Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years
Abstract
:1. Introduction
2. Materials and Methods
- Healthy volunteers not occupationally exposed to phthalates;
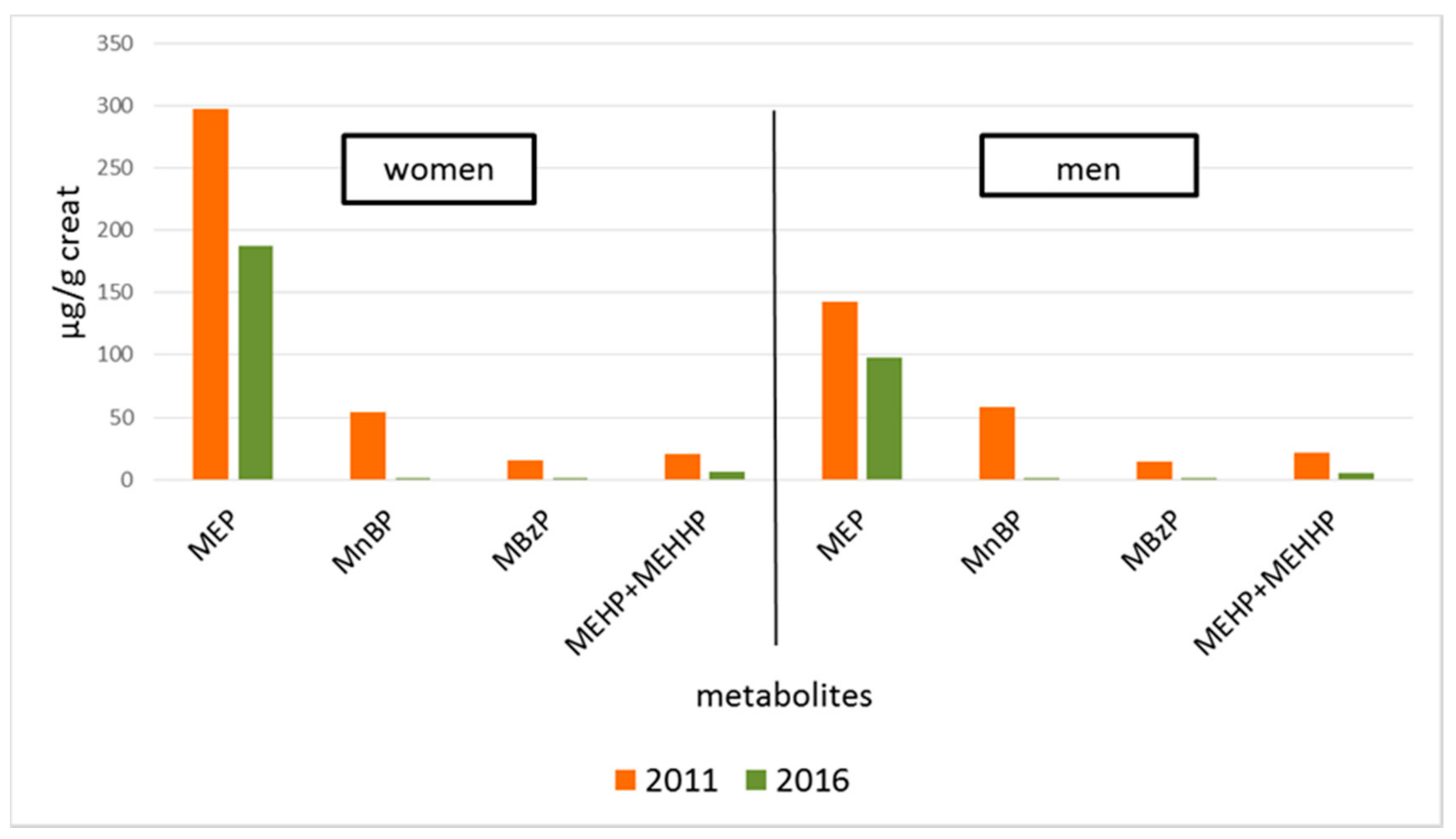
3. Results
4. Discussion
4.1. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.; Ingerman, L.; Gray, D.A.; Little, S.; Amata, R. Toxicological Profile for n-butylphthalate. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=859&tid=167 (accessed on 31 August 2018).
- Richardson, M.; Bosh, S.; Swarts, S.; Llados, S.; Gray, F. Toxicological Profile for di(2-ethylexyl)phthalate (DEHP). Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=684&tid=65 (accessed on 31 August 2018).
- Houliham, J.; Brody, C.; Schwan, B. Not too Pretty; Phthalates, Beauty Products & the FDA. Available online: https://noharm-asia.org/sites/default/files/documents-files/110/Not_Too_Pretty.pdf (accessed on 20 June 2018).
- Nassan, F.L.; Coull, B.A.; Skakkebaek, N.E.; Andersson, A.M.; Williams, M.A.; Minques-Alarcon, L.; Krawets, S.A.; Hall, J.E.; Hait, E.J.; Korzenik, J.R.; et al. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and serum reproductive hormones in men. Environ. Res. 2018, 160, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Coull, B.A.; Skakkebaek, N.E.; Williams, M.A.; Dadd, R.; Minques-Alarcon, L.; Krawets, S.A.; Hait, E.J.; Korzenik, J.R.; Moss, A.C.; et al. A crossover–crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ. Int. 2016, 95, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Lorz, P.M.; Towae, F.K.; Enke, W.; Jäckh, R.; Bhargava, N.; Hillesheim, W. Phthalic Acid and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eroksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Main, K.M.; Mortensen, G.K.; Kaleva, M.M.; Boisen, K.A.; Damgaard, I.N.; Chellakooty, M.; Schmidt, I.M.; Suomi, A.M.; Virtanen, H.E.; Petersen, J.H.; et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006, 114, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.H.; Breindahl, T. Plasticizers in total diet samples, baby food and infant formulae. Food Addit. Contam. 2000, 17, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, G.K.; Main, K.M.; Andersson, A.M.; Leffers, H.; Skakkebæk, N.E. Determination of phthalates monoesters in human milk, consumer milk, and infant formula by tandem mass spectrometry (LC-MS-MS). Anal. Bioanal. Chem. 2005, 382, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Bosnir, J.; Puntaric, D.; Skes, I.; Klaric, M.; Simkic, S.; Zoric, I. Migration of phthalates from plastic products to model solutions. Coll. Antropol. 2003, 27, 23–30. [Google Scholar] [PubMed]
- Becker, K.; Seiwert, M.; Angerer, J.; Heger, W.; Koch, H.M.; Nagorka, R.; Rosskamp, E.; Schlüter, C.; Seifert, B.; Ullrich, D. DEHP metabolites in urine of children and DEHP in house dust. Int. J. Hyg. Environ. Health 2004, 207, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Jarfelt, K.; Dalgaard, M.; Hass, U.; Borch, J.; Jacobsen, H.; Ladefoged, O. Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl)phthalate and di(2-ethylhexyl)adipate. Reprod. Toxicol. 2005, 19, 505–515. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.H.; Butala, J.H.; David, R.M.; Gans, G. NTP center for the evaluation of risks to human reproduction reports on phthalates: Addressing the data gaps. Reprod. Toxicol. 2004, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Calafat, A. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Soto, B.D.; Lacroix, G.M. The E-screen assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. 1995, 103, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrillo, L.; Hernandez-Ramirez, R.U.; Calafat, A.M.; Torres-Sanchez, L.; Galvan-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrian, M.E. Exposure to phthalates and breast cancer. Risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program, Center for the Evaluation of Risks to Human Reproduction. NTP-CERHR Expert Panel Report on di-Isononylphthalate. Available online: http://cerhr.niehs.nih.gov/news/index.html (accessed on 31 August 2018).
- Koch, H.M.; Rossbach, B.; Drexler, H.; Angerer, J. Internal exposure of the general population to DEHP and other phthalates determination of secondary and primary phthalate monoester metabolites in urine. Environ. Res. 2003, 93, 177–185. [Google Scholar] [CrossRef]
- Barr, D.B.; Silva, M.J.; Kato, K.; Reidy, J.A.; Malek, N.A.; Hurtz, D.; Sadowski, M.; Needham, L.L.; Calafat, A.M. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ. Health Perspect. 2003, 111, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 112, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Samandar, E.; Preau, J.L.J.; Needham, L.L.; Calafat, A.M. Urinary oxidative metabolites of di(2-ethylhexyl)phthalate in humans. Toxicology 2006, 219, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Kato, K.; Malek, N.A.; Hodge, C.C.; Hurtz, D.; Calafat, A.M.; Needham, L.L.; Brock, J.W. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 2003, 77, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Latini, G. Monitoring phthalate exposure in humans. Clin. Chim. Acta 2005, 361, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Drexler, H.; Angerer, J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int. J. Hyg. Environ. Health 2004, 207, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; Perera, F.P.; Jedrychowski, W.; Camann, D.E.; Barr, D.; Jacek, R.; Whyatt, R.M. Prenatal exposures to phthalates in New York City and Krakow, Poland. Environ. Health Perspect. 2003, 111, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Caporossi, L.; Paci, E.; Aragona, C.; Romanzi, C.; De Carolis, C.; De Rosa, M.; Capanna, S.; Papaleo, B.; Pera, A. Urinary phthalates monoesters concentration in couples with infertility problems. Toxicol. Lett. 2012, 213, 15–20. [Google Scholar] [CrossRef] [PubMed]
- European Chemical Agency. Chemicals Database. Available online: https://echa.europa.eu (accessed on 20 June 2018).
- Kurata, Y.; Shimamura, N.; Katoh, M. Metabolite profiling and identification in human urine after single oral administration of DEHP. J. Toxicol. Sci. 2012, 37, 401–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, S.; Shin, M.Y.; Kim, K.N.; Hong, Y.C. Risk assessment for phthalate exposures in the elderly: A repeated biomonitoring study. Sci. Total Environ. 2018, 618, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Christensen, K.L.; Harth, V.; Lorber, M.; Brüning, T. Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch. Toxicol. 2012, 86, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Eigenberg, D.A.; Bozigian, H.P.; Carter, D.E.; Sipes, I.G. Distribution, excretion, and metabolism of butylbenzyl phthalate in the rat. J. Toxicol. Environ. Health 1986, 17, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Skakkebaek, N.E.; Andresson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 7, 899–911. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO), International Programme on Chemical Safety (IPCS). Concise International Chemical Assessment Document (CICADS) n. 52: Diethyl Phthalate 2003. Available online: http://www.inchem.org/documents /cicads/cicads/cicad52.htm (accessed on 17 April 2008).
- Tranfo, G.; Papaleo, B.; Caporossi, L.; Capanna, S.; De Rosa, M.; Pigini, D.; Corsetti, F.; Paci, E. Urinary metabolite concentrations of phthalate metabolites in Central Italy healthy volunteers determined by a validated HPLC/MS/MS analytical method. Int. J. Hyg. Environ. Health 2013, 216, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Jarošova, A.; Bogdanovičova, S. Phthalates in meat products in dependence on the fat content. Potravinarstvo Slovak J. Food Sci. 2016. [Google Scholar] [CrossRef]
- David, F.; Sandra, P.; Tienpont, B.; Vanwallengem, F.; Ikonomou, M. Analysis of Phthalates in Biota (Vegetation, Milk, Fish). In The Handbook of Environmental Chemistry; Staples, C.A., Ed.; Springer: Fairfax, VA, USA, 2003. [Google Scholar]
- David, R.M. Commentary regarding the article by Koch et al.: An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 2004, 207, 75–76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- The European Workplace Drug Testing Society (EWDTS). European Guidelines for Workplace Drug Testing in Urine. Available online: http://www.ewdts.org/ewdts-guidelines.html (accessed on 26 September 2016).
- Smerieri, A.; Testa, C.; Lazzeroni, P.; Nuti, F.; Grossi, E.; Cesari, S.; Montanini, L.; Latini, G.; Bernasconi, S.; Papini, A.M.; et al. Di-(2-Ethylhexyl) Phthalate Metabolites in Urine Show Age-Related Changes and Associations with Adiposity and Parameters of Insulin Sensitivity in Childhood. PLoS ONE 2015, 10, e0117831. [Google Scholar] [CrossRef] [PubMed]
- Göen, T.; Dobler, L.; Koschorreck, J.; Müller, J.; Wiesmüller, G.A.; Drexler, H.; Lolossa-Gehring, M. Trends of the internal phthalate exposure of young adults in Germany—Follow up of a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health 2011, 215, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Gallo, F.; Dipaola, L.; De Angelis, S.; Olivieri, A. Pre-plus postnatal exposures to di-(2-ethylhexyl)-phthalate and thyroid dysfunction in prematurely born children. J. Endocrinol. Investig. 2014, 37, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on human exposure to environmental chemicals. Department of Health and Human Services. Available online: https://www.cdc.gov/biomonitoring/pdf/fourthReport_UpdatedTables_Volume1_Jan2017.pdf (accessed on 10 July 2018).
- Testa, C.; Nuti, F.; Hayek, J.; De Felice, C.; Chelli, M.; Rovero, P.; Latini, G.; Papini, A.M. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro 2012, 4, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latini, G.; Massaro, M.; Mandich, A. Prenatal plus postnatal exposures to phthalates and child health risks. J Biol. Res. 2011, 84, 60–62. [Google Scholar] [CrossRef]
- Fromme, H.; Bolte, G.; Koch, H.M.; Angerer, J.; Boehmer, S.; Drexler, H.; Mayer, R.; Liebl, B. Occurrence and daily variation of phthalate metabolites in the urine of adult population. Int. J. Hyg. Environ. Health 2007, 210, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Celnikier, D.H.; Calafat, A.M.; Needham, L.L.; Amitai, Y.; Wormser, U.; Richter, E. Phthalate exposure among pregnant women in Jerusalem, Israel: Results of a pilot study. Environ. Int. 2009, 35, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Calafat, A.M.; Woodruff, T. Temporal trends in phthalate exposure: Findings from the National Health and Nutrition examination survey, 2001–2010. Environ. Health Perspect. 2014, 122, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Valentin-Blasini, L.; Ye, X. Trends in Exposure to chemicals in personal care and consumer products. Curr. Environ. Health Rep. 2015, 2, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; De Felice, C.; Presta, G.; Del Vecchio, A.; Paris, I.; Ruggieri, F.; Mazzeo, P. Exposure to Di(2-ethylhexyl)phthalate in Humans during Pregnancy. Biol. Neonate 2003, 83, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Luisi, S.; Latini, G.; De Felice, C.; Sanseverino, F.; Di Pasquale, D.; Mazzeo, P.; Petraglia, F. Low serum concentrations of di-(2-ethylhexyl)phthalate in women with uterine fibromatosis. Gynecol. Endocrinol. 2006, 22, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Luginbühl, M.; Weinmann, W. Creatinine in urine—A method comparison. Drug Test. Anal. 2017, 9, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Kuo, P.L.; Guo, Y.L.; Liao, P.C.; Lee, C.C. Association between urinary phthalate monoesters and thyroid hormones in pregnant women. Human Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammar, I.; Glynn, A.; Jönsson, B.A.G.; Lindh, C.H.; Darnerud, P.O.; Svensson, K.; Lignell, S. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, cuased by substitution of legacy EDCs? Environ. Res. 2017, 153, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Hauser, R.; Duty, S.; Angerer, J.; Park, M.M.; Burdorf, A.; Hofman, A.; Jaddoe, V.W.V.; Mackenbach, P.J.; et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The generation R study. Environ. Res. 2008, 108, 260–267. [Google Scholar] [CrossRef] [PubMed]
| Substance | Acronymous | In List for Authorization from | In Annex XIV of REACH from | Cause for Being in the List |
|---|---|---|---|---|
| Di-(2-ethylhexyl)-phthalate | DEHP | 28 October 2008 | 21 August 2013 | Endocrine disrupters Toxic for reproduction |
| Di-(n-butyl) phthalate | DnBP | 28 October 2008 | 21 August 2013 | Endocrine disrupters Toxic for reproduction |
| Benzylbutyl phthalate | BBP | 28 October 2008 | 21 August 2013 | Endocrine disrupters Toxic for reproduction |
| Diisobutyl phthatale | DiBP | 13 October 2010 | 21 August 2013 | Endocrine disrupters Toxic for reproduction |
| Diisopentyl phthalate | DIPP | 19 December 2012 | 04 January 2019 | Toxic for reproduction |
| Dipentyl phthalate | DPP | 19 December 2012 | 04 January 2019 | Toxic for reproduction |
| n-Pentylisopentyl phthalate | nPiPP | 19 December 2012 | 04 January 2019 | Toxic for reproduction |
| Bis(2-Methoxyethyl) phthalate | DMEP | 19 December 2011 | - | Toxic for reproduction |
| Substance | Acronymous | Main Metabolites 1 | Where You Can Find It |
|---|---|---|---|
| Di-(2-ethylhexyl)-phthalate | DEHP | MEHP (6%) MEHHP (33–49%) 5-oxo-MEHP (14%) [22,33,34] | Production of PVC 2 and vinyl chloride resins, where it is added to plastics to make them flexible. Adhesives and sealants, arts, crafts and hobby materials. Building/construction materials, electrical and electronic products, fabric, textile and leather products, paints and coatings, plastic and rubber products, playground and sporting equipment. It’s a possible food contaminant for indirect contact. |
| Di-(n-butyl)phthalate | DnBP | MnBP (84%) [35] | Production of plastics to help make it soft and flexible. Shower curtains, raincoats, food wraps, bowls, car interiors, vinyl fabrics, floor tiles, and other products. Adhesives and sealants, explosive materials, floor coverings, ink, toner and colorant products. Plastic and rubber products, excipient in drugs. |
| Benzylbutyl phthalate | BBP | MnBP (44%) MBzP (16%) [36] | Adhesive and sealants, floor coverings |
| Dibenzyl phthalate | DBzP | MBzP (%not defined) [37] | Ingredient in drugs for: disorders of the urinary system, prostate, bladder; dermatological disorders; skeletal disorders; antipsoriatics; arthritis, arthrosis, antiasthmatics, muscular and neuromuscular system disorders, nervous system disorders and many other type of drugs. |
| Diethyl-phthalate | DEP | MEP (70%) [38] | Odor agents, plasticizers, adhesives and sealants, air care products, automotive care products, cleaning and furnishing care products, ink, toner and colorant products, laundry and dishwashing products, paints and coatings, personal care products, plastics and rubber products |
| Characteristics | 2011 | 2016 | ||
|---|---|---|---|---|
| Women (n = 83) | Men (n = 74) | Women (n = 111) | Men (n = 60) | |
| Age (SD 1) | 36.5 (7.2) | 40.4 (7.3) | 42.4 (8.3) | 41 (9.2) |
| Smoking (% current) | 15.7 | 32.4 | 39.3 | 47.5 |
| Regular and occasional alcohol intake (%) | 37.3 | 58.1 | 58 | 90.2 |
| Area of residence (%) | ||||
| Urban | 73.5 | 71.6 | 63.4 | 57.4 |
| Rural | 21.7 | 20.3 | 36.8 | 26.2 |
| Coast | 4.8 | 8.1 | 6.3 | 16.4 |
| Other | - | - | 3.5 | - |
| Use of plastic containers for fat food storage (%) | ||||
| Never | 59.0 | 54.1 | 59.3 | 55.2 |
| Daily | 10.8 | 9.5 | 12.3 | 7.1 |
| weekly | 15.7 | 17.6 | 15.4 | 14.2 |
| monthly | 14.5 | 18.8 | 13.0 | 23.5 |
| Use of canned foods at least weekly (%) | 31.3 | 31.1 | 25.9 | 23.0 |
| Eating fat fish at least weekly (%) | 26.5 | 25.7 | 58 | 68.9 |
| Job (%) | ||||
| Office/school | 35.7 | 28.6 | 30.8 | 23.3 |
| Trade | 26.9 | 32.3 | 12.6 | 15.5 |
| Craftsman/manual worker | 1.2 | 9.5 | 4.3 | 17.4 |
| Cleaning man/woman | 12.0 | 2.7 | 26.4 | 8.2 |
| Hairdresser/beautician | 3.6 | 1.4 | 7.5 | |
| Armed forces | 1.2 | 12.2 | 18.9 | |
| Healthcare/Laboratory | 18.1 | 13.5 | 12.5 | 10.1 |
| Other | 0.1 | - | 5.9 | 6.6 |
| LODs (µg/L) | MEP 1 | MnBP 2 | MBzP 3 | MEHHP 4 | MEHP 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 3.0 | 0.2 | 1.0 | 1.0 | |||||||
| 2011 | 2016 | 2011 | 2016 | 2011 | 2016 | 2011 | 2016 | 2011 | 2016 | ||
| Men | N 6 < LOD | 22 | 19 | 1 | 54 | 0 | 33 | 4 | 6 | 0 | 49 |
| % 7 > LOD | 71 | 68 | 99 | 10 | 100 | 45 | 95 | 90 | 100 | 18 | |
| Women | N 6 < LOD | 0 | 11 | 0 | 99 | 2 | 38 | 0 | 13 | 35 | 85 |
| % 7 > LOD | 100 | 90 | 100 | 11 | 98 | 66 | 100 | 88 | 57 | 23 | |
| MEP 4 | MnBP 5 | MBzP 6 | MEHP 7 + MEHHP 8 | |||||
|---|---|---|---|---|---|---|---|---|
| 2011 1 | 2016 2 | 2011 1 | 2016 2 | 2011 1 | 2016 2 | 2011 1 | 2016 2 | |
| Average (SD 3) | 297.7 (881.1) | 187.7 (321.0) | 54.5 (55.7) | 1.3 (5.3) | 15.0 (21.9) | 1.3 (3.7) | 20.9 (17.0) | 5.9 (5.6) |
| Median | 73.1 | 49.9 | 38.8 | 0.0 | 7.0 | 0.5 | 15.6 | 4.5 |
| 5th percentile | 6.4 | 0.0 | 4.8 | 0.0 | 0.7 | 0.0 | 3.6 | 0.8 |
| 95th percentile | 1177.9 | 781.3 | 163.0 | 6.4 | 72.8 | 5.1 | 61.4 | 16.9 |
| MEP 4 | MnBP 5 | MBzP 6 | MEHP 7 + MEHHP 8 | |||||
|---|---|---|---|---|---|---|---|---|
| 2011 1 | 2016 2 | 2011 1 | 2016 2 | 2011 1 | 2016 2 | 2011 1 | 2016 2 | |
| Average (SD 3) | 142.7 (243.6) | 97.7 (218.1) | 57.7 (62.6) | 1.4 (7.2) | 14.5 (27.8) | 0.9 (2.2) | 21.1 (26.0) | 5.7 (7.1) |
| Median | 49.9 | 21.6 | 37.6 | 0.0 | 4.8 | 0.0 | 14.1 | 3.1 |
| 5th percentile | 4.2 | 0.0 | 7.3 | 0.0 | 0.8 | 0.0 | 3.8 | 0.0 |
| 95th percentile | 637.9 | 406.7 | 145.6 | 4.8 | 51.7 | 3.0 | 62.1 | 20.1 |
| Country | Italy [This Study] | Italy [This Study] | Taiwan [51] | Israel [52] | USA [46] | USA [46] | Sweden [48] | the Netherlands [53] | Germany [54] |
|---|---|---|---|---|---|---|---|---|---|
| Sampling year | 2011 | 2016 | 2005–2006 | 2006 | 1999–2000 | 2011–2012 | 2009–2014 | 2004 | 2005 |
| N | 83 | 111 | 76 | 19 | 1326 | 1229 | 178 | 100 | 27 |
| phthalates | |||||||||
| MEP 2 | 73.1 | 49.9 | 68.0 | 140.5 | 123.0 1 | 51.8 1 | 24.3 | 112.0 1 | - |
| MnBP 3 | 38.8 | 0.0 | 195.0 | 45.9 | 28.6 1 | 9.8 1 | 42.7 | 43.2 1 | 46.8 |
| MBzP 4 | 7.0 | 0.5 | 3.7 | 9.6 | 11.0 1 | 5.87 1 | 8.76 | 8.9 1 | 7.6 |
| ∑DEHP 5 met. | 15.6 | 4.5 | 60.8 | 31.3 | 3.36 1 | 1.70 1 | 11.4 | 21.2 1 | 4.3 |
| Country | Italy [This Study] | Italy [This Study] | USA [46] | USA [46] | Germany [54] |
|---|---|---|---|---|---|
| Sampling year | 2011 | 2016 | 1999–2000 | 2011–2012 | 2005 |
| N | 74 | 60 | 1215 | 1258 | 23 |
| phthalates | |||||
| MEP 2 | 49.9 | 21.6 | 92.8 1 | 35.8 1 | - |
| MnBP 3 | 37.6 | 0.0 | 17.3 1 | 7.61 1 | 41.4 |
| MBzP 4 | 4.8 | 0.0 | 9.14 1 | 4.5 1 | 5.1 |
| ∑DEHP 5 met. | 14.1 | 3.1 | 2.89 1 | 1.41 1 | 4.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tranfo, G.; Caporossi, L.; Pigini, D.; Capanna, S.; Papaleo, B.; Paci, E. Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years. Int. J. Environ. Res. Public Health 2018, 15, 1950. https://doi.org/10.3390/ijerph15091950
Tranfo G, Caporossi L, Pigini D, Capanna S, Papaleo B, Paci E. Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years. International Journal of Environmental Research and Public Health. 2018; 15(9):1950. https://doi.org/10.3390/ijerph15091950
Chicago/Turabian StyleTranfo, Giovanna, Lidia Caporossi, Daniela Pigini, Silvia Capanna, Bruno Papaleo, and Enrico Paci. 2018. "Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years" International Journal of Environmental Research and Public Health 15, no. 9: 1950. https://doi.org/10.3390/ijerph15091950
APA StyleTranfo, G., Caporossi, L., Pigini, D., Capanna, S., Papaleo, B., & Paci, E. (2018). Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years. International Journal of Environmental Research and Public Health, 15(9), 1950. https://doi.org/10.3390/ijerph15091950









