Groundwater Flow Processes and Human Impact along the Arid US-Mexican Border, Evidenced by Environmental Tracers: The Case of Tecate, Baja California
Abstract
:1. Introduction
2. Study Area
3. Materials and Methods
3.1. Field Work and Laboratory
3.2. Interpretation
4. Results
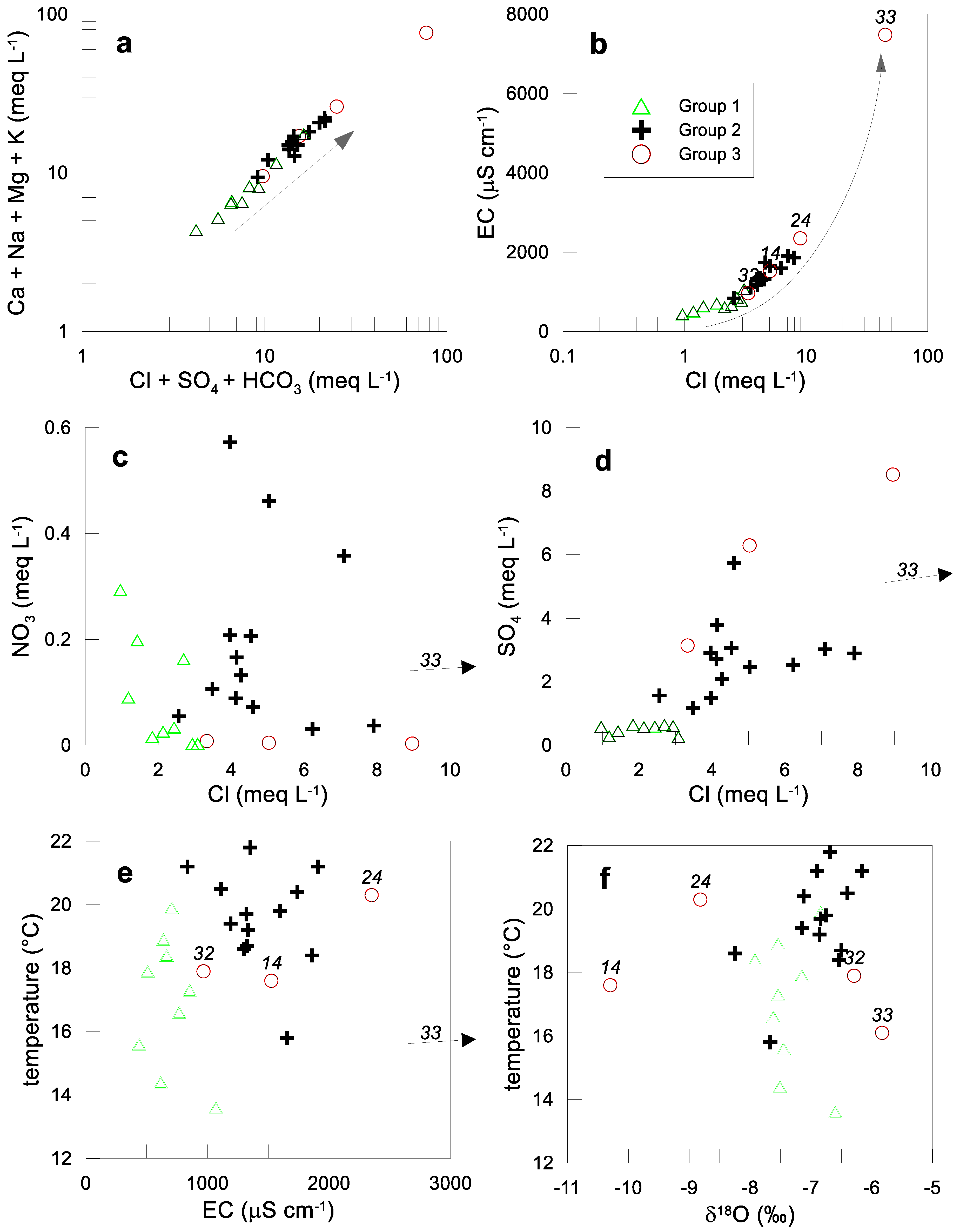
4.1. Chemical Characterization
4.2. Hydrochemical Clusters
4.3. Statistical Variation of Selected Variables
4.4. Identification of Hydrogeochemical Processes
4.5. Geochemical Trends
4.6. Stable Isotopes
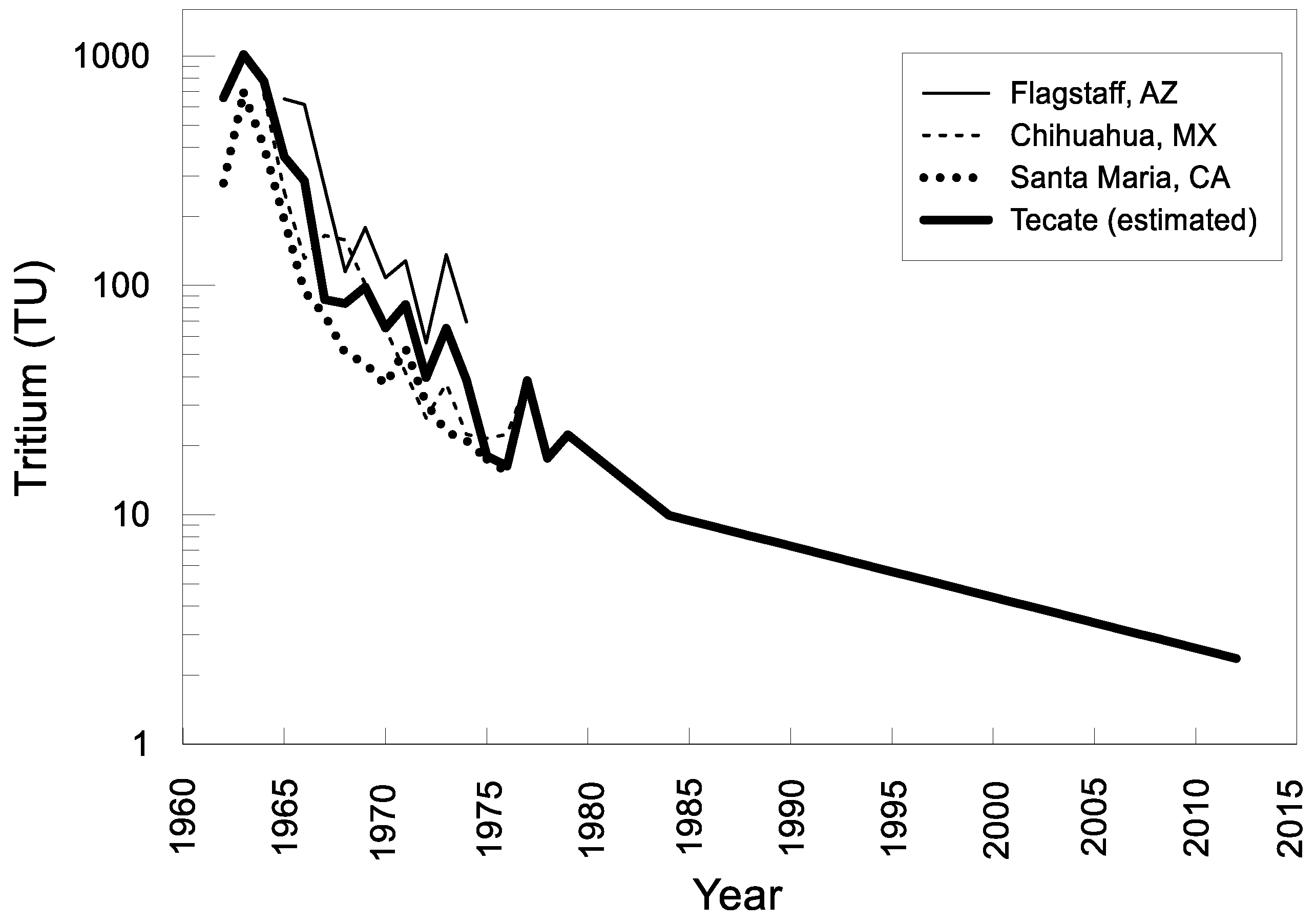
4.7. Groundwater Age
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castro-Ruíz, J.L. Tecate’s water supply. In Tecate, Baja California: Realities and Challenges in a Mexican Border Community; Ganster, P., Cuamea Velázquez, F., Castro Ruiz, J.L., Villegas, A., Eds.; SDSU Press: San Diego, CA, USA, 2002. [Google Scholar]
- Instituto Tecnológico y de Estudios Superiores de Monterrey. Estudio hidrogeológico de los acuíferos Las Palmas, Tecate y Rumorosa-Tecate; Instituto Tecnológico y de Estudios Superiores de Monterrey: Monterrey, Mexico, 2017. [Google Scholar]
- Wakida, F.T.; Lara-Ruiz, D.; Temores-Peña, J.; Rodriguez-Ventura, J.G.; Diaz, C.; Garcia-Flores, E. Heavy metals in sediments of the Tecate River, Mexico. Environ. Geol. 2008, 54, 637–642. [Google Scholar] [CrossRef]
- Lüderitz, V.; Gerlach, F.; Jüpner, R.; Calleros, J.; Pitt, J.; Gersberg, M.; Ju, R. Biological Assessment of Tecate Creek (U.S.–Mexico) with Special Regard to Self-Purification with Special Regard to Self-Purification. Bull.-South. Calif. Acad. Sci. 2005, 104, 1–13. [Google Scholar]
- Eckstein, G.E. Rethinking Transboundary Ground Water Resources Management: A Local Approach along the Mexico-US Border. Georg. Int. Environ. Law Rev. 2013, 25, 95–128. [Google Scholar]
- Michel, S.M. Rediscovering the Tecate river. In Urban Rivers in Tecate and Tijuana: Strategies for Sustainable Cities; Michel, S.M., Graizbord, C., Eds.; SCERP and IRSC publications: San Diego, CA, USA, 2002. [Google Scholar]
- Sanchez, V.; Cortez-Lara, A.A. Minute 319 of the International Boundary and Water Commission between the US and Mexico: Colorado River binational water management implications. Int. J. Water Resour. Dev. 2015, 31, 17–27. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua. Determinación de la Disponibilidad de Agua en el Acuífero Tecate (0202), Estado de Baja California; Comisión Nacional del Agua: México City, México, 2015. [Google Scholar]
- Comisión Nacional del Agua. Reactivación de Redes de Monitoreo Piezométricas de los Acuíferos de Valle Chico-San Pedro Mártir, Tecate y Laguna Salada en el Estado de Baja California; Comisión Nacional del Agua: México City, México, 2009. [Google Scholar]
- Comisión Nacional del Agua. Estudio de actualización de mediciones piezométricas en los Acuíferos: El Rosario, San Rafael, Santo Tomás, Tecate y Laguna Salada, en el estado de Baja California; Comisión Nacional del Agua: México City, México, 2007. [Google Scholar]
- Cook, P.G.; Solomon, D.K. Recent advances in dating young groundwater: Chlorofluorocarbons 3H/3He and 85Kr. J. Hydrol. 1997, 191, 245–265. [Google Scholar] [CrossRef]
- Newman, B.D.; Osenbrück, K.; Aeschbach-Hertig, W.; Solomon, D.K.; Cook, P.; Rózánski, K.; Kipfer, R. Dating of “young” groundwaters using environmental tracers: Advantages, applications, and research needs. Isot. Environ. Health Stud. 2010, 46, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, C.J.; Watts, C.J.; Ploughe, M.; Wright, W.E. Future use of tritium in mapping pre-bomb groundwater volumes. Ground Water 2012, 50, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997; ISBN 1566702496. [Google Scholar]
- Li, J.; Liu, J.; Pang, Z.; Wang, X. Characteristics of Chemistry and Stable Isotopes in Groundwater of the Chaobai River Catchment, Beijing. Procedia Earth Planet. Sci. 2013, 7, 487–490. [Google Scholar] [CrossRef]
- Kalin, R.M. Radiocarbon Dating of Groundwater Systems. In Environmental Tracers in Subsurface Hydrology; Springer: Boston, MA, USA, 2000; pp. 111–144. ISBN 978-1-4613-7057-4. [Google Scholar]
- Vogel, J.C. Vogel Carbon-14 dating of groundwater. Isot. Hydrol. 1970, 1970, 225–239. [Google Scholar]
- Fontes, J.-C.; Garnier, J.-M. Determination of the initial 14C activity of the total dissolved carbon: A review of the existing models and a new approach. Water Resour. Res. 1979, 15, 399–413. [Google Scholar] [CrossRef]
- Ingerson, E.; Pearson, F.J. Estimation of age and rate of motion of groundwater by the 14C-method. Recent Res. Fields Atmos. Hydrosph. Nucl. Geochem. 1964, 263–283. [Google Scholar]
- Mahlknecht, J.; Merchán, D.; Rosner, M.; Meixner, A.; Ledesma-Ruiz, R. Assessing seawater intrusion in an arid coastal aquifer under high anthropogenic influence using major constituents, Sr and B isotopes in groundwater. Sci. Total Environ. 2017, 587–588, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Horst, A.; Mahlknecht, J.; Merkel, B.J.; Aravena, R.; Ramos-Arroyo, Y.R. Evaluation of the recharge processes and impacts of irrigation on groundwater using CFCs and radiogenic isotopes in the Silao-Romita basin, Mexico. Hydrogeol. J. 2008, 16, 1601–1614. [Google Scholar] [CrossRef]
- SMN—Servicio Meteorológico Nacional Información Climatológica. Available online: http://smn.conagua.gob.mx/es/climatologia/informacion-climatologica (accessed on 20 April 2018).
- Thomas, W. Mountain block recharge in Santo Tomas Valley, Baja California, Mexico, Centro de Investigación Científica y de Educación Superior de Ensenada. In Proceedings of the 2009 AGU Fall Meeting Abstracts, San Francisco, CA, USA, 14–18 December 2009. [Google Scholar]
- Wilson, J.L.; Guan, H. Mountain-block hydrology and mountain-front recharge. In Groundwater Recharge in a Desert Environment: The Southwestern United States; American Geophysical Union: Washington, DC, USA, 2004; pp. 113–137. [Google Scholar]
- Comisión Estatal de Servicios Públicos Tijuana-Sistema Tecate. Informe Hidrogeológico de la Cuenca del Arroyo Tecate; Comisión Estatal de Servicios Públicos Tijuana-Sistema Tecate: Tijuana, Mexico, 1988. [Google Scholar]
- Servicio Geológico Mexicano. Carta Geológica-Minera Tijuana I11-11; Servicio Geológico Mexicano: Pachuca, Mexico, 2003. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA/AWWA/WEF: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Östlund, H.G.; Werner, P.E. The Electrolytic Enrichment of Tritium and Deuterium for Natural Tritium Measurements. In Tritium in the Physical and Biological Sciences; International Atomic Energy Agency: Viena, Austria, 1962; Volume 1, pp. 95–105. [Google Scholar]
- McCrea, J.M. On the Isotopic Chemistry of Carbonates and a Paleotemperature Scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Denver, CO, USA, 2013. [Google Scholar]
- Pearson, F.J.; Hanshaw, B.B. Sources of dissolved carbonate species in groundwater and their effects on carbon-14 dating. In Isotope hydrology 1970; International Atomic Energy Agency: Viena, Austria, 1970; pp. 271–285. [Google Scholar]
- Plummer, L.N.; Prestemon, E.C.; Parkhurst, D.L. An Interactive Code (NETPATH) for Modeling Net Geochemical Reactions along a Flow Path; USGS Earth Science Information Center: Reston, VA, USA, 1991. [Google Scholar]
- Plummer, L.N.; Glynn, P.D. Radiocarbon dating in groundwater systems. In Isotope Methods for Dating Old Groundwater; International Atomic Energy Agency: Viena, Austria, 2013; pp. 33–89. ISBN 978-92-0-137210-9. [Google Scholar]
- Ganster, P. Tecate: Challenges for the Twenty-First Century. In Tecate, Baja California: Realities and Challenges in a Mexican Border Community; Ganster, P., Cuamea Velázquez, F., Castro Ruiz, J.L., Villegas, A., Eds.; SDSU Press: San Diego, CA, USA, 2002. [Google Scholar]
- Vogel, J.C.; Grootes, P.M.; Mook, W.G. Isotopic fractionation between gaseous and dissolved carbon dioxide. Zeitschrift für Physik 1970, 230, 225–238. [Google Scholar] [CrossRef]
- Mook, W.G.; Bommerson, J.C.; Staverman, W.H. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet. Sci. Lett. 1974, 22, 169–176. [Google Scholar] [CrossRef]
- Deines, P.; Langmuir, D.; Harmon, R.S. Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate ground waters. Geochim. Cosmochim. Acta 1974, 38, 1147–1164. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; A.A. Balkema Publishers: Leiden, The Netherlands, 2005. [Google Scholar]
- Pastén-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramírez, A.I.; Mahlknecht, J. Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci. Total Environ. 2014, 470–471, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Morán-Ramírez, J.; Ledesma-Ruiz, R.; Mahlknecht, J.; Ramos-Leal, J.A. Rock-water interactions and pollution processes in the volcanic aquifer system of Guadalajara, Mexico, using inverse geochemical modeling. Appl. Geochem. 2016, 68, 79–94. [Google Scholar] [CrossRef]
- Kuldip-Singh; Hundal, H.S. Dhanwinder-Singh Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ. Earth Sci. 2011, 64, 1823–1833. [Google Scholar] [CrossRef]
- Patterson, R.A. Domestic Wastewater and the Sodium Factor. In Site Characterization and Design of On-Site Septic Systems; ASTM International: West Conshohocken, PA, USA, 1997; pp. 23–35. [Google Scholar]
- Esteller, M.V.; Kondratenko, N.; Expósito, J.L.; Medina, M.; Martin del Campo, M.A. Hydrogeochemical characteristics of a volcanic-sedimentary aquifer with special emphasis on Fe and Mn content: A case study in Mexico. J. Geochem. Explor. 2017, 180, 113–126. [Google Scholar] [CrossRef]
- Rozanski, K.; Araguás-Araguás, L.; Gonfiantini, R. Isotopic Patterns in Modern Global Precipitation. In Climate Change in Continental Isotopic Records; Swart, P.K., Lohmann, K.C., Mckenzie, J., Savin, S., Eds.; American Geophysical Union: Washington, DC, USA, 1993; pp. 1–36. ISBN 0-87590-037-2. [Google Scholar]
- Kretzschmar, T.G.; Frommen, T. Stable Isotope Composition of Surface and Groundwater in Baja California, Mexico. Procedia Earth Planet. Sci. 2013, 7, 451–454. [Google Scholar] [CrossRef]
- Ledesma-Ruiz, R.; Mahlknecht, J. Geochemical and Isotopic Characterization of Groundwater in Tecate, Baja California, Mexico. Procedia Earth Planet. Sci. 2017, 17, 516–519. [Google Scholar] [CrossRef]
- Peng, H.; Mayer, B.; Harris, S.; Krouse, H.R. The influence of below-cloud secondary effects on the stable isotope composition of hydrogen and oxygen in precipitation at Calgary, Alberta, Canada. Tellus B Chem. Phys. Meteorol. 2007, 59, 698–704. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Van Wilgenburg, S.L.; Larson, K.; Hobson, K.A. A groundwater isoscape (δD, δ18O) for Mexico. J. Geochem. Explor. 2009, 102, 123–136. [Google Scholar] [CrossRef]
- Eastoe, C.J. Isotopic Data for Tucson Basin: Summary of Work to 1998; Laboratory of Isotope Geochemistry: Tucson, AZ, USA, 1998. [Google Scholar]
- Harris, R.C. Tritium as a Tracer of Groundwater Sources and Movement in the Safford Basin, Graham County, Arizona; Arizona Geological Survey: Tucson, AZ, USA, 2000. [Google Scholar]
- Daesslé, L.W.; van Geldern, R.; Orozco-Durán, A.; Barth, J.A.C. The 2014 water release into the arid Colorado River delta and associated water losses by evaporation. Sci. Total Environ. 2016, 542, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Daesslé, L.W.; Orozco, A.; Struck, U.; Camacho-Ibar, V.F.; van Geldern, R.; Santamaría-del-Angel, E.; Barth, J.A.C. Sources and sinks of nutrients and organic carbon during the 2014 pulse flow of the Colorado River into Mexico. Ecol. Eng. 2017, 106, 799–808. [Google Scholar] [CrossRef]
- Secretaria de Salud. NORMA Oficial Mexicana NOM-127-SSA1-1994, Salud Ambiental, Agua Para uso y Consumo Humano-Límites Permisibles de Calidad y Tratamientos a Que Debe Someterse el Agua Para su Potabilización; Secretaria de Salud: Mexico City, Mexico, 1994; pp. 1–7. [Google Scholar]








| Well ID | Temp | pH | EC | OD | Na | K | Ca | Mg | HCO3 | Cl | SO4 | NO3 | F | Sr | Fe | Si | Charge Balance Error | 18O | 2H | 3H | 13C | 14C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (-) | (°C) | (std. Unit) | (µS cm−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (mg L−1) | (%) | (‰) | (‰) | (TU) | (‰) | (pMC) |
| 1 | 19.9 | 7.7 | 706 | 5.0 | 76.1 | 2.5 | 56.1 | 23.8 | 414.3 | 65.1 | 31.3 | 1.0 | <0.5 | 0.264 | 0.010 | 9.2 | −6.8 | −6.84 | −46.47 | 2.2 | −13.0 | 107.2 |
| 2 | 20.5 | 7.9 | 1111 | 7.2 | 149.0 | 6.0 | 61.6 | 37.4 | 604.8 | 123.5 | 56.2 | 6.6 | 0.7 | 0.422 | 0.010 | 10.2 | −6.8 | −6.40 | −43.75 | --- | --- | --- |
| 3 | 18.7 | 7.8 | 1323 | 6.7 | 179.2 | 5.7 | 83.9 | 40.6 | 444.7 | 146.1 | 130.4 | 5.5 | 0.6 | 0.550 | 0.010 | 17.3 | 4.7 | −6.50 | −43.94 | 2.8 | −13.4 | 104.0 |
| 4 | 21.2 | 7.8 | 835 | 7.3 | 79.0 | 6.1 | 68.7 | 28.5 | 305.7 | 90.7 | 75.4 | 3.4 | <0.5 | 0.278 | 0.010 | 22.7 | 1.0 | −6.16 | −42.51 | --- | --- | --- |
| 5 | 20.4 | 7.5 | 1738 | 5.5 | 199.1 | 5.3 | 131.8 | 65.1 | 587.9 | 163.0 | 275.3 | 4.5 | <0.5 | 0.761 | 0.010 | 19.5 | 1.9 | −7.12 | −50.20 | --- | --- | --- |
| 6 | 21.8 | 7.7 | 1351 | 3.8 | 154.9 | 5.4 | 102.5 | 44.2 | 376.4 | 146.9 | 182.1 | 10.3 | <0.5 | 0.524 | 0.010 | 21.6 | 5.2 | −6.69 | −47.75 | 2.7 | −14.2 | 100.8 |
| 7 | 18.4 | 7.8 | 1860 | 6.6 | 268.6 | 4.4 | 103.7 | 52.7 | 646.9 | 280.3 | 139.2 | 2.3 | <0.5 | 0.626 | 0.010 | 20.7 | −0.5 | −6.54 | −44.94 | 1.8 | −13.4 | 98.4 |
| 8 | 19.8 | 7.8 | 1594 | 5.3 | 190.9 | 7.0 | 111.5 | 50.4 | 533.1 | 220.8 | 121.8 | 1.9 | <0.5 | 0.418 | 0.010 | 16.3 | 1.7 | −6.75 | −47.20 | 2.1 | −13.8 | 97.3 |
| 9 | 21.2 | 7.3 | 1907 | 7.9 | 202.9 | 4.8 | 134.1 | 78.5 | 681.9 | 251.5 | 145.5 | 22.2 | <0.5 | 0.841 | 0.010 | 22.0 | 1.6 | −6.90 | −47.92 | --- | --- | --- |
| 10 | 19.2 | 7.8 | 1332 | 4.3 | 164.8 | 4.4 | 85.1 | 41.9 | 405.7 | 140.5 | 140.1 | 12.9 | 0.6 | 0.589 | 0.010 | 19.4 | 4.8 | −6.86 | −47.57 | --- | --- | --- |
| 13 | 19.7 | 7.5 | 1319 | 5.3 | 170.2 | 4.2 | 83.0 | 40.8 | 459.1 | 160.6 | 147.6 | 12.8 | 0.6 | 0.562 | 0.010 | 18.9 | −1.0 | −6.84 | −47.80 | --- | --- | --- |
| 14 | 17.6 | 8.6 | 1525 | 13.1 | 200.8 | 6.4 | 98.5 | 39.4 | 250.9 | 178.3 | 302.2 | 0.3 | 0.4 | 1.262 | 0.013 | 5.2 | 4.9 | −10.30 | −88.04 | 7.4 | −8.7 | 90.6 |
| 15 | 15.6 | 7.0 | 437 | 9.2 | 43.1 | 3.5 | 28.1 | 12.2 | 162.5 | 34.0 | 28.1 | 18.2 | <0.5 | 0.238 | 0.010 | 13.3 | −0.3 | −7.45 | −48.98 | 2.2 | −15.8 | 96.0 |
| 16 | 13.6 | 7.9 | 1070 | 5.3 | 125.2 | 5.1 | 77.0 | 25.5 | 503.1 | 109.2 | 12.6 | 0.2 | 0.3 | 0.826 | 0.065 | 10.9 | −1.2 | −6.60 | −46.88 | 1.8 | −12.6 | 101.7 |
| 17 | 19.4 | 7.7 | 1189 | 8.3 | 121.9 | 2.6 | 85.0 | 30.1 | 303.4 | 140.7 | 71.4 | 35.5 | 0.4 | 0.651 | 0.010 | 26.8 | 6.6 | −7.15 | −48.88 | --- | --- | --- |
| 18 | 18.6 | 7.7 | 1299 | 10.8 | 136.5 | 2.5 | 102.8 | 35.5 | 445.3 | 151.3 | 100.2 | 8.2 | 0.6 | 0.682 | 0.010 | 19.7 | 0.6 | −8.25 | −60.33 | 2.3 | −14.2 | 103.5 |
| 19 | 17.3 | 7.8 | 854 | 11.8 | 197.4 | 7.2 | 117.8 | 35.5 | 795.7 | 95.6 | 31.0 | 10.1 | <0.5 | 0.457 | 0.010 | 9.0 | 2.8 | −7.54 | −51.83 | 1.9 | −13.6 | 107.4 |
| 20 | 18.9 | 7.9 | 637 | 10.8 | 71.3 | 2.7 | 48.9 | 13.0 | 288.2 | 50.5 | 21.6 | 12.3 | <0.5 | 0.439 | 0.010 | 10.2 | −1.4 | −7.54 | −51.35 | 1.8 | −12.5 | 108.8 |
| 24 | 20.3 | 7.4 | 2350 | 2.8 | 307.8 | 2.4 | 132.1 | 74.8 | 447.6 | 317.6 | 409.4 | 0.2 | 0.8 | 0.030 | 0.180 | 16.3 | 2.3 | −8.82 | −72.73 | 5.5 | −14.7 | 102.2 |
| 25 | 18.4 | 7.4 | 664 | 7.3 | 74.9 | 2.9 | 42.3 | 12.5 | 214.0 | 86.2 | 28.5 | 2.1 | <0.5 | 0.051 | 0.010 | 22.0 | −2.8 | −7.92 | −56.76 | <0.5 | −13.3 | 98.9 |
| 26 | 15.8 | 7.6 | 1655 | 5.2 | 253.3 | 4.8 | 75.7 | 24.7 | 421.1 | 178.5 | 118.6 | 28.6 | 2.2 | 0.110 | 0.010 | 19.4 | 7.3 | −7.67 | −54.40 | 1.0 | −13.3 | 102.6 |
| 27 | 16.6 | 7.4 | 767 | 5.8 | 92.7 | 1.3 | 57.1 | 16.1 | 286.6 | 104.0 | 29.4 | 0.2 | 0.6 | 0.086 | 0.019 | 18.9 | −2.1 | −7.62 | −51.65 | <0.9 | −14.8 | 95.7 |
| 28 | 14.4 | 7.7 | 616 | 6.1 | 81.9 | 2.0 | 37.9 | 12.7 | 293.0 | 75.6 | 27.7 | 1.6 | <0.5 | 0.058 | 0.023 | 13.3 | −9.3 | −7.51 | −51.60 | 2.2 | −13.6 | 101.3 |
| 29 | 17.9 | 7.6 | 508 | 5.2 | 50.4 | 2.3 | 37.7 | 13.0 | 248.9 | 41.8 | 13.8 | 5.6 | <0.5 | 0.022 | 0.010 | 10.9 | −6.5 | −7.15 | −49.04 | 1.5 | −15.3 | 107.3 |
| 32 | 17.9 | 7.1 | 967 | 5.0 | 115.8 | 2.4 | 46.0 | 25.9 | 200.5 | 118.0 | 150.9 | 0.5 | <0.5 | 0.039 | 0.026 | 20.6 | −3.3 | −6.29 | −47.64 | 1.8 | −15.5 | 101.2 |
| 33 | 16.1 | 7.7 | 7480 | 5.8 | 747.6 | 185.6 | 374.3 | 247.7 | 1238.7 | 1584.4 | 574.8 | 15.8 | <0.5 | 0.002 | 0.078 | 18.7 | −0.7 | −5.83 | −47.67 | 2.2 | −10.2 | 106.1 |
| Year | Measures | CE (µS/cm) | pH | Ca (mg L−1) | Mg (mg L−1) | Na (mg L−1) |
| 1981 | Avg. ± St.dev. | 1122 ± 739 | 8.1 ± 0.4 | 51.3 ± 16.6 | 40.6 ± 33.2 | 138.3 ± 139.5 |
| 2014 | Avg. ± St.dev. | 1622 ± 1642 | 7.6 ± 0.3 | 103.0 ± 79.4 | 49.3 ± 56.7 | 193.0 ± 163.3 |
| p-value | 0.001 * | 0.001 * | 0.021 * | 0.584 | 0.269 | |
| K (mg L−1) | Cl (mg L−1) | HCO3 (mg L−1) | SO4 (mg L−1) | NO3 (mg L−1) | ||
| 1981 | Avg. ± St.dev. | 4.3 ± 2.1 | 175.9 ± 184.7 | 207.6 ± 45.6 | 159.0 ± 205.7 | 7.2 ± 6.3 |
| 2014 | Avg. ± St.dev. | 15.2 ± 45.5 | 232.5 ± 367.1 | 472.7 ± 261.6 | 151.5 ± 161.6 | 8.8 ± 11.1 |
| p-value | 0.346 | 0.577 | 0.001 * | 0.887 | 0.640 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlknecht, J.; Daessle, L.W.; Esteller, M.V.; Torres-Martinez, J.A.; Mora, A. Groundwater Flow Processes and Human Impact along the Arid US-Mexican Border, Evidenced by Environmental Tracers: The Case of Tecate, Baja California. Int. J. Environ. Res. Public Health 2018, 15, 887. https://doi.org/10.3390/ijerph15050887
Mahlknecht J, Daessle LW, Esteller MV, Torres-Martinez JA, Mora A. Groundwater Flow Processes and Human Impact along the Arid US-Mexican Border, Evidenced by Environmental Tracers: The Case of Tecate, Baja California. International Journal of Environmental Research and Public Health. 2018; 15(5):887. https://doi.org/10.3390/ijerph15050887
Chicago/Turabian StyleMahlknecht, Jürgen, Luis Walter Daessle, Maria Vicenta Esteller, Juan Antonio Torres-Martinez, and Abrahan Mora. 2018. "Groundwater Flow Processes and Human Impact along the Arid US-Mexican Border, Evidenced by Environmental Tracers: The Case of Tecate, Baja California" International Journal of Environmental Research and Public Health 15, no. 5: 887. https://doi.org/10.3390/ijerph15050887







