Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
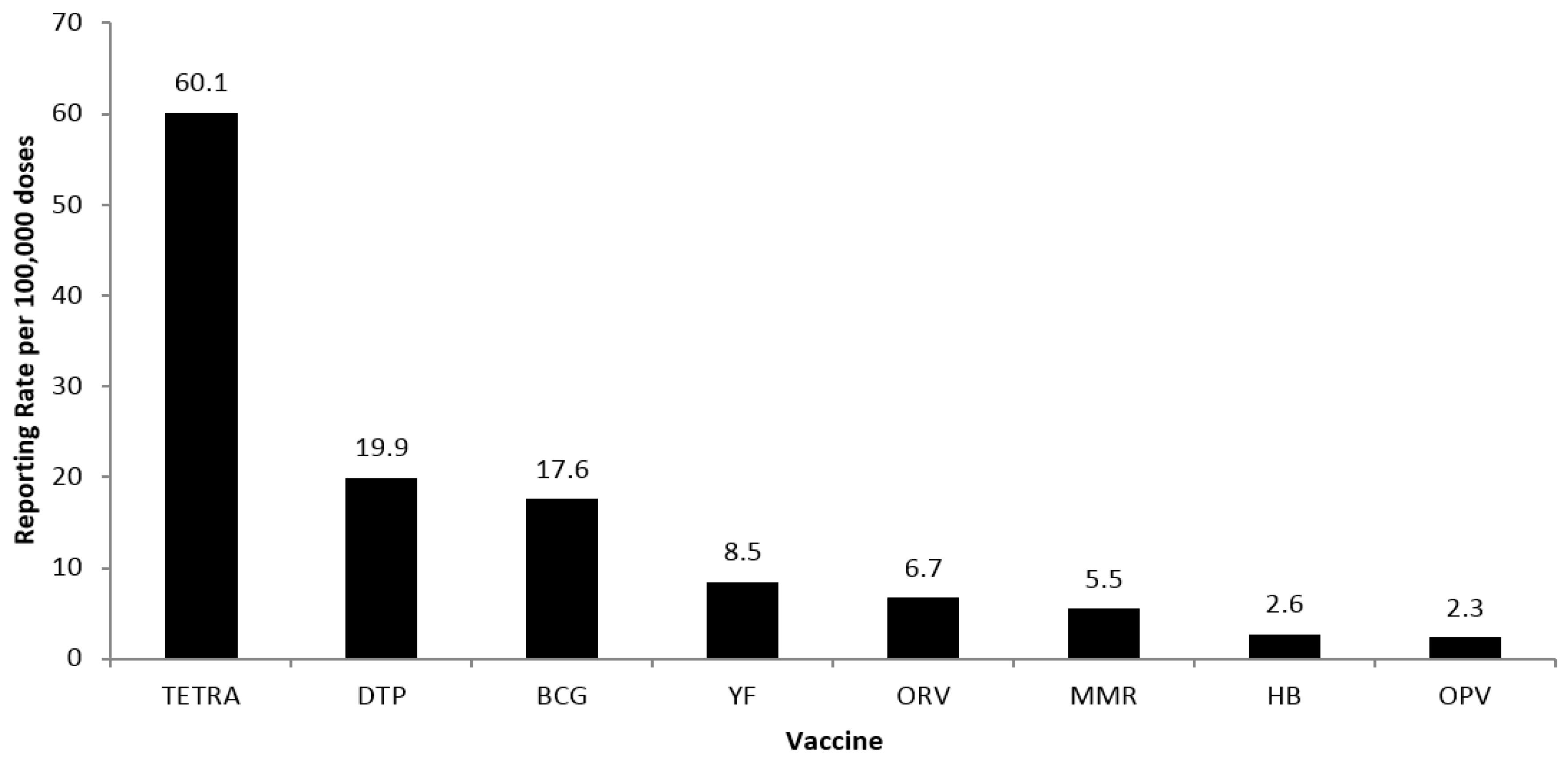
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| AEFI | Vaccine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TETRA | DTP | BCG | OPV | MMR | ORV | HB | YF | p | |
| Test 1 | <0.0001 | ||||||||
| HHE | |||||||||
| Yes | 9115 (38.5%) | 803 (16.6%) | 1 0.00% | 15 (4.4%) | 14 (1.4%) | 4 −0.60% | 30 (3.9%) | 10 (1.4%) | |
| [60.4] | [−19.5] | [−37.6] | [−9.9] | [−19.1] | [−15.8] | [−15.2] | [−16.1] | ||
| No | 14,580 (61.5%) | 4037 (83.4%) | 3252 (100.0%) | 327 (95.6%) | 975 (98.6%) | 643 (99.4%) | 740 (96.1%) | 699 (98.6%) | |
| [−60.4] | [19.5] | [37.6] | [9.9] | [19.1] | [15.8] | [15.2] | [16.1] | ||
| Total | 23,695 (100.0%) | 4840 (100.0%) | 3253 (100.0%) | 342 (100.0%) | 989 (100.0%) | 647 (100.0%) | 770 (100.0%) | 709 (100.0%) | |
| Test 2 | <0.0001 | ||||||||
| Afebrile seizure | 2724 (11.5%) | 599 (12.4%) | 0 0.00% | 18 (5.3%) | 34 (3.4%) | 4 −0.60% | 23 (3.0%) | 13 (1.8%) | |
| [16.4] | [6.8] | [−19.6] | [−2.8] | [−6.7] | [−7.9] | [−6.4] | [−7.1] | ||
| Febrile convulsion | 562 (2.4%) | 92 (1.9%) | 0 0.00% | 1 −0.30% | 7 −0.70% | 2 −0.30% | 5 −0.60% | 7 −1.00% | |
| [8.9] | [−0.1] | [−8.4] | [−2.2] | [−2.8] | [−3.0] | [−2.6] | [−1.8] | ||
| Fever without seizure | 8040 (33.9%) | 1502 (31.0%) | 44 (1.4%) | 74 (21.6%) | 326 (33.0%) | 65 (10.0%) | 116 (15.1%) | 155 (21.9%) | |
| [27.4] | [2.9] | [−36.7] | [−3.1] | [2.6] | [−10.9] | [−8.8] | [−4.4] | ||
| Cases without | 12,369 (52.2%) | 2647 (54.7%) | 3209 (98.6%) | 249 (72.8%) | 622 (62.9%) | 576 (89.0%) | 626 (81.3%) | 534 (75.3%) | |
| fever or convulsion | [−37.8] | [−6.7] | [48.1] | [5.2] | [2.5] | [15.6] | [12.7] | [8.9] | |
| Total | 23,695 (100.0%) | 4840 (100.0%) | 3253 (100.0%) | 342 (100.0%) | 989 (100.0%) | 647 (100.0%) | 770 (100.0%) | 709 (100.0%) | |
| Test 3 | <0.0001 | ||||||||
| Generalized rash | |||||||||
| Yes | 722 (3.0%) | 112 (2.3%) | 7 −0.20% | 50 (14.6%) | 462 (46.7%) | 7 −1.10% | 41 (5.3%) | 177 (25.0%) | |
| [−18.6] | [−7.8] | [−12.3] | [9.1] | [65.1] | [−4.2] | [1.1] | [26.6] | ||
| No | 22,973 (97.0%) | 4728 (97.7%) | 3246 (99.8%) | 292 (85.4%) | 527 (53.3%) | 640 (98.9%) | 729 (94.7%) | 532 (75.0%) | |
| [18.6] | [7.8] | [12.3] | [−9.1] | [−65.1] | [4.2] | [−1.1] | [−26.6] | ||
| Total | 23,695 (100.0%) | 4840 (100.0%) | 3253 (100.0%) | 342 (100.0%) | 989 (100.0%) | 647 (100.0%) | 770 (100.0%) | 709 (100.0%) | |
| Test 4 | <0.0001 | ||||||||
| Increased lymph nodes | |||||||||
| Yes | 1079 (4.6%) | 345 (7.1%) | 1513 (46.5%) | 4 (1.2%) | 38 (3.8%) | 0 0.00% | 82 (10.6%) | 10 (1.4%) | |
| [−39.7] | [−4.2] | [80.2] | [−5.0] | [−5.5] | [−7.9] | [1.9] | [−7.0] | ||
| No | 22,616 (95.4%) | 4495 (92.9%) | 1740 (53.5%) | 338 (98.8%) | 951 (96.2%) | 647 (100.0%) | 688 (89.4%) | 699 (98.6%) | |
| [39.7] | [4.2] | [−80.2] | [5.0] | [5.5] | [7.9] | [−1.9] | [7.0] | ||
| Total | 23,695 (100.0%) | 4840 (100.0%) | 3253 (100.0%) | 342 (100.0%) | 989 (100.0%) | 647 (100.0%) | 770 (100.0%) | 709 (100.0%) | |
| Vaccine | Median Age (in Months) | Homogeneous Groups * |
|---|---|---|
| DTP | 19 | A |
| OPV | 16 | B |
| MMR | 13 | B |
| YF | 10 | B |
| TETRA | 5 | C |
| ORV | 4 | D |
| BCG | 3 | D |
| HB | 2 | E |
| Predictor | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR | IC 95% | OR | IC 95% | |
| Gender | ||||
| Female | 1 | - | 1 | - |
| Male | 1.0 | (0.99; 1.08) | 1.0 | (0.99; 1.08) |
| Age | ||||
| 5–9 years | 1 | - | 1 | - |
| 1–4 years | 1.5 | (1.38; 1.71) | 1.2 | (1.09; 1.39) |
| <1 year | 1.5 | (1.35; 1.65) | 1.1 | (0.98; 1.27) |
| Vaccine | ||||
| TETRA | 1 | - | 1 | - |
| DTP | 0.8 | (0.76; 0.86) | 0.8 | (0.69; 0.89) |
| BCG | 0.7 | (0.65; 0.76) | 0.7 | (0.67; 0.79) |
| OPV | 1.1 | (0.91; 1.43) | 1.3 | (0.99; 1.63) |
| MMR | 1.0 | (0.84; 1.08) | 0.9 | (0.79; 1.07) |
| ORV | 1.8 | (1.48; 2.11) | 1.8 | (1.52; 2.16) |
| HB | 1.0 | (0.90; 1.21) | 1.1 | (0.91; 1.22) |
| YF | 1.4 | (1.21; 1.68) | 1.5 | (1.25; 1.74) |
| Applied Dosage | ||||
| 2nd booster | 1 | - | 1 | - |
| 1st booster | 1.5 | (1.37; 1.73) | 1.5 | (1.31; 1.70) |
| 3rd dose | 1.7 | (1.52; 1.88) | 1.4 | (1.19; 1.62) |
| 2nd dose | 1.6 | (1.42; 1.74) | 1.3 | (1.13; 1.52) |
| 1st dose | 1.5 | (1.33; 1.61) | 1.3 | (1.09; 1.46) |
References
- Hinman, A.R.; Orenstein, W.A.; Schuchat, A. Centersfor Disease Control and Prevention (CDC). Vaccine-preventable diseases, immunizations, and MMWR—1961–2011. MMWR Surveill. Summ. 2011, 60, 49–57. [Google Scholar]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Adversomics: The emerging field of vaccine adverse event immunogenetics. Pediatr. Infect. Dis. J. 2009, 28, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Pool, V.; Takahashi, H.; Weniger, B.G.; Patel, B. Combination vaccines: Postlicensure safety evaluation. Clin. Infect. Dis. 2001, 33, S327–S333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Pool, V.; Iskander, J.K.; English-Bullard, R.; Ball, R.; Wise, R.P.; Haber, P.; Pless, R.P.; Mootrey, G.; Ellenberg, S.S.; et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveill. Summ. 2003, 52, 1–24. [Google Scholar] [PubMed]
- Autret-Leca, E.; Bensouda-Grimaldi, L.; Jonville-Béra, A.P.; Beau-Salinas, F. Pharmacovigilance of vaccines. Arch. Pediatr. 2006, 13, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Bonanni, P.; Garçon, N.; Stanberry, L.R.; El-Hodhod, M.; Da Tavares Silva, F. Vaccine safety evaluation: Practical aspects in assessing benefits and risks. Vaccine 2016, 34, 6672–6680. [Google Scholar] [CrossRef] [PubMed]
- Waldman, E.A.; Luhm, K.R.; Monteiro, S.A.; Freitas, F.R. Surveillance of adverse effects following vaccination and safety of immunization programs. Revista de Saude Publica 2011, 45, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.A.; Takano, O.A.; Waldman, E.A. Surveillance for adverse events after DTwP/Hib vaccination in Brazil: Sensitivity and factors associated with reporting. Vaccine 2010, 28, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Homma, A.; Tanuri, A.; Duarte, A.J.; Marques, E.; de Almeida, A.; Martins, R.; Silva-Junior, J.B.; Possas, C. Vaccine research, development, and innovation in Brazil: A translational science perspective. Vaccine 2013, 31 (Suppl. 2), B54–B60. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.L.; Teixeira, M.G.; Bastos, F.I.; Ximenes, R.A.; Barata, R.B.; Rodrigues, L.C. Successes and failures in the control of infectious diseases in Brazil: Social and environmental context, policies, interventions, and research needs. Lancet 2011, 377, 1877–1889. [Google Scholar] [CrossRef]
- Agresti, A. An Introduction to Categorical Data Analysis; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Daniel, W.W. Applied Nonparametric Statistics; Houghton Mifflin Co.: Boston, MA, USA, 1978. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Kleinbaum, D.G.; Klein, M. Logistic Regression: A Self-Learning Text, 3rd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Dórea, J.G. Breastfeeding is an essential complement to vaccination. Acta Paediatr. 2009, 98, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Breast-feeding and responses to infant vaccines: Constitutional and environmental factors. Am. J. Perinatol. 2012, 29, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Alguacil-Ramos, A.M.; Muelas-Tirado, J.; Garrigues-Pelufo, T.M.; Portero-Alonso, A.; Diez-Domingo, J.; Pastor-Villalba, E.; Lluch-Rodrigo, J.A. Surveillance for adverse events following immunization (AEFI) for 7 years using a computerised vaccination system. Public Health 2016, 135, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gkampeta, A.; Pavlidou, E.; Pavlou, E. Vaccination and neurological disorders. J. Pediatr. Sci. 2015, 7, e237. [Google Scholar] [CrossRef]
- Thomas, R.E.; Lorenzetti, D.L.; Spragins, W.; Jackson, D.; Williamson, T. Active and passive surveillance of yellow fever vaccine 17D or 17DD-associated serious adverse events: systematic review. Vaccine 2011, 29, 4544–4555. [Google Scholar] [CrossRef] [PubMed]
- De Martins, R.M.; Pavão, A.L.; de Oliveira, P.M.; dos Santos, P.R.; Carvalho, S.M.; Mohrdieck, R.; Fernandes, A.R.; Sato, H.K.; de Figueiredo, P.M.; dos Reis von Doellinger, V.; et al. Adverse events following yellow fever immunization: Report and analysis of 67 neurological cases in Brazil. Vaccine 2014, 32, 6676–6682. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Adverse events following immunization: Real causality and myths. Expert Opin. Drug Saf. 2016, 15, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; López-Collada, V.R.; Bulhões, M.M.; De Oliveira, L.H.; Márquez, A.B.; Flannery, B.; Esparza-Aguilar, M.; Montenegro Renoiner, E.I.; Luna-Cruz, M.E.; Sato, H.K.; et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N. Engl. J. Med. 2011, 364, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Moylett, E.H.; Hanson, I.C. Mechanistic actions of the risks and adverse events associated with vaccine administration. J. Allergy Clin. Immunol. 2004, 114, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Low-dose Thimerosal in pediatric vaccines: Adverse effects in perspective. Environ. Res. 2017, 152, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.S.; Miller, N.Z. Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990–2010. Hum. Exp. Toxicol. 2012, 31, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.A.; Ovsyannikova, I.G.; Poland, G.A. Adversomics: A new paradigm for vaccine safety and design. Expert Rev. Vaccines 2015, 14, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the VaccineAdverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.L.; Dórea, J.G.; Monsores, N.S. Judicialização dos eventos adversos pós-vacinais. Rev. Bioet. 2017, 25, 482–492. [Google Scholar]
| Vaccine | Total of Adverse Events | ||
|---|---|---|---|
| SI | CI | SI/CI (%) | |
| TETRA | 30315 | 32362 | 93.7 |
| DTP | 6405 | 6840 | 93.6 |
| BCG | 3501 | 3595 | 97.4 |
| MMR | 1284 | 1567 | 81.9 |
| HB | 934 | 1513 | 61.7 |
| YF | 886 | 1009 | 87.8 |
| ORV | 700 | 1620 | 43.2 |
| OPV | 407 | 1881 | 21.6 |
| Event | Age (Years) | |||||||
|---|---|---|---|---|---|---|---|---|
| <1 | 1–4 | 5–9 | Total | |||||
| N | % | N | % | N | % | N | % | |
| HB | ||||||||
| Abscess hot spot | 218 | 29.18 | 1 | 0.13 | 0 | 0 | 219 | 29.32 |
| Pain, redness and heat | 107 | 14.32 | 4 | 0.54 | 4 | 0.54 | 115 | 15.39 |
| Induration | 72 | 9.64 | 3 | 0.4 | 0 | 0 | 75 | 10.04 |
| Fever ≥ 39.5 °C | 69 | 9.24 | 1 | 0.13 | 0 | 0 | 70 | 9.37 |
| Other severe events | 58 | 7.76 | 1 | 0.13 | 0 | 0 | 59 | 7.9 |
| HR after 2 h | 51 | 6.83 | 0 | 0 | 1 | 0.13 | 52 | 6.96 |
| Fever < 39.5 °C | 49 | 6.56 | 2 | 0.27 | 0 | 0 | 51 | 6.83 |
| Generalized rash | 38 | 5.09 | 2 | 0.27 | 1 | 0.13 | 41 | 5.49 |
| Lump | 31 | 4.15 | 2 | 0.27 | 0 | 0 | 33 | 4.42 |
| Other local reactions | 26 | 3.48 | 4 | 0.54 | 2 | 0.27 | 32 | 4.28 |
| Total | 719 | 96.25 | 20 | 2.68 | 8 | 1.07 | 747 | 100 |
| TETRA | ||||||||
| HHE | 8294 | 30.32 | 783 | 2.86 | 38 | 0.14 | 9115 | 33.32 |
| Fever ≥ 39.5 °C | 4589 | 16.77 | 539 | 1.97 | 23 | 0.08 | 5151 | 18.83 |
| Fever < 39.5 °C | 3270 | 11.95 | 196 | 0.72 | 7 | 0.03 | 3473 | 12.7 |
| Pain, redness, and heat | 2559 | 9.35 | 179 | 0.65 | 14 | 0.05 | 2752 | 10.06 |
| Febrile convulsion | 2067 | 7.56 | 415 | 1.52 | 18 | 0.07 | 2500 | 9.14 |
| Induration | 993 | 3.63 | 59 | 0.22 | 3 | 0.01 | 1055 | 3.86 |
| Other severe events | 962 | 3.52 | 90 | 0.33 | 1 | 0 | 1053 | 3.85 |
| Afebrile seizure | 687 | 2.51 | 97 | 0.35 | 7 | 0.03 | 791 | 2.89 |
| Lump | 707 | 2.58 | 36 | 0.13 | 2 | 0.01 | 745 | 2.72 |
| Generalized rash | 648 | 2.37 | 69 | 0.25 | 5 | 0.02 | 722 | 2.64 |
| Total | 24,776 | 90.57 | 2463 | 9 | 118 | 0.43 | 27,357 | 100 |
| DTP | ||||||||
| Pain, redness, and heat | 53 | 0.97 | 816 | 14.87 | 438 | 7.98 | 1307 | 23.81 |
| Fever | 61 | 1.11 | 652 | 11.88 | 185 | 3.37 | 898 | 16.36 |
| HHE | 82 | 1.49 | 564 | 10.28 | 157 | 2.86 | 803 | 14.63 |
| Fever < 39.5 °C | 42 | 0.77 | 506 | 9.22 | 152 | 2.77 | 700 | 12.75 |
| Febrile convulsion | 36 | 0.66 | 458 | 8.34 | 95 | 1.73 | 589 | 10.73 |
| Induration | 16 | 0.29 | 234 | 4.26 | 73 | 1.33 | 323 | 5.88 |
| Lump | 10 | 0.18 | 152 | 2.77 | 39 | 0.71 | 201 | 3.66 |
| Abscess hot spot | 9 | 0.16 | 179 | 3.26 | 62 | 1.13 | 250 | 4.55 |
| Difficulty walking | 6 | 0.11 | 180 | 3.28 | 52 | 0.95 | 238 | 4.34 |
| Headache and vomiting | 6 | 0.11 | 124 | 2.26 | 50 | 0.91 | 180 | 3.28 |
| Total | 321 | 5.85 | 3865 | 70.41 | 1303 | 23.74 | 5489 | 100 |
| BCG | ||||||||
| Lymphadenopathy | 758 | 23.53 | 18 | 0.56 | 12 | 0.37 | 788 | 24.46 |
| Abscess hot spot | 368 | 11.42 | 36 | 1.12 | 76 | 2.36 | 480 | 14.9 |
| Cold abscess site | 382 | 11.86 | 62 | 1.92 | 23 | 0.71 | 467 | 14.49 |
| Ulcer > 1 cm | 276 | 8.57 | 29 | 0.9 | 42 | 1.3 | 347 | 10.77 |
| Lymphadenitis > 3 cm | 332 | 10.3 | 4 | 0.12 | 5 | 0.16 | 341 | 10.58 |
| Lump | 208 | 6.46 | 19 | 0.59 | 5 | 0.16 | 232 | 7.2 |
| Suppurated lymphadenitis | 214 | 6.64 | 11 | 0.34 | 1 | 0.03 | 226 | 7.01 |
| Lymphadenopathy > 3 cm | 132 | 4.1 | 8 | 0.25 | 2 | 0.06 | 142 | 4.41 |
| Pain, redness, and heat | 91 | 2.82 | 3 | 0.09 | 9 | 0.28 | 103 | 3.2 |
| Other local reactions | 85 | 2.64 | 4 | 0.12 | 7 | 0.22 | 96 | 2.98 |
| Total | 2846 | 88.33 | 194 | 6.02 | 182 | 5.65 | 3222 | 100 |
| MMR | ||||||||
| Generalized rash | 44 | 3.95 | 399 | 35.82 | 19 | 1.71 | 462 | 41.47 |
| Fever < 39.5 °C | 17 | 1.53 | 149 | 13.38 | 11 | 0.99 | 177 | 15.89 |
| Fever ≥ 39.5 °C | 9 | 0.81 | 142 | 12.75 | 5 | 0.45 | 156 | 14 |
| HR after 2 h | 5 | 0.45 | 84 | 7.54 | 3 | 0.27 | 92 | 8.26 |
| HR up to 2 h | 1 | 0.09 | 30 | 2.69 | 5 | 0.45 | 36 | 3.23 |
| Pain, redness, and heat | 5 | 0.45 | 37 | 3.32 | 22 | 1.97 | 64 | 5.75 |
| Other severe events | 5 | 0.45 | 31 | 2.78 | 4 | 0.36 | 40 | 3.59 |
| Febrile convulsion | 4 | 0.36 | 28 | 2.51 | 1 | 0.09 | 33 | 2.96 |
| Mumps | 1 | 0.09 | 25 | 2.24 | 6 | 0.54 | 32 | 2.87 |
| Headache and vomiting | 1 | 0.09 | 17 | 1.53 | 4 | 0.36 | 22 | 1.97 |
| Total | 92 | 8.26 | 942 | 84.56 | 80 | 7.18 | 1114 | 100 |
| YF | ||||||||
| Generalized rash | 140 | 19.31 | 32 | 4.41 | 5 | 0.69 | 177 | 24.41 |
| HR up to 2 h | 95 | 13.1 | 19 | 2.62 | 5 | 0.69 | 119 | 16.41 |
| HR after 2 h | 84 | 11.59 | 27 | 3.72 | 2 | 0.28 | 113 | 15.59 |
| Fever ≥ 39.5 °C | 59 | 8.14 | 24 | 3.31 | 5 | 0.69 | 88 | 12.14 |
| Fever < 39.5 °C | 44 | 6.07 | 23 | 3.17 | 7 | 0.97 | 74 | 10.21 |
| Other severe events | 34 | 4.69 | 5 | 0.69 | 3 | 0.41 | 42 | 5.79 |
| Pain, redness, and heat | 27 | 3.72 | 5 | 0.69 | 3 | 0.41 | 35 | 4.83 |
| Meningitis | 5 | 0.69 | 9 | 1.24 | 16 | 2.21 | 30 | 4.14 |
| Headache | 7 | 0.97 | 4 | 0.55 | 13 | 1.79 | 24 | 3.31 |
| Headache and vomiting | 15 | 2.07 | 5 | 0.69 | 3 | 0.41 | 23 | 3.17 |
| Total | 510 | 70.34 | 153 | 21.1 | 62 | 8.55 | 725 | 100 |
| Event | Age (Years) | |||||||
|---|---|---|---|---|---|---|---|---|
| < 1 | 4-Jan | 9-May | Total | |||||
| N | % | N | % | N | % | N | % | |
| OPV (Oral poliovirus) | ||||||||
| Other severe events | 31 | 9.78 | 30 | 9.46 | 0 | 0 | 61 | 19.24 |
| Generalized rash | 21 | 6.62 | 28 | 8.83 | 1 | 0.32 | 50 | 15.77 |
| Fever ≥ 39.5 °C | 13 | 4.1 | 25 | 7.89 | 0 | 0 | 38 | 11.99 |
| Fever < 39.5 °C | 13 | 4.1 | 25 | 7.89 | 0 | 0 | 38 | 11.99 |
| HR up to 2 h | 15 | 4.73 | 17 | 5.36 | 0 | 0 | 32 | 10.09 |
| HR after 2 h | 12 | 3.79 | 24 | 7.57 | 1 | 0.32 | 37 | 11.67 |
| HHE | 12 | 3.79 | 3 | 0.95 | 0 | 0 | 15 | 4.73 |
| Other local reactions | 7 | 2.21 | 7 | 2.21 | 0 | 0 | 14 | 4.42 |
| Febrile convulsion | 4 | 1.26 | 10 | 3.15 | 0 | 0 | 14 | 4.42 |
| Headache and vomiting | 3 | 0.95 | 15 | 4.73 | 0 | 0 | 18 | 5.68 |
| Total | 131 | 41.32 | 184 | 58.04 | 2 | 0.63 | 317 | 100 |
| ORV (Oral rotavirus) | ||||||||
| Other severe events | 329 | 48.24 | 3 | 0.44 | 0 | 0 | 332 | 48.68 |
| Intussusception | 173 | 25.37 | 3 | 0.44 | 1 | 0.15 | 177 | 25.95 |
| Other local reactions | 43 | 6.3 | 1 | 0.15 | 1 | 0.15 | 45 | 6.6 |
| Fever ≥ 39.5 °C | 35 | 5.13 | 0 | 0 | 0 | 0 | 35 | 5.13 |
| Headache and vomiting | 33 | 4.84 | 1 | 0.15 | 0 | 0 | 34 | 4.99 |
| Fever < 39.5 °C | 32 | 4.69 | 0 | 0 | 0 | 0 | 32 | 4.69 |
| HR after 2 h | 9 | 1.32 | 0 | 0 | 0 | 0 | 9 | 1.32 |
| Generalized rash | 7 | 1.03 | 0 | 0 | 0 | 0 | 7 | 1.03 |
| Angioedema | 7 | 1.03 | 0 | 0 | 0 | 0 | 7 | 1.03 |
| HHE | 4 | 0.59 | 0 | 0 | 0 | 0 | 4 | 0.59 |
| Total | 672 | 98.53 | 8 | 1.17 | 2 | 0.29 | 682 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, S.R.C.; Perin, J.L.R.; Prass, T.S.; Carvalho, S.M.D.; Lessa, S.C.; Dórea, J.G. Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression. Int. J. Environ. Res. Public Health 2018, 15, 1149. https://doi.org/10.3390/ijerph15061149
Lopes SRC, Perin JLR, Prass TS, Carvalho SMD, Lessa SC, Dórea JG. Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression. International Journal of Environmental Research and Public Health. 2018; 15(6):1149. https://doi.org/10.3390/ijerph15061149
Chicago/Turabian StyleLopes, Sílvia R.C., João L.R. Perin, Taiane S. Prass, Sandra Maria D. Carvalho, Sérgio C. Lessa, and José G. Dórea. 2018. "Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression" International Journal of Environmental Research and Public Health 15, no. 6: 1149. https://doi.org/10.3390/ijerph15061149
APA StyleLopes, S. R. C., Perin, J. L. R., Prass, T. S., Carvalho, S. M. D., Lessa, S. C., & Dórea, J. G. (2018). Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression. International Journal of Environmental Research and Public Health, 15(6), 1149. https://doi.org/10.3390/ijerph15061149





