A Systematic Review of the Time Series Studies Addressing the Endemic Risk of Acute Gastroenteritis According to Drinking Water Operation Conditions in Urban Areas of Developed Countries
Abstract
:1. Introduction
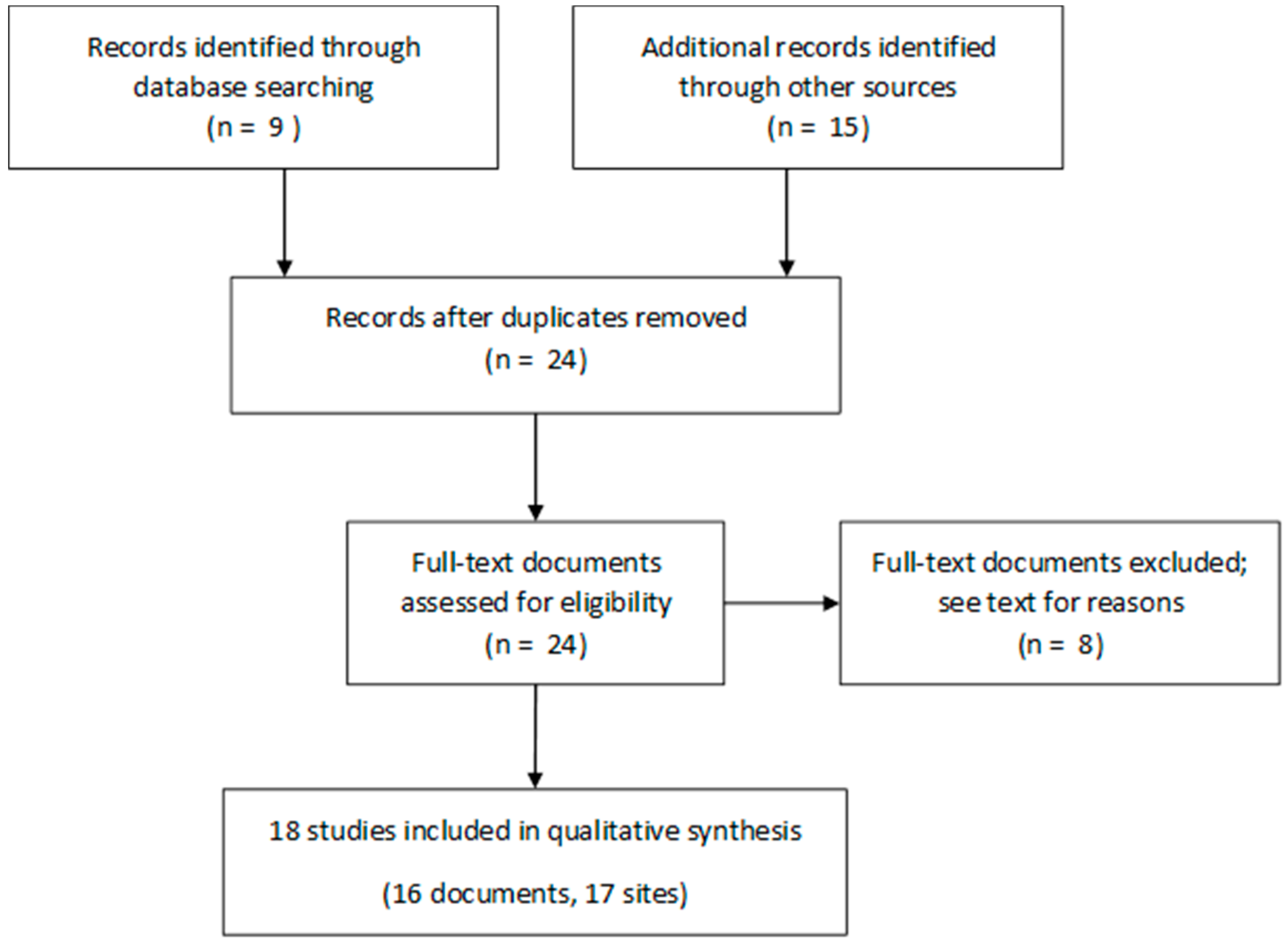
2. Materials and Methods
- Studies focusing on an outbreak;
- Studies which design prevents the marginal risk estimate;
- Prospective studies which used self-reported health outcome were excluded to prevent reporting biases due to individuals’ perception of exposure. Only health care-related databases were used, including on-line remote diagnosis data from calls for medical advice;
- Studies showing inadequate mathematical control of potential confounding factors (i.e., resulting in bias);
- Studies where the number of AGE cases included was under 1000, (excluded to prevent insufficient statistical power);
- Studies with a duration of two years or less (excluded to achieve minimal representativeness of the hydro-epidemiological conditions diversity); and
- Studies mapping complex distribution zones (DZ) resulting in major exposure misclassification.
- Minimal significance (p < 0.1) of the turbidity-risk function (TRF);
- Sufficient plausibility of the shape of the TRF: an increasing TRF was required for the shape to be considered plausible; and
- Good fit between (1) the turbidity-AGE latency observed in the model and (2) the delay for both water transit through the distribution network and infection incubation in sick people.
3. Results
3.1. Selected Studies
- The Egorov et al. study [22] because of its short duration, the small population size, and the use of self-reported health outcomes;
- The Drayna et al. study [23] because it focused on the relationship between precipitation and AGE in Wisconsin (USA), irrespective of distribution zone (DZ) locations and organization;
- The first study carried out in Le Havre [26] that used a moving average model yielding conditional risk estimates.
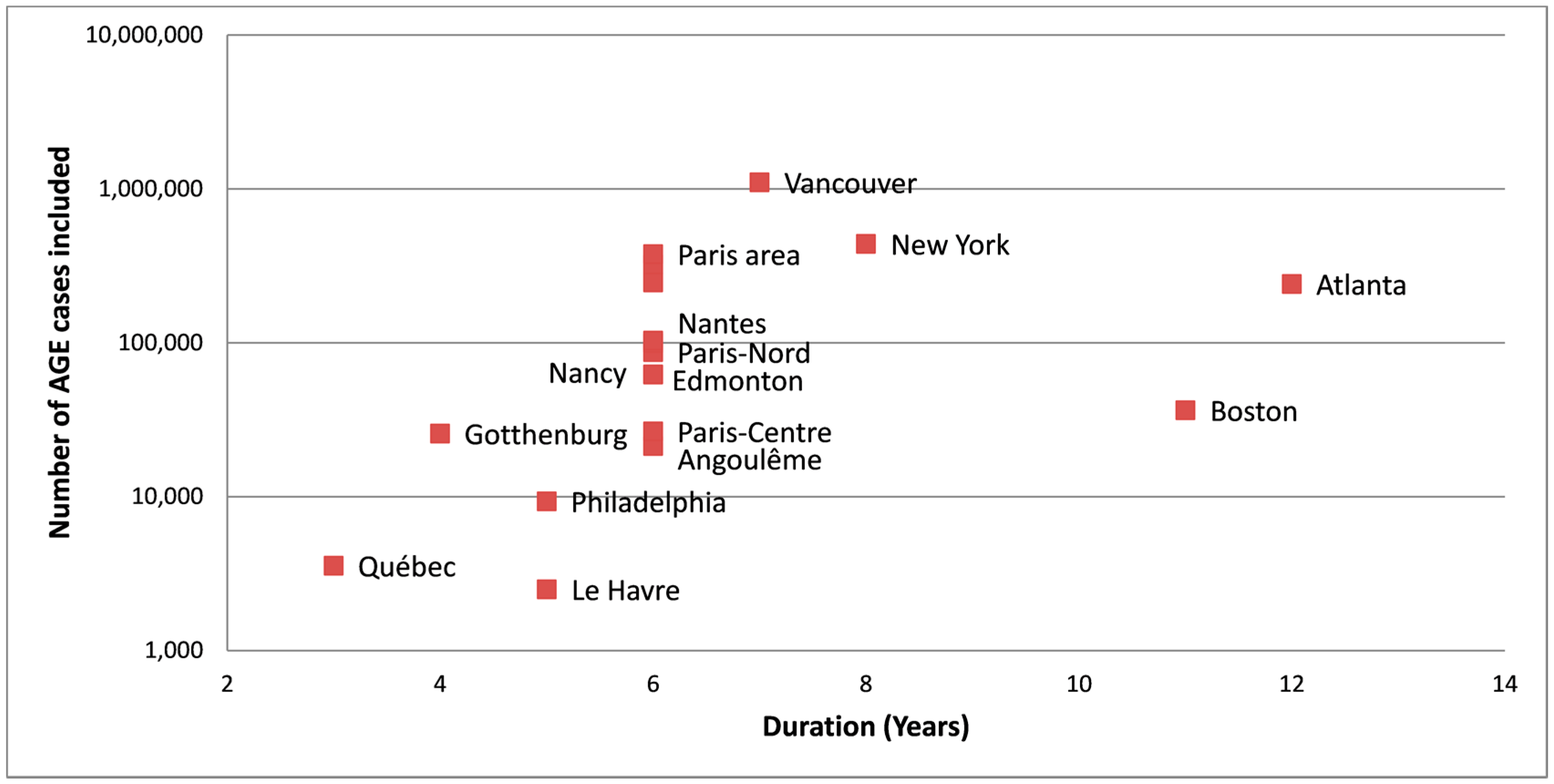
3.2. Serviced Populations and Water Systems
3.3. Health Outcomes
3.4. Model Designs
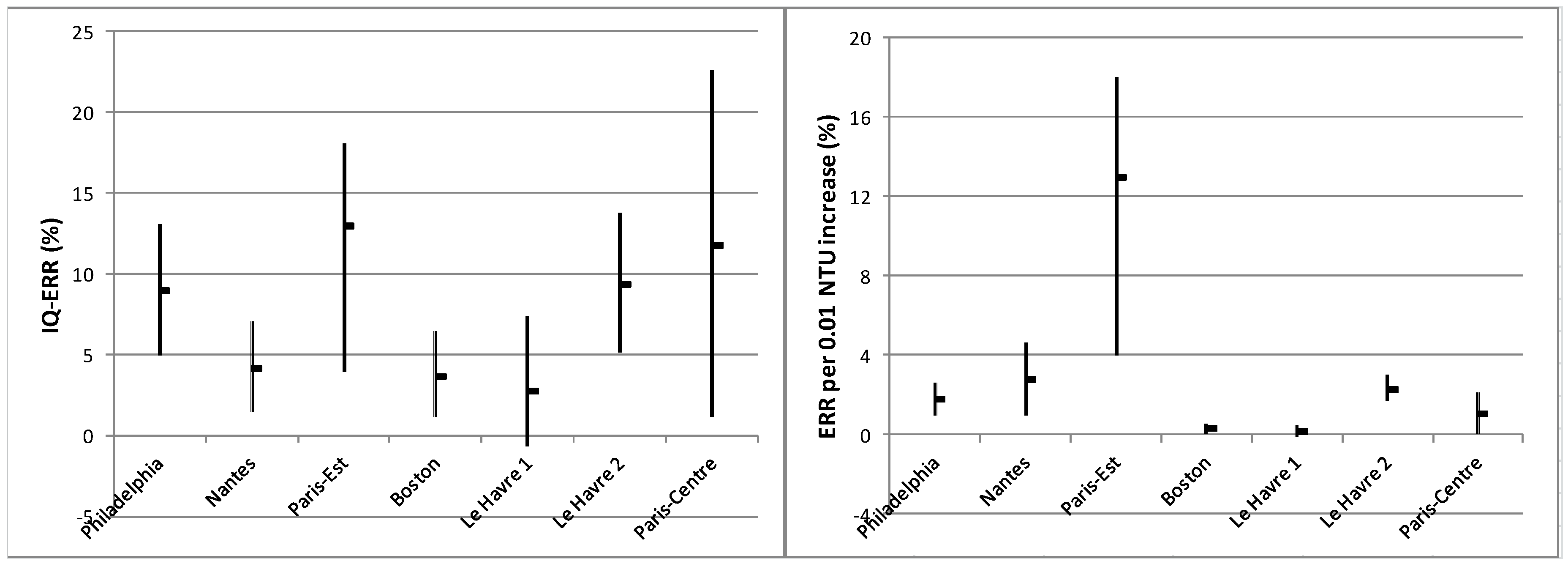
3.5. Turbidity-Related Risk
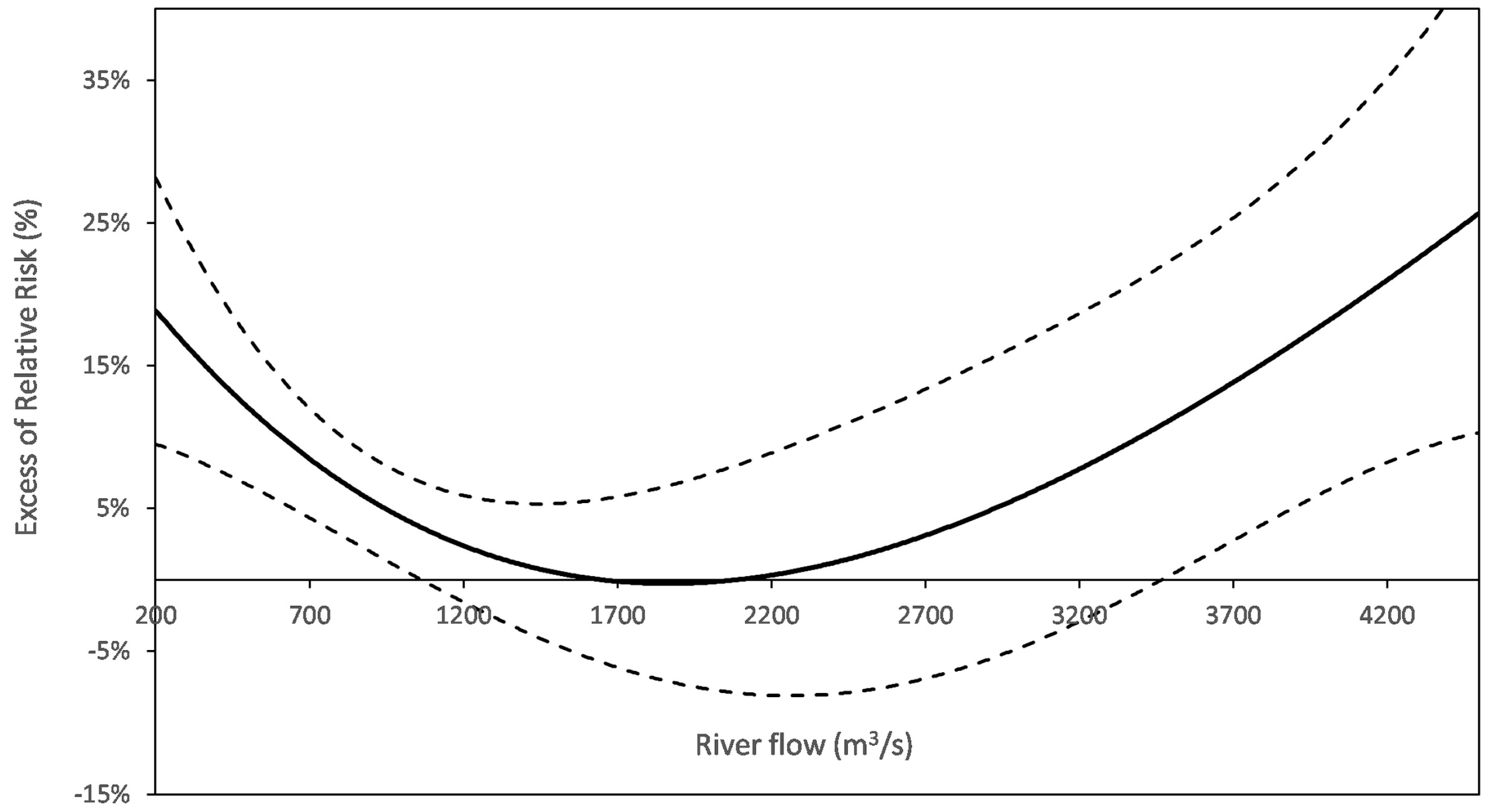
3.6. In Search for Additional Exposure Proxies from Water Operation Data
4. Discussion
4.1. An Underestimated Established Risk
4.2. How to Improve Inter-Site Comparison of Turbidity-Related Risks
4.2.1. About Health Outcomes
4.2.2. Need to Standardize the Time Window for Exposure Assessment
4.2.3. Need for Long Duration Studies
4.3. Microbiological vs. Operational Interpretations of Turbidity
4.4. Public Health Issues
4.4.1. TSS Do Not Support the Quantitative Health Impact Assessment
4.4.2. TSS Do Not Help Identify Causative Pathogens
4.4.3. But TSS Can Teach Water Operators about High Risk Conditions
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Acute gastroenteritis |
| CFD | Coagulation-flocculation-decantation |
| DWS | Drinking water system |
| DWTP | Drinking water treatment plant |
| DZ | Distribution zone |
| ERR | Excess of relative risk |
| GAM | Generalized additive model |
| GP | General Practitioner |
| ICD | International classification of diseases |
| IQ | Interquartile |
| NTU | Nephelometric turbidity unit |
| P10 | 10th Percentile |
| PA | Paris area |
| PRISMA | Preferred reporting items for Systematic Reviews and Meta-Analysis |
| TRF | Turbidity risk function |
| TSS | Time series study |
| Tu_FW | Turbidity of finished water |
| Tu_RW | Turbidity of raw water |
| USA | United States of America |
| UV | Ultraviolet |
References
- Morris, R.D.; Naumova, E.N.; Griffiths, J.K. Did Milwaukee experience waterborne cryptosporidiosis before the large documented outbreak in 1993? Epidemiology 1998, 9, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Payment, P.; Richardson, L.; Siemiatycki, J.; Dewar, R.; Edwardes, M.; Franco, E. A randomized trial to evaluate the risk of gastrointestinal disease due to consumption of drinking water meeting current microbiological standards. Am. J. Public Health 1991, 81, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Payment, P.; Siemiatycki, J.; Richardson, L.; Renaud, G.; Franco, E.; Prevost, M. A prospective epidemiological study of gastrointestinal health effects due to the consumption of drinking water. Int. J. Environ. Health Res. 1997, 7, 5–31. [Google Scholar] [CrossRef]
- Hellard, M.E.; Sinclair, M.I.; Forbes, A.B.; Fairley, C.K. A randomized, blinded, controlled trial investigating the gastrointestinal health effects of drinking water quality. Environ. Health Perspect. 2001, 109, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Reacher, M.; Casemore, D.P.; Verlander, N.Q.; Charlett, A.; Chalmers, R.M.; Knowles, M.; Pennington, A.; Williams, J.; Osborn, K.; et al. Sporadic cryptosporidiosis decline after membrane filtration of public water supplies, england, 1996–2002. Emerg. Infect. Dis. 2005, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Harrison, F.C.; Chalmers, R.M.; Bentham, G.; Nichols, G.; Hunter, P.R.; Kovats, R.S.; Grundy, C. Case-control study of environmental and social factors influencing cryptosporidiosis. Eur. J. Epidemiol. 2007, 22, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.I.; Fairley, C.K. Drinking water and endemic gastrointestinal illness. J. Epidemiol. Community Health 2000, 54, 728. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Levin, R. Drinking water turbidity and health. Epidemiology 1999, 10, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P. Surveillance Syndromique Des Gastroentérites Aigües: Une Opportunité Pour la Prévention du Risque Infectieux Attribuable à L’ingestion D’eau du Robinet. [Syndromic Surveillance of Acute Gastroenteritis: An Opportunity for the Prevention of the Infectious Risk Attributable to Tap Water]. Ph.D. Thesis, Université de Rennes, Rennes, France, 2012. Available online: https://tel.archives-ouvertes.fr/tel-00795215/document (accessed on 1 November 2017).
- Schwartz, J.; Levin, R.; Hodge, K. Drinking water turbidity and pediatric hospital use for gastrointestinal illness in Philadelphia. Epidemiology 1997, 8, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Dockery, D.W. Particulate air pollution and daily mortality in Steubenville, Ohio. Am. J. Epidemiol. 1992, 135, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.G.; Tam, C.C.; Higgins, C.D.; Rodrigues, L.C. The association between drinking water turbidity and gastrointestinal illness: A systematic review. BMC Public Health 2007, 7, 256–262. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.J.; Gurian, P.L.; Robinson, L.F.; Rai, A.; Zakeri, I.; Kondo, M.C. Review of epidemiological studies of drinking-water turbidity in relation to acute gastrointestinal illness. Environ. Health Perspect. 2017, 125, 086003. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P.; Rambaud, L.; Zeghnoun, A.; Corso, M. Qualité de L’eau Distribuée à Angoulême et Incidence Des Gastro-Entérites Aiguës, 2002–2007 [Quality of Water Distributed in Angoulême (France) and Incidence of Acute Gastroenteritis, 2002-7]; Institut de Veille Sanitaire: Saint-Maurice, France, 2013; p. 36. ISBN 978-2-11-131096-4. Available online: http://invs.santepubliquefrance.fr (accessed on 1 November 2017).
- Rambaud, L.; Zeghnoun, A.; Corso, M.; Beaudeau, P. Qualité de L’eau Distribuée à Paris-Centre et Incidence Des Gastro-Entérites Aiguës, 2002–2007 [Quality of Water Distributed in Paris (France) and Incidence of Acute Gastroenteritis, 2002-7]; Institut de Veille Sanitaire: Saint-Maurice, France, 2013; p. 43. ISBN 978-2-11-138332-6. Available online: http://invs.santepubliquefrance.fr (accessed on 1 November 2017).
- Rambaud, L.; Zeghnoun, A.; Corso, M.; Beaudeau, P. Qualité de L’eau Distribuée à Paris-Est et Incidence Des Gastroentérites aiguës, 2002–2007 [Quality of Water Distributed in East of Paris (France) and Incidence of Acute Gastroenteritis, 2002-7]; Institut de Veille Sanitaire: Saint-Maurice, France, 2014; p. 46. ISBN 979-10-289-0071-4. Available online: http://invs.santepubliquefrance.fr (accessed on 1 November 2017).
- Rambaud, L.; Zeghnoun, A.; Corso, M.; Beaudeau, P. Qualité de L’eau Distribuée en Banlieue Parisienne et Incidence Des Gastro-Entérites aiguës, 2002–2007 [Quality of Water Distributed in Paris Suburbs (France) and Incidence of Acute Gastroenteritis, 2002-7]; Institut de Veille Sanitaire: Saint-Maurice, France, 2015; p. 83. ISBN 979-10-289-0170-7. Available online: http://invs.santepubliquefrance.fr (accessed on 1 November 2017).
- Rambaud, L.; Zeghnoun, A.; Corso, M.; Beaudeau, P. Qualité de L’eau Distribuée à Nancy et Incidence Des Gastro-Entérites Aiguës, 2002–2007 [Quality of Water Distributed in Nancy (France) and Incidence of Acute Gastroenteritis, 2002-7]; Institut de Veille Sanitaire: Saint-Maurice, France, 2016; p. 41. ISBN 979-10-289-0195-0. Available online: http://invs.santepubliquefrance.fr (accessed on 1 November 2017).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.D.; Naumova, E.N.; Levin, R.; Munasinghe, R.L. Temporal variation in drinking water turbidity and diagnosed gastroenteritis in Milwaukee. Am. J. Public Health 1996, 86, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.N.; Egorov, A.I.; Morris, R.D.; Griffiths, J.K. The elderly and waterborne Cryptosporidium infection: Gastroenteritis hospitalizations before and during the 1993 Milwaukee outbreak. Emerg. Infect. Dis. 2003, 9, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.I.; Naumova, E.N.; Tereschenko, A.A.; Kislitsin, V.A.; Ford, T.E. Daily variations in effluent water turbidity and diarrhoeal illness in a Russian city. Int. J. Environ. Health Res. 2003, 13, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Drayna, P.; McLellan, S.L.; Simpson, P.; Li, S.H.; Gorelick, M.H. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ. Health Perspect. 2010, 118, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.L.; Edge, V.L.; Schuster-Wallace, C.J.; Berke, O.; McEwen, S.A. Weather, water quality and infectious gastrointestinal illness in two Inuit communities in Nunatsiavut, Canada: Potential implications for climate change. Ecohealth 2011, 8, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Uejio, C.K.; Yale, S.H.; Malecki, K.; Borchardt, M.A.; Anderson, H.A.; Patz, J.A. Drinking water systems, hydrology, and childhood gastrointestinal illness in central and Northern Wisconsin. Am. J. Public Health 2014, 104, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P.; Payment, P.; Bourderont, D.; Mansotte, F.; Boudhabay, O.; Laubiès, B.; Verdière, J. A time series study of anti-diarrheal drug sales and tap-water quality. Int. J. Environ. Health Res. 1999, 9, 293–311. [Google Scholar] [CrossRef]
- Schwartz, J.; Levin, R.; Goldstein, R. Drinking water turbidity and gastrointestinal illness in the elderly of Philadelphia. J. Epidemiol. Community Health 2000, 54, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Investigating the Relationship Between Drinking Water and Gastroenteritis in Edmonton: 1993–1998. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/investigating-relationship-between-drinking-water-gastroenteritis-edmonton-1993-1998.html?wbdisable=true (accessed on 1 November 2017).
- Gilbert, M.L.; Levallois, P.; Rodriguez, M.J. Use of a health information telephone line, info-sante clsc, for the surveillance of waterborne gastroenteritis. J. Water Health 2006, 4, 225–232. [Google Scholar] [PubMed]
- Tinker, S.C.; Moe, C.L.; Klein, M.; Flanders, W.D.; Uber, J.; Amirtharajah, A.; Singer, P.; Tolbert, P.E. Drinking water turbidity and emergency department visits for gastrointestinal illness in atlanta, 1993–2004. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P.; Zeghnoun, A.; Corso, M.; Lefranc, A.; Rambaud, L. A time series study of gastroenteritis and tap water quality in the Nantes area, France, 2002–2007. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Tornevi, A.; Axelsson, G.; Forsberg, B. Association between precipitation upstream of a drinking water utility and nurse advice calls relating to acute gastrointestinal illnesses. PLoS ONE 2013, 8, e69918. [Google Scholar] [CrossRef] [PubMed]
- Aramini, J.; Allen, B.; Copes, R.; Holt, J.; Mc Lean, M.; Sears, W.; Wilson, J. Drinking Water Quality and Health Care Utilization for Gastrointestinal Illness in Greater Vancouver; University of Guelph and Vancouver Richmond Health Board, Guelf: Guelph, ON, Canada, 2000; p. 78. [Google Scholar]
- Beaudeau, P.; Schwartz, J.; Levin, R. Drinking water quality and hospital admissions of elderly people for gastrointestinal illness in Eastern Massachusetts, 1998–2008. Water Res. 2014, 52, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.L.; Nguyen, T.Q.; Matte, T.; Ito, K. Drinking water turbidity and emergency department visits for gastrointestinal illness in new york city, 2002–2009. PLoS ONE 2015, 10, e0125071. [Google Scholar] [CrossRef] [PubMed]
- Beaudeau, P.; Le, T.A.; Zeghnoun, A.; Zanobetti, A.; Schwartz, J. A time series study of drug sales and turbidity of tap water in Le Havre, France. J. Water Health 2012, 10, 221–235. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of the Interior, U.S. Geological Survey. USGS Water-Quality Data for USA. Available online: https://waterdata.usgs.gov/nwis/qw (accessed on 1 November 2016).
- Gregory, M.B.; Frick, E.A. Fecal-coliform bacteria concentrations in streams of the Chattahoochee River National Recreation Area, Metropolitan Atlanta, Georgia, May–October 1994 and 1995. U.S. Geological Survey Water-Resources Investigations Report 00-4139; 2000; p. 8. Available online: https://pubs.usgs.gov/wri/wri004139/pdf/wrir00-4139.pdf (accessed on 1 November 2016).
- NYC Environmental Protection. New York City 2009 Drinking Water Supply and Quality Report. 2010; p. 16. Available online: http://www.nyc.gov/html/dep/pdf/wsstate09.pdf (accessed on 1 November 2016).
- Jones, T.F.; McMillian, M.B.; Scallan, E.; Frenzen, P.D.; Cronquist, A.B.; Thomas, S.; Angulo, F.J. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996–2003. Epidemiol. Infect. 2007, 135, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Van Cauteren, D.; de Valk, H.; Vaux, S.; Le, S.Y.; Vaillant, V. Burden of acute gastroenteritis and healthcare-seeking behaviour in France: A population-based study. Epidemiol. Infect. 2012, 140, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Bounoure, F.; Beaudeau, P.; Mouly, D.; Skiba, M.; Lahiani-Skiba, M. Syndromic surveillance of acute gastroenteritis based on drug consumption. Epidemiol. Infect. 2010, 139, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Hall, G.; Scallan, E.; Adak, G.K.; Gauci, C.; Jones, T.F.; Sockett, P.N. A common, symptom-based case definition for gastroenteritis. Epidemiol. Infect. 2008, 136, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.D.; Dominici, F.; Louis, T.A. Model choice in time series studies of air pollution and mortality. J. R. Stat. Soc. A 2006, 169, 179–203. [Google Scholar] [CrossRef]
- Perrakis, K.; Gryparis, A.; Schwartz, J.; Tertre, A.L.; Katsouyanni, K.; Forastiere, F.; Stafoggia, M.; Samoli, E. Controlling for seasonal patterns and time varying confounders in time-series epidemiological models: A simulation study. Stat. Med 2014, 33, 4904–4918. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.H.; Quade, G.; Hartemann, P.; Exner, M. Waterborne diseaeses in Europe: 1986–1996. Am. Water Work. Assoc. 2001, 93, 48–53. [Google Scholar] [CrossRef]
- Craun, G.F.; Calderon, R.L.; Nwachuku, N. Causes of waterborne outbreaks reported in the United States, 1991–1998. In Drinking Water and Infectious Disease: Estblishing the Links; Hunter, P.R., Waite, M., Ronchi, E., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 119–126. [Google Scholar]
- Ercumen, A.; Gruber, J.S.; Colford, J.M., Jr. Water distribution system deficiencies and gastrointestinal illness: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Jagai, J.S.; Li, Q.; Wang, S.; Messier, K.P.; Wade, T.J.; Hilborn, E.D. Extreme precipitation and emergency room visits for gastrointestinal illness in areas with and without combined sewer systems: An analysis of Massachusetts data, 2003–2007. Environ. Health Perspect. 2015, 123, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ling, F.Q.; van der Mark, E.J.; Zhang, X.D.; Knezev, A.; Verberk, J.Q.; van der Meer, W.G.; Medema, G.J.; Liu, W.T.; van Dijk, J.C. Comparison of particle-associated bacteria from a drinking water treatment plant and distribution reservoirs with different water sources. Sci. Rep. 2016, 6, 20367. [Google Scholar] [CrossRef] [PubMed]
- Medema, G.J.; Schets, F.M.; Teunis, P.F.; Havelaar, A.H. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Appl. Environ. Microbiol. 1998, 64, 4460–4466. [Google Scholar] [PubMed]
- Krometis, L.A.; Characklis, G.W.; Simmons, O.D., III; Dilts, M.J.; Likirdopulos, C.A.; Sobsey, M.D. Intra-storm variability in microbial partitioning and microbial loading rates. Water Res. 2007, 41, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Mahler, B.J.; Personné, J.-C.; Lods, G.F.; Drogue, C. Transport of free and particulate-associated bacteria in karst. J. Hydrol. 2000, 238, 179–193. [Google Scholar] [CrossRef]
- Jamieson, R.; Joy, D.M.; Lee, H.; Kostaschuk, R.; Gordon, R. Transport and deposition of sediment-associated escherichia coli in natural streams. Water Res. 2005, 39, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Servais, P.; Garcia-Armisen, T. Partitioning and fate of particle-associated E. coli in river waters. Water Environ. Res. 2009, 81, 21–28. [Google Scholar]
- Hijnen, W.A.M.; Medema, G.J. Elimination of Micro-Organisms by Water Treatment Processes; IWA Publishing: London, UK, 2010; ISBN 9781843393733. [Google Scholar]
- LeChevallier, M.W.; Au, K. Water Treatment and Pathogen Control: Process Efficiency in Achieving Safe Drinking-Water; World Health Organization and IWA Publishing: London, UK, 2004; ISBN 1-84339-069-8. [Google Scholar]
- LeChevallier, M.W.; Evans, T.M.; Seidler, R.J. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl. Environ. Microbiol. 1981, 42, 159–167. [Google Scholar] [PubMed]
- Kaegi, R.; Wagner, T.; Hetzer, B.; Sinnet, B.; Tzvetkov, G.; Boller, M. Size, number and chemical composition of nanosized particles in drinking water determined by analytical microscopy and libd. Water Res. 2008, 42, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Craun, G.F.; Calderon, R.L. Observational epidemiologic studies of endemic waterborne risks: Cohort, case-control, time-series, and ecologic studies. J. Water Health 2006, 4 (Suppl. S2), 101–119. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Ballester, F.; Chanel, O.; Declercq, C.; Pascal, M. Quantifying the health impacts of outdoor air pollution: Useful estimations for public health action. J. Epidemiol. Community Health 2013. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessment; John Wiley & Sons: New York, NY, USA, 1999; p. 449. ISBN 9781118145296. [Google Scholar]
- Beaudeau, P.; Tousset, N.; Bruchon, F.; Lefevre, A.; Taylor, H.D. In situ measurement and statistical modelling of Escherichia coli decay in small rivers. Water Res. 2001, 35, 3168–3178. [Google Scholar] [CrossRef]
- Menon, P.; Billen, G.; Servais, P. Mortality rates of autochthonous and fecal bacteria in natural aquatic ecosystems. Water Res. 2003, 37, 4151–4158. [Google Scholar] [CrossRef]
- Gerba, C.P. Chapter 5 Virus occurrence and survival in the environmental waters. In Perspectives in Medical Virology; Bosch, A., Ed.; Elsevier: New York, NY, USA, 2007; Volume 17, pp. 91–108. ISBN 0168-7069. [Google Scholar]
- Gregory, J. Particles in Water, Properties and Processes; CRC Press and IWA Publishing: Boca Raton, FL, USA, 2006; p. 180. ISBN 9781587160851. [Google Scholar]

| Site | Period | Serviced Population | AGE Indicator | Age Classes (Years) | Number of Resources, DWTP and DZ Covered by the Study | Type of Resource | Turbidity in Raw Water: Mean (Max) (NTU) | [E.coli] in Raw Water: Mean (Max.) (CFU/100 mL) | Treatment Facilities a | Turbidity in Finished Water: Mean (Max) (NTU) | Number of AGE Cases Included | Exposure Scenario | ERR Related to Exposure Scenario | Significant Lags (Days) b | Proxies Tested as Exposure with Significance and Reproducibility c | Study |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Philadelphia (USA) | 1989–1993 | 1.2 M | Visits and admissions to hospital | <16 | 2-3-3 | Rivers | 9–19 (100–150) d | 20–40 (1000) | PCh, CFD, RFi, pChm | 0.17–0.20 | 3282 | Tu_FW IQ change (lag 4): 0.16–0.20 NTU | all cases: 7% [3; 12] | 1, 4, 6–7 †, 7–9 †, 8, 10, 13 | Tu_FW **◊ | Schwartz et al., 1997 [10] |
| Philadelphia (USA) | 1992–1993 | 1.2 M | Admissions to hospital | >64 | 2-3-3 | Rivers | 9–19 (100–150) d | 20–40 (1000) | PCh, CFD, RFi, pChm | 0.17–0.20 | 6021 | Tu_FW IQ change (lag 9–11): 0.16–0.21 NTU | all cases: 9% [5; 13] | 4–6 †, 9, 10, 11 | Tu_FW ** | S2: Schwartz et al., 2000 [27] |
| Edmonton (Canada) | 1993–1998 | 845,000 | Admissions to hospital, visits to emergency department, visits to GP | All, 2–18, 19–65, >65 | 1-2-3 | River | 35 (1500) | 400 (15,000) | CFD, Rfi, Ch, pChm | 0.04 (0.38) | 62,060 | Precipitation, Tu_RW, coliforms in raw water, Tu_FW, particle count, air temperature, change in the location of water abstraction point (0/1) | Lim et al., 2003 [28] | |||
| Québec (Canada) | 2000–2002 | 240,000 | Calls for medical advice | All | 1-1-1 | River | 1.7–3.2 | 62 (340) | PCh, CFD, Rfi, Oz, pCh | 0.27 (0.75) | 3555 | Tu_FW daily change from min. to max.: 0.11–0.75 NTU | 33–76% depending on the lag | 11, 15, 17 | Tu_FW **, precipitation | Gilbert et al., 2006 [29] |
| Atlanta (USA) | 1993–2004 | 3.0 M | Visits to emergency department | All | 3-9-8 | Rivers | Hourly max: 1.5–55 (1984) | 100 (1000) e | CFD, RFi, Ch (UV for 3 DWTPs), pCh | 0.03–0.17 | 240,925 | IQ change in Tu_FW: 0.04–0.09 NTU (lags 4–6)10 NTU change in Tu_RW over three weeks | 0.5% [−0.2; 1.2] (NS except for 1/8 distribution zones: 6% [4; 8]) | 4–11 | Tu_RW *** | Tinker et al., 2010 [30] |
| Nantes (France) | 2002–2007 | 410,000 | Consultations of GP | <16, >15 | 1-1-1 | River | 20 (124) | 120 (7000) | CFD, Rfi, Oz, pCh | 0.05 (0.35) | 103,149 | Tu_FW IQ change (lags 7–9): 0.04–0.06 NTU | 4.2% [1.5; 6.9] (child.), 2.9 [0.5; 5.4] (ad.) | 7–15 | Precipitation, Tu_RW, Tu_FW **◊, air temperature ***◊, river flow ***◊, produced flow **◊, free chlorine, interventions for broken pipe *, hydrant flushes | Beaudeau et al., 2014 [31] |
| Gothenburg (Sweden) | 2008–2011 | 500,000 | Calls for medical advice | All | 2-1-1 | River and lake | 5 (40) | 36 (6500) | CFD, Rfi, Ch | <0.05 | 25,659 | 40 mm precipitation in 24 h (lag 5) | 17% [7; 27] | 4–7 | Precipitation **, number of consecutive dry days, number of consecutive wet days ** | Tornevi et al., 2013 [32] |
| Paris-Est (France) | 2002–2007 | 379,000 | Consultations of GP | <16, >15 | 2-2-1 | Rivers | 15–16 (124–149) | 6200–6700 (125,000–240,000) | [CFD], RFi or Flot, Sfi, Oz, pCh (syst. 1) ; CoagRFi, Sfi, Oz, pCh (syst. 2) | 0.03–0.05 (0.14–0.19) | 99,315 | Tu_FW P10-P50 change (lags 6–8): 0.03–0.04 NTU | 13% [4; 18] (child.), 14% [4; 16] (ad.) | 6–8 † | Precipitation, Tu_RW **, Tu_FW **◊, water temperature ***◊, river flow **◊, produced flow *, free chlorine | Rambaud et al., 2014 [16] |
| Paris area—Nord (France) | 2002–2007 | 673,000 | Consultations of GP | <16, >15 | 1-2-1 | River | 20 (147) | 1600 (40,000) | CFD, RFi, Oz, NanoFi, UV, pCh (DWTP 1); CFD, RFI, Oz, Ch (DWTP 2) | 0.04 (0.05) | 246,165 | IQ change of particle count in filtered water (lags 6–8): 147–333 units/mL (0.03–0.05 NTU) | ERR = 12.1% [7.5; 17.0] (child.), 8.5% [4.3; 12.9] (ad.) | 6–8 †, 5–13 | Tu_RW, turbidity in filtered water *, particle count in finished water ***◊, TOC in raw water ***◊, water temperature ***◊, river flow ***◊, proportion of nanofiltered water, produced flow ***◊ | Rambaud et al., 2015 [17] |
| Paris area—Est (France) | 2002–2007 | 874,000 | Consultations of GP | <16, >15 | 1-1-1 | River | 30 (320) | 3100 (48,000) | CFD, RFI, Oz, Ch | 0.04 (0.05) | 322,773 | IQ change in particle count in filtered water (lags 6–8): 52–150 units/mL (0.04–0.04 NTU) | NS | Tu_RW, turbidity in filtered water **, Tu_FW, particle count in filtered water, TOC in raw water ***◊, TOC in filtered water, water temperature ***◊, river flow ***◊, produced flow *** | Rambaud et al., 2015 [17] | |
| Paris area—Sud (France) | 2002–2007 | 1.4 M | Consultations of GP | <16, >15 | 1-1-1 | River | 16 (220) | 1600 (43,000) | CFD, RFI, Oz, Ch | 0.03 (0.14) | 375,613 | IQ change in particle count in filtered water (lags 6–8): 25–65 units/mL (0.03–0.03 NTU) | ERR = 3.8% [1.0; 6.7] (child.), 2.7% [−0.3; 5.7] (ad.) | 6–10 | Tu_RW *, turbidity in filtered water **, Tu_FW, particle count in filtered water ***◊, TOC in raw water **◊, TOC in filtered water *, water temperature ***◊, river flow *◊, produced flow *** | Rambaud et al., 2015 [17] |
| Nancy (France) | 2002–2007 | 247,000 | Consultations of GP | <16, >15 | 1-2-1 | River | 8 (290) | 2,000 (16,000) | PCh, CFD, Oz, pCh | 0,07 (0,23) | 87,007 | Tu_FW IQ change (lags 5–7): 0.06–0.08 NTU | NS | Tu_RW, Tu_FW, water temperature ***◊, river flow, produced flow **, water cuts, interventions for broken pipe | Rambaud et al., 2016 [18] | |
| Vancouver (Canada) | 1992–1998 | 2.1 M | Visits to GP, admissions to hospital | All, <16, >64 | 3-3-3 | Reservoirs | 0.5–1.3 (8–19) | 1–2 (38–51) | Ch | 0.5–1.3 (8–19) | 14,571 H admission; 1.102 M visits to GP | Tutbidity > 1 NTU | Attributable Risk: 0.8–2.1 visits to GP; 0.2–1.3% H visits | 3–6 †, 6–9 †, 12–16 †, 21–29 † | Turbidity ***◊, precipitation, fecal coliform | Aramini et al., 2000 [33] |
| Boston (USA) | 1998–2008 | 1.5 M | Visits to hospital | >64 | 1-1-1 | Reservoirs | 0.34 (0.68) | 1.5 (43) | Ch/Oz, pChm | 0.34 (0.68) | 36,456 | Turbidity IQ change (lags 8–12): 0.28–0.39 NTU | ERR = 3.7% [1.2; 6.3] | 8–12 †, 13–17 †, 18–22 †, 23–27 †, 28–32 †, 33–37 † | Precipitation, turbidity corrected from algae **, water temperature **, fecal coliforms *, cyanobacteria *, ozone *, abs.UV350, CT | Beaudeau et al., 2014 [34] |
| New York (USA) | 2002–1999 | 9.2 M | Visits to emergency department | All, 1–4, 5–17 | 3-3-1 | Reservoirs | 0.98–1.0 (2.80–2.85) | 1–2 (14–57) f | Ch | 0.97 (2.38) | 438,000 | Turbidity IQ change (lag 6): NA | 5% [3;6] in spring, NS in other seasons | 3–11 | Turbidity ***◊ (only in spring) | Hsieh et al., 2015 [35] |
| Le Havre (France) | 1994–1996, 1997–2000 | 80,000 | Drug sales | All | 2-2-1 | Karstic springs | 4 (>200) (syst. 1); 0.1 (1) (syst. 2) | 80 (1000) (syst. 1); 8 (50) (syst. 2) | [CFD], Rfi, Ch (syst. 1); Ch (syst. 2) | 0.3 (>1.5) (syst. 1); 0.1 (1.0) (syst. 2) | 14,600 drug boxes (2500 cases) | IQ change in Tu_FW over lags 6–8:0.13–0.27 NTU (syst. 1); 0.08–0.11 NTU (syst. 2) | 2.8 [−0.6;7.2] (syst. 1); 9.4 [5.2; 13.7] (syst. 2) | 6–8 †, 9–10 (syst. 2), 13–15 (syst. 1) | Precipitation, turbidity ** (syst. 2), Tu_RW (syst. 1), Tu_FW* (syst. 1), produced flow, free chlorine (hourly min.) ** (syst. 2), decantation * (syst. 1) | Beaudeau et al. 2012 [36] |
| Angoulême (France) | 2002–2007 | 50,000 | Consultations of GP | <16, >15 | 1-1-1 | Karstic spring | 4 (27) | 31 (1700) | CoagRFi, Ch | 0.14 (2) | 21,336 | P10-P50 change in Tu_RW over lags 7–9: 1.1–2.9 NTU | 30% [0; 60] (child.), 15% [−15; 45] (ad.) | 7–9 †, 13–15 † | Precipitation, Tu_RW *, Tu_FW, air temperature *◊, produced flow *◊, interventions for broken pipe *◊ | Rambaud et al., 2013a [14] |
| Paris-Centre (France) | 2002–2007 | 160,000 | Consultations of GP | <16, >15 | 3-1-1 | Karstic springs | 0.08–0.23 (0.50–0.73) | 1–8 (14–150) | Ch | 0.17 (0.66) | 26,526 | IQ change in Tu_FW over lags 7–9: 0.11–0.22 NTU | 11.8% [1.2; 22.5] (child.), 4.1% [−0.2; 8.8] (ad.) | 7–9 †, 10–11 | Precipitation, turbidity **◊, air temperature ***, free chlorine, produced flow *◊, contribbution of the most fecally contaminated resource in the produced flow * | Rambaud et al., 2013b [15] |
| Indicator | Shape of the Risk Function | Commentary | Frequency of Positive Tests a | Sites |
|---|---|---|---|---|
| Concentration of fecal coliform or Escherichia coli in raw water | Increasing, linear | Lack of sensitivity to viral or protozoan contamination. | 1/3 | Edmonton, Boston *, Vancouver |
| Cyanobacteria | Increasing, linear | Possibly relevant for reservoir waters in the absence of clarification facilities. | 1/1 | Boston * |
| Turbidity in finished water (in the presence of clarification facilities) | Increasing | Suspended particles may carry pathogens. May indicate resource contamination and/or treatment transient weaknesses. May interact with river flow. | 5/12 | Philadelphia *, Edmonton, Québec *, Atlanta, Nantes *, Paris-Est *, PA-Nord, PA-Est, PA-Sud, Nancy, Le Havre * (syst. 1), Angoulême |
| Particle count in filtered/finished water (in the presence of clarification facilities) | Increasing | Alternative to turbidity in finished water. More precise when turbidity is very low. | 2/4 | Edmonton, PA-Nord *, PA-Est, PA-Sud * |
| Turbidity in raw/finished water (in the absence of clarification facilities) | Increasing | The availability of algae data makes possible to correct turbidity from algae influence (Boston). May interact with water temperature or season. | 5/5 | Vancouver *, Boston *, New York *, Le Havre (sys. 2) *, Paris-Centre * |
| Turbidity in raw water (in the presence of clarification facilities) | Increasing | May better correlate to AGE than turbidity in finished water. | 5/10 | Atlanta *, Edmonton, Nantes, Paris-Est *, PA-Nord, PA-Est, Paris-Sud *, Nancy, Le Havre (sys. 1) *, Angoulême * |
| Precipitation | Increasing with threshold | Alternative to turbidity in raw water. | 1/10 | Edmonton, Québec, Nantes, Paris-Est, Gothenburg *, Vancouver, Boston, Le Havre, Angoulême, Paris-Centre |
| Numbers of consecutive days of wet weather | Increasing | Derived from precipitation. Surrogate for wetness of soils (facilitating surface runoff). | 1/1 | Gothenburg * |
| Numbers of consecutive days of dry weather | No expectation | Derived from precipitation. Unclear. | 1/1 | Gothenburg |
| total organic carbon (TOC) in raw water | No expectation | Unclear. May interact with river flow. | 3/3 | PA-Nord *, PA-Est *, PA-Sud * |
| total organic carbon (TOC) in filtered water | No expectation | Unclear. | 1/2 | PA-Est, PA-Sud * |
| River flow | U-shaped | High or low flows may be associated to fecal pollution. Heavy precipitations bring about both high river flows and river contaminations. Low flows result in less dilution of urban effluents. May modify the turbidity risk function. | 5/6 | Nantes *, Paris-Est *, PA-Nord *, PA-Est *, PA-Sud *, Nancy |
| Water temperature | U-shaped | High or low temperature may enhance the AGE risk (via waterborne or other route exposure), possibly depending on climate. May modify the turbidity or TOC -AGE association. | 6/6 | Paris-Est *, PA-Nord *, PA-Est *, PA-Sud *, Nancy *, Boston * |
| Air temperature | U-shaped | Beside a possible direct and synchronous effect on health care pursue (Boston and New York), may also serve as a surrogate to water temperature (exposure). | 3/4 | Edmonton, Nantes *, Angoulême *, Paris-Centre * |
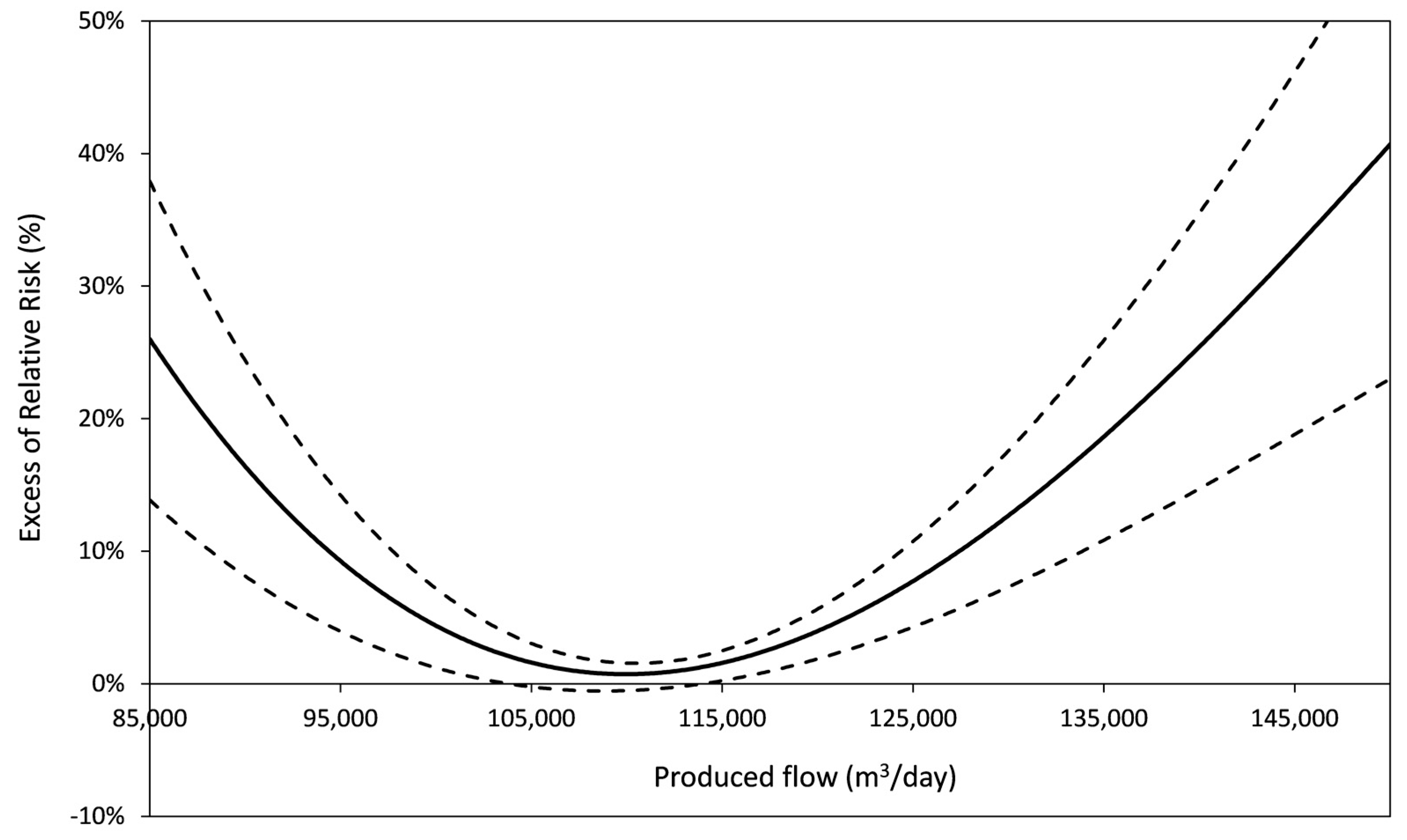
| Produced flow | U-shaped or increasing | Sub optimal operation conditions at low or high produced flow. | 8/9 | Nantes *, Paris-Est *, PA-Nord *, PA-Est *, PA-Sud *, Nancy *, Le Havre, Angoulême *, Paris-Centre * |
| CT (disinfectant concentration × time of contact) | Decreasing | Measure of the disinfection power; available in the USA. | 0/1 | Boston |
| Free chlorine concentration at the outlet of the treatment plant | Decreasing with threshold | Hourly minimum may be relevant to highlight a risk associated to transient breakdowns, if direct distribution (i.e., no buffer effect of storage). | 1/4 | Nantes, Le Havre *, Paris-Est, Paris-Centre |
| Permanent change in abstraction or treatment facilities (Boolean) | Improvement | E.g., change in abstraction point, implementation of ozonation instead of chlorination. | 1/2 | Edmonton, Boston * (respectively) |
| Episodic change in treatment (Boolean) | Improvement | Decantation implementation interacts with turbidity on AGE incidence. | 1/1 | Le Havre * |
| Daily number of water cuts | Increasing, linear | Adverse impact limited to the inhabitants next downstream of the intervention point. TSS are poorly adequate to address this risk. | Nancy | |
| Daily number of interventions for broken pipe | Increasing, linear | Idem. | 2/3 | Nantes *, Nancy, Angoulême * |
| Daily number of hydrant flushings | Increasing, linear | Idem. | 0/1 | Nantes |
| Daily number of consumers’ complaints | Increasing | Idem. Additional limitation: few complaints are specific to fecal contamination. | 0/1 | Nantes |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudeau, P. A Systematic Review of the Time Series Studies Addressing the Endemic Risk of Acute Gastroenteritis According to Drinking Water Operation Conditions in Urban Areas of Developed Countries. Int. J. Environ. Res. Public Health 2018, 15, 867. https://doi.org/10.3390/ijerph15050867
Beaudeau P. A Systematic Review of the Time Series Studies Addressing the Endemic Risk of Acute Gastroenteritis According to Drinking Water Operation Conditions in Urban Areas of Developed Countries. International Journal of Environmental Research and Public Health. 2018; 15(5):867. https://doi.org/10.3390/ijerph15050867
Chicago/Turabian StyleBeaudeau, Pascal. 2018. "A Systematic Review of the Time Series Studies Addressing the Endemic Risk of Acute Gastroenteritis According to Drinking Water Operation Conditions in Urban Areas of Developed Countries" International Journal of Environmental Research and Public Health 15, no. 5: 867. https://doi.org/10.3390/ijerph15050867
APA StyleBeaudeau, P. (2018). A Systematic Review of the Time Series Studies Addressing the Endemic Risk of Acute Gastroenteritis According to Drinking Water Operation Conditions in Urban Areas of Developed Countries. International Journal of Environmental Research and Public Health, 15(5), 867. https://doi.org/10.3390/ijerph15050867




