieira, J.L.; Oikawa, T.; Amoras, W.W.; Guimaraes, G.A.; Costa, C.A.; Crespo-Lopez, M.E.; Herculano, A.M.; Silveira, L.C.; et al. Mercury exposure and antioxidant defenses in women: A comparative study in the Amazon. Environ. Res. 2008, 107, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.C.; Oikawa, T.; Vieira, J.L.; Gomes, M.S.; Guimaraes, G.A.; Crespo-Lopez, M.E.; Muller, R.C.; Amoras, W.W.; Ribeiro, D.R.; Rodrigues, A.R.; et al. Comparative study of human exposure to mercury in riverside communities in the Amazon region. Braz. J. Med. Biol. Res. 2006, 39, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lopez, M.E.; Macedo, G.L.; Arrifano, G.P.; Pinheiro Mda, C.; do Nascimento, J.L.; Herculano, A.M. Genotoxicity of mercury: Contributing for the analysis of Amazonian populations. Environ. Int. 2011, 37, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.D.; Souza Gda, S.; Silveira, L.C.; Costa, C.A.; Araujo, A.A.; Pinheiro Mda, C. Neurological manifestations in riverine populations from areas exposed to mercury in the Brazilian Amazon. Cad. Saude Publ. 2013, 29, 2307–2318. [Google Scholar] [CrossRef]
- Faial, K.; Deus, R.; Deus, S.; Neves, R.; Jesus, I.; Santos, E.; Alves, C.N.; Brasil, D. Mercury levels assessment in hair of riverside inhabitants of the Tapajos river, Para State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015, 30, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Franca, S.L.; Lima, S.S.; Vieira, J.R. Metabolic syndrome and associated factors in adults of the Amazon region. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Ramirez-Mateos, V.; da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the Amazon and human exposure to mercury: The case-study of the tucurui dam. Ecotoxicol. Environ.Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Valentini, J.; Passos, C.J.S.; Garcia, S.C.; Davidson, R.; Lucotte, M.; Mertens, F.; Romana, C.; Valadão, L.M.; Charão, M.F.; Baierle, M.; et al. Blood antioxidant nutrients in riparian villagers of the Brazilian Amazon: Its associations with wet/dry seasons and modulation by sociodemographic determinants. Cad. Saúde Colet. 2016, 24, 21–31. [Google Scholar] [CrossRef]
- Krewer, C.C.; Ribeiro, E.E.; Ribeiro, E.A.; Moresco, R.N.; da Rocha, M.I.; Montagner, G.F.; Machado, M.M.; Viegas, K.; Brito, E.; da Cruz, I.B. Habitual intake of guarana and metabolic morbidities: An epidemiological study of an elderly Amazonian population. Phytother. Res. 2011, 25, 1367–1374. [Google Scholar]
- Gomes, R.; Nascimento, E.F.; Araujo, F.C. Why do men use health services less than women? Explanations by men with low versus higher education. Cad. Saude Publ. 2007, 23, 565–574. [Google Scholar] [CrossRef]
- Anish, T.S.; Shahulhameed, S.; Vijayakumar, K.; Joy, T.M.; Sreelakshmi, P.R.; Kuriakose, A. Gender difference in blood pressure, blood sugar, and cholesterol in young adults with comparable routine physical exertion. J. Fam. Med. Prim. Care 2013, 2, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Meng, F.C.; Shih, Y.L.; Su, F.Y.; Lin, Y.P.; Lin, F.; Lin, J.W.; Chang, W.K.; Lee, C.J.; Li, Y.H.; et al. Sex-specific association between metabolic abnormalities and elevated alanine aminotransferase levels in a military cohort: The chief study. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Hadi, A.; Razzaque, A.; Ashraf, A.; Juvekar, S.; Ng, N.; Kanungsukkasem, U.; Soonthornthada, K.; Van Minh, H.; Huu Bich, T. Clustering of chronic non-communicable disease risk factors among selected Asian populations: Levels and determinants. Glob. Health Action 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dai, J. BMI better explains hypertension in Chinese senior adults and the relationship declines with age. Aging Clin. Exp. Res. 2015, 27, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gabb, G.M.; Mangoni, A.A.; Anderson, C.S.; Cowley, D.; Dowden, J.S.; Golledge, J.; Hankey, G.J.; Howes, F.S.; Leckie, L.; Perkovic, V.; et al. Guideline for the diagnosis and management of hypertension in adults 2016. Med. J. Aust. 2016, 205, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.F.; Mourao Dde, S.; Gomes, N.; Costa, J.M.; Souza, A.V.; Bastos, W.R.; Fonseca Mde, F.; Mariani, C.F.; Abbad, G.; Hacon, S.S. Prevalence of arterial hypertension in communities along the madeira river, western Brazilian Amazon. Cad. Saude Publ. 2013, 29, 1617–1630. [Google Scholar] [CrossRef]
- Singh, P.P.; Singh, M.; Mastana, S.S. Apoe distribution in world populations with new data from India and the UK. Ann. Hum. Biol. 2006, 33, 279–308. [Google Scholar] [CrossRef] [PubMed]
- De Franca, E.; Alves, J.G.; Hutz, M.H. Apolipoprotein e polymorphism and its association with serum lipid levels in Brazilian children. Hum. Biol. 2004, 76, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.R.; Nakachima, L.; Biagioni, R.B.; Nakazone, M.A.; Pinhel, M.A.; Trindade, D.M.; Mafra, V.T.; Tacito, L.H.; Martin, J.F.; Pinheiro Junior, S.; et al. Relevance of apolipoprotein e4 for the lipid profile of brazilian patients with coronary artery disease. Braz. J. Med. Biol. Res. 2007, 40, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.C.; Costa, T.F.; Aguiar, S.L.; Marques, A.R.; Ramos, S.A.; Gomes, K.B.; Alvarez-Leite, J.I. Association of apoliprotein e polymorphisms and metabolic syndrome in subjects with extreme obesity. Clin. Chim. Acta 2011, 412, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Sery, O.; Hlinecka, L.; Balcar, V.J.; Janout, V.; Povova, J. Diabetes, hypertension and stroke-Does alzheimer protect you? Neuro Endocrinol. Lett. 2014, 35, 691–696. [Google Scholar] [PubMed]
- Pavan, L.; Casiglia, E.; Braga, L.M.; Winnicki, M.; Puato, M.; Pauletto, P.; Pessina, A.C. Effects of a traditional lifestyle on the cardiovascular risk profile: The amondava population of the Brazilian Amazon. Comparison with matched African, Italian and Polish populations. J. Hypertens. 1999, 17, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Berzas Nevado, J.J.; Rodriguez Martin-Doimeadios, R.C.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Mercury in the Tapajos river basin, Brazilian Amazon: A review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Martin-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Arrifano, G.P.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ.Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef] [PubMed]
- Isaac, V.J.; Almeida, M.C.; Giarrizzo, T.; Deus, C.P.; Vale, R.; Klein, G.; Begossi, A. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An. Acad. Bras. Cienc. 2015, 87, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury exposure and heart diseases. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of mercury in cardiovascular disease. J. Cardiovasc. Dis. Diagn. 2014, 2. [Google Scholar] [CrossRef]
- Valera, B.; Dewailly, E.; Poirier, P. Cardiac autonomic activity and blood pressure among nunavik inuit adults exposed to environmental mercury: A cross-sectional study. Environ. Health Glob. Access Sci. Source 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Paraense, R.; Machado, C.R.; Farina, M.; Macchi, B.; do Nascimento, J.L.M.; et al. Assessing mercury intoxication in isolated/remote populations: Increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology 2018, 68, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gugelmin, S.A.; Santos, R.V. Human ecology and nutritional anthropometry of adult xavante Indians in mato grosso, Brazil. Cad. Saude Publ. 2001, 17, 313–322. [Google Scholar] [CrossRef]
- Lindgarde, F.; Ercilla, M.B.; Correa, L.R.; Ahren, B. Body adiposity, insulin, and leptin in subgroups of peruvian Amerindians. High Altit. Med. Biol. 2004, 5, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Benefice, E.; Lopez, R.; Monroy, S.L.; Rodriguez, S. Fatness and overweight in women and children from riverine amerindian communities of the Beni River (Bolivian Amazon). Am. J. Hum. Biol. 2007, 19, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, A.D.; Pryor, G., 3rd; Pozo, J.; Tiwia, W.; Sugiyama, L.S. Growth and market integration in Amazonia: A comparison of growth indicators between shuar, shiwiar, and nonindigenous school children. Am. J. Hum. Biol. 2009, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.R.; Ferreira, A.A.; Santos, R.V.; Gugelmin, S.A.; Werneck, G.; Coimbra, C.E.A. Nutrition transition, socioeconomic differentiation, and gender among adult xavante Indians, Brazilian Amazon. Hum. Ecol. 2009, 37, 13–26. [Google Scholar] [CrossRef]


| Characteristics | Total | Sex | Gender Difference p-Value | |
|---|---|---|---|---|
| Women | Men | |||
| Subjects, n (%) | 763 (100.0) | 487 (63.8) | 276 (36.2) | <0.0001 a |
| Age, years | 47 (34–57) | 44 (31–55) | 51 (40–62) | <0.0001 b |
| Height, cm | 155 (151–162) | 152 (148–156) | 164 (158–169) | <0.0001 b |
| Weight, kg | 64.3 (56.0–74.6) | 61.7 (53.7–71.0) | 69.3 (60.1–78.3) | <0.0001 b |
| BMI, kg/m2 | 26.0 (23.3–29.6) | 26.7 (23.3–30.4) | 25.5 (23.3–28.5) | 0.0032 b |
| FBS, mg/dL | 90 (81–103) | 90 (81–106) | 90 (80–100) | ns b |
| SBP, mmHg | 125 (113–139) | 121 (110–137) | 129 (120–142) | <0.0001 b |
| DBP, mmHg | 79 (70–88) | 77 (69–86) | 80 (73–89) | <0.0001 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrifano, G.P.F.; Alvarez-Leite, J.I.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.; Macchi, B.M.; Pinto, A.; Oriá, R.B.; Do Nascimento, J.L.M.; Crespo-Lopez, M.E. In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population. Int. J. Environ. Res. Public Health 2018, 15, 1957. https://doi.org/10.3390/ijerph15091957
Arrifano GPF, Alvarez-Leite JI, Souza-Monteiro JR, Augusto-Oliveira M, Paraense R, Macchi BM, Pinto A, Oriá RB, Do Nascimento JLM, Crespo-Lopez ME. In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population. International Journal of Environmental Research and Public Health. 2018; 15(9):1957. https://doi.org/10.3390/ijerph15091957
Chicago/Turabian StyleArrifano, Gabriela P. F., Jacqueline I. Alvarez-Leite, José Rogério Souza-Monteiro, Marcus Augusto-Oliveira, Ricardo Paraense, Barbarella M. Macchi, André Pinto, Reinaldo B. Oriá, José Luiz Martins Do Nascimento, and Maria Elena Crespo-Lopez. 2018. "In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population" International Journal of Environmental Research and Public Health 15, no. 9: 1957. https://doi.org/10.3390/ijerph15091957
APA StyleArrifano, G. P. F., Alvarez-Leite, J. I., Souza-Monteiro, J. R., Augusto-Oliveira, M., Paraense, R., Macchi, B. M., Pinto, A., Oriá, R. B., Do Nascimento, J. L. M., & Crespo-Lopez, M. E. (2018). In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population. International Journal of Environmental Research and Public Health, 15(9), 1957. https://doi.org/10.3390/ijerph15091957








