Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria
Abstract
:1. Introduction
2. .Materials and Methods
2.1. Maize Plots
2.2. Chemical Application
2.3. Sampling Methods:
- 10 g of grains from five different randomly selected plants of each entry in a plot,
- 10 cm piece of stem from five different randomly selected plants of each entry in a plot, and
- five soil samples of 10 g at 20 cm deep in different randomly selected areas in each plot.
2.4. Protocol of Pesticide Residues Analysis
- Pesticide residues in soil samples were analyzed using Reverse Phase HPLC (Agilent Technologies, Santa Clara, CA, USA) with C18, 5 µm 120 Â, 4.6 × 250 mm column as stationary phase and methanol/H2O (90:10) as mobile phase at a flow rate of 1 mL/min for 30 min.
- Five (5) insecticides standards, which are part of the active ingredients in the commercial products, were used as reference and for quantifications: Cypermethrin, Permethrin, Deltamenthrin, Lambdacyhalothrin, and Chorpyrifos.
- Duplicates of the soil samples were examined. Extraction was done with 1 mL acetonitrile and 1 g soil sample using Microwave Extraction method. Each replicate was divided into 2, and one was spiked with prepared cocktail of the standards. Thus, the total run per sample on the HPLC was 4.
- The duplicates and the corresponding spiked samples were overlaid for the identification of possible insecticide residues.
- Duplicates of the maize samples were examined. 5 g of maize first was grinded to powdered form. Slurry was made from the powder by blending in 10 mL of ddH2O water. 10 mL acetonitrile was added and then shaken at 8 rpm for 30 min to allow for the repartitioning of the residues of the insecticides into acetonitrile. A modified QuECHERS method was used to separate the water/acetonitrile phases. 8 mL of the acetonitrile was then concentrated to 1 mL and use for the HPLC analysis.
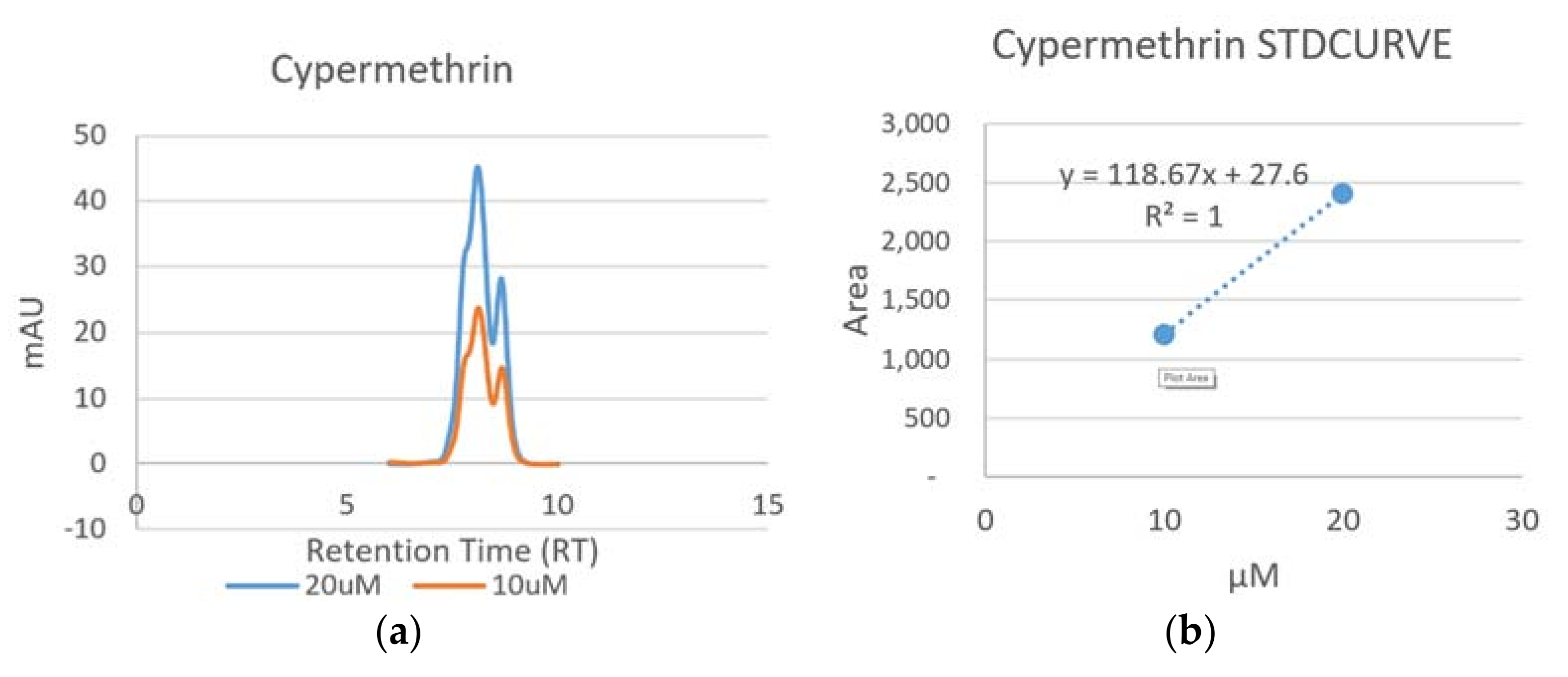
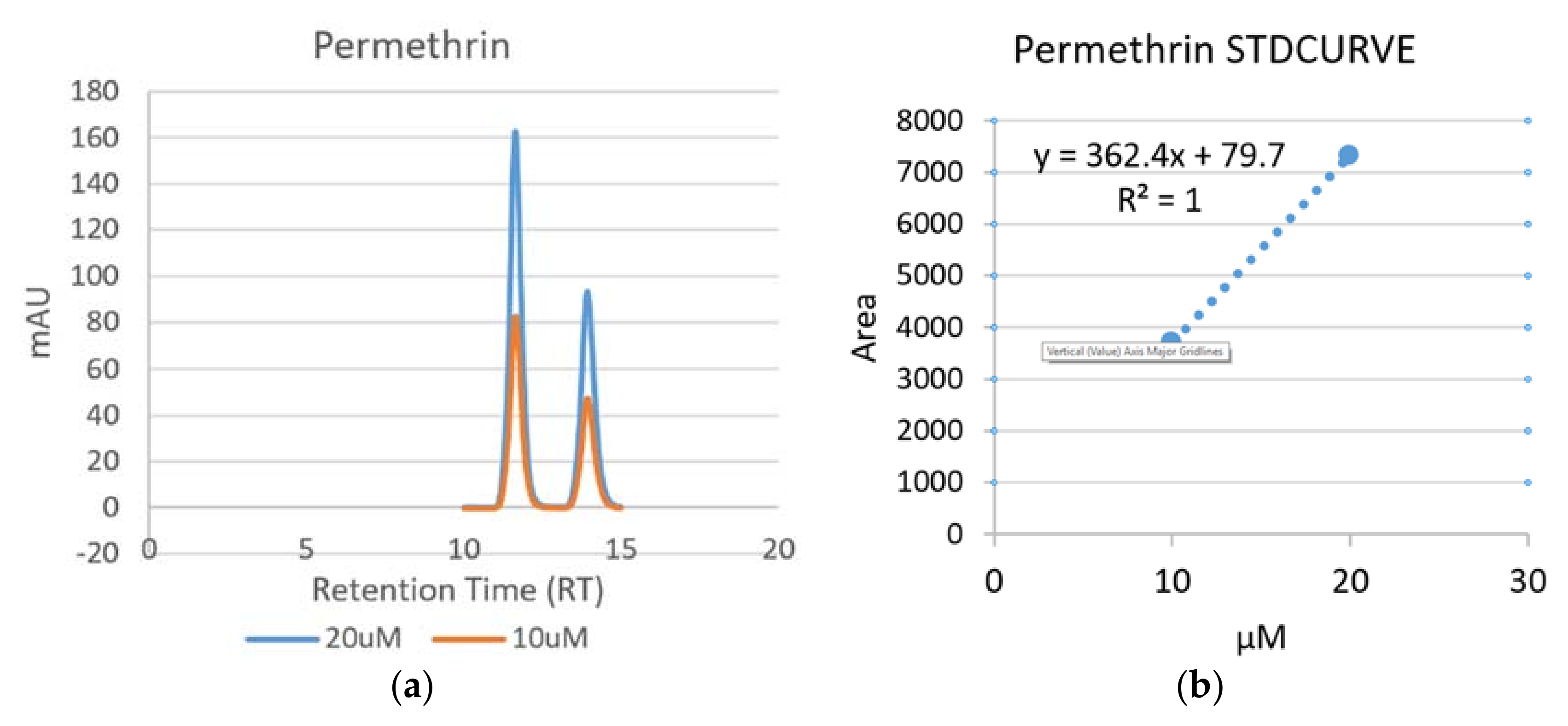
- Area of peaks were used for the standard curves of the pyrethroid insecticides, while the height of peaks used for the Chlorpyrifos-Ethyl.
2.4.1. The LOD, LOQ Recovery, Precision and Accuracy
2.4.2. Chromatography and HPLC Analysis
2.4.3. Quality Assurance/Quality Control (QA/QC) Information
3. Results
3.1. Detection of Chemical Compound in Soil Samples
3.1.1. Detection of Cypermethrin
3.1.2. Detection of Lamda-Cyhalothrin
3.1.3. Detection of Deltamethrin
3.1.4. Detection of Permethrin
3.2. Detection of Chemical Compound in Maize Stem and Seeds Samples
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 10, e0165632. [Google Scholar]
- FAO. Fall Armyworm. Available online: http://wwwfao.org/food-chain-crisis/how-we-work/plant-protection/fall-armyworm/en/ (accessed on 16 February 2018).
- Buntin, G.D. A review of plant response to fall armyworm, Spodoptera frugiperda (JE Smith), injury in selected field and forage crops. FL Entomol. 1986, 69, 549–559. [Google Scholar] [CrossRef]
- Cruz, I.; Figueiredo, M.L.C.; Oliveira, A.C.; Vasconcelos, C.A. Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminium saturation. Int. J. Pest Manag. 1999, 45, 293–296. [Google Scholar] [CrossRef]
- Belfroid, A.C.; Drunen, M.V.; Beek, M.A.; Schrap, S.M.; Gestel, C.A.M.V.; Hattum, B.V. Relative risks of transformation products of pesticides for aquatic ecosystems. Sci. Total Environ. 1998, 222, 167–183. [Google Scholar] [CrossRef]
- Shanker, K.; Gupta, S.; Srivastava, P.; Srivastava, S.K.; Singh, S.C.; Gupta, M.M. Simultaneous determination of three steroidal glycoalkaloids in Solanum xanthocarpum by high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2011, 54, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.A.; Peris, S.J. Effects of forest spraying with two application rates of cypermethrin on food supply and on breeding success of the blue tit (Parus caeruleus). Environ. Toxicol. Chem. 1992, 11, 1271–1280. [Google Scholar] [CrossRef]
- Stephenson, R.R. Aquatic toxicology of cypermethrin. I. Acute toxicity to some freshwater fish and invertebrates in laboratory tests. Aquat. Toxicol. 1982, 2, 175–185. [Google Scholar] [CrossRef]
- Martinez-Cabrillo, J.L.; Schouest, L.P., Jr.; Miller, T.A. Responses of populations of the tobacco budworm (Lepidopterea: Noctuidae) from northwest Mexico to pyrethroids. J. Econ. Entomol. 1991, 84, 363–366. [Google Scholar] [CrossRef]
- Yu, S.J.; Nguyen, S.N.; Abo-Elghar, G.E. Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (JE Smith). Pesticide Biochem. Physiol. 2003, 77, 1–11. [Google Scholar] [CrossRef]
- Yu, S.J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (JE Smith). Pesticide Biochem. Physiol. 1991, 39, 84–91. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; Santos, A.C.D.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Bouwman, H.; Sereda, B.; Meinhardt, H.M. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ. Pollut. 2006, 144, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Köprücü, K.; Aydın, R. The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pesticide Biochem. Physiol. 2004, 80, 47–53. [Google Scholar] [CrossRef]
- Ian, R.H. Aquatic organisms and pyrethroids. Pesticide Sci. 1989, 27, 429–457. [Google Scholar]
- Robert, L.M. Insect Control in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Leistra, M.; Zweers, A.J.; Warinton, J.S.; Crum, S.J.; Hand, L.H.; Beltman, W.H.; Maund, S.J. Fate of the insecticide lambda-cyhalothrin in ditch enclosures differing in vegetation density. Pest Manag. Sci. 2004, 60, 75–84. [Google Scholar] [CrossRef] [PubMed]
- NPIC. Lambda-Cyhalothrin (Genreal Fact Sheet Pdf). Available online: http://npic.orst.edu/factsheets/l_cyhalogen.pdf (accessed on 5 March 2018).
- Wikepedia. Cyhalothrin. Available online: https://enwikipediaorg/wiki/Cyhalothrin (accessed on 5 March 2018).
- Rathod, A.L.; Garg, R.K. Chlorpyrifos poisoning and its implications in human fatal cases: A forensic perspective with reference to Indian scenario. J. Forensic Legal Med. 2017, 47, 29–34. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009; WHO Report; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D.B.; Slotkin, T.A.; Peterson, B.S. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. USA 2012, 109, 7871–7876. [Google Scholar] [CrossRef] [PubMed]
- Giddings, J.M.; Williams, W.M.; Solomon, K.R.; Giesy, J.P. Risks to Aquatic Organisms from Use of Chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States; Giesy, J.P., Solomon, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2014; Volume 231, pp. 119–162. [Google Scholar]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yu, G. Agricultural chemicals. In Point Sources of Pollution: Local Effects and Their Control; Yi, Q., Ed.; Encyclopedia of Life Support Systems (EOLSS): Oxford, UK, 2009; Volume 2, p. 43. [Google Scholar]
- Chouaibou, M.S.; Fodjo, B.K.; Fokou, G.; Allassane, O.F.; Koudou, B.G.; David, J.; Antonio-Nkondjio, C.; Ranson, H.; Bonfoh, B. Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Côte d’ivoire. Malar. J. 2016, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Akogbeto, M.; Djouaka, R.; Kinde-Gazard, D.A. Screening of pesticide residues in soil and water samples from agricultural settings. Malar. J. 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]



| Date of Application | Chemical | Active Ingredients | Quantity | Plots |
|---|---|---|---|---|
| 27–31/7/2016 | PYRINEX and and BEST ACTION | Chlorpyrifos/Xylene | 15 mL each | Experiment plots |
| 1–25/8/2016 | CRUSH and MAGIC FORCE | Cypermethrin/Dimethoate | 20 mL and 30 mL | Experiment plots |
| 26/8/–8/9/2016 | DD FORCE and SHARP SHORTER | β Cyfluthrin | 40 mL and 30 mL | Experiment plots |
| 9–12/9/2016 | ATTACK and COURAGE | Lambda-Cyhalothrin/Dimethoate | 50 mL and 30 mL | Experiment plots |
| 13–15/9/2016 | GOODBYE and SHARP SHOOTER | Dichlorvos | 30 mL and 70 mL | Experiment plots |
| 16–19/9/2016 | GOODBYE and PYRINEX | Citric Acid | 30 mL and 70 mL | Experiment plots |
| 20–22/9/2016 | V.I.P and SHARP SHOOTER | Permethrin/Pirimiphos-methyl | 30 mL and 70 mL | Experiment plots |
| 23/9–9/10/2016 | DD FORCE and IRON FORCE | Imidacloprid | 30 mL and 70 mL | Experiment plots |
| 10–28/10/2016 | IRON FORCE | Pyrethrum | 100 mL | Seed multiplication |
| 22–24/11/2016 | PYRINEX | Profenofos/Cypermethrin | 100 mL | Seed multiplication |
| SN | Sample Name | No of Replicates Tested | Active Ingredients Detected |
|---|---|---|---|
| 1 | Plot 15/ENTRY 1 | 2 | Lambda/Cyperm |
| 2 | Plot 14/ENTRY 2 | 2 | Cyperm/Delta |
| 3 | Plot 12/ENTRY 3 | 2 | Perm |
| 4 | Plot 10/ENTRY 4 | 2 | Lambda/Cyperm |
| 5 | Plot 16/ENTRY 5 | 2 | Cyperm/Delta |
| 6 | Plot 9/ENTRY 6 | 2 | Lambda/Cyperm/Delta |
| 7 | Plot 11/ENTRY 7 | 2 | Lambda/Cyperm/Delta |
| 8 | Plot 13/ENTRY 8 | 2 | Lambda/Cyperm/Delta |
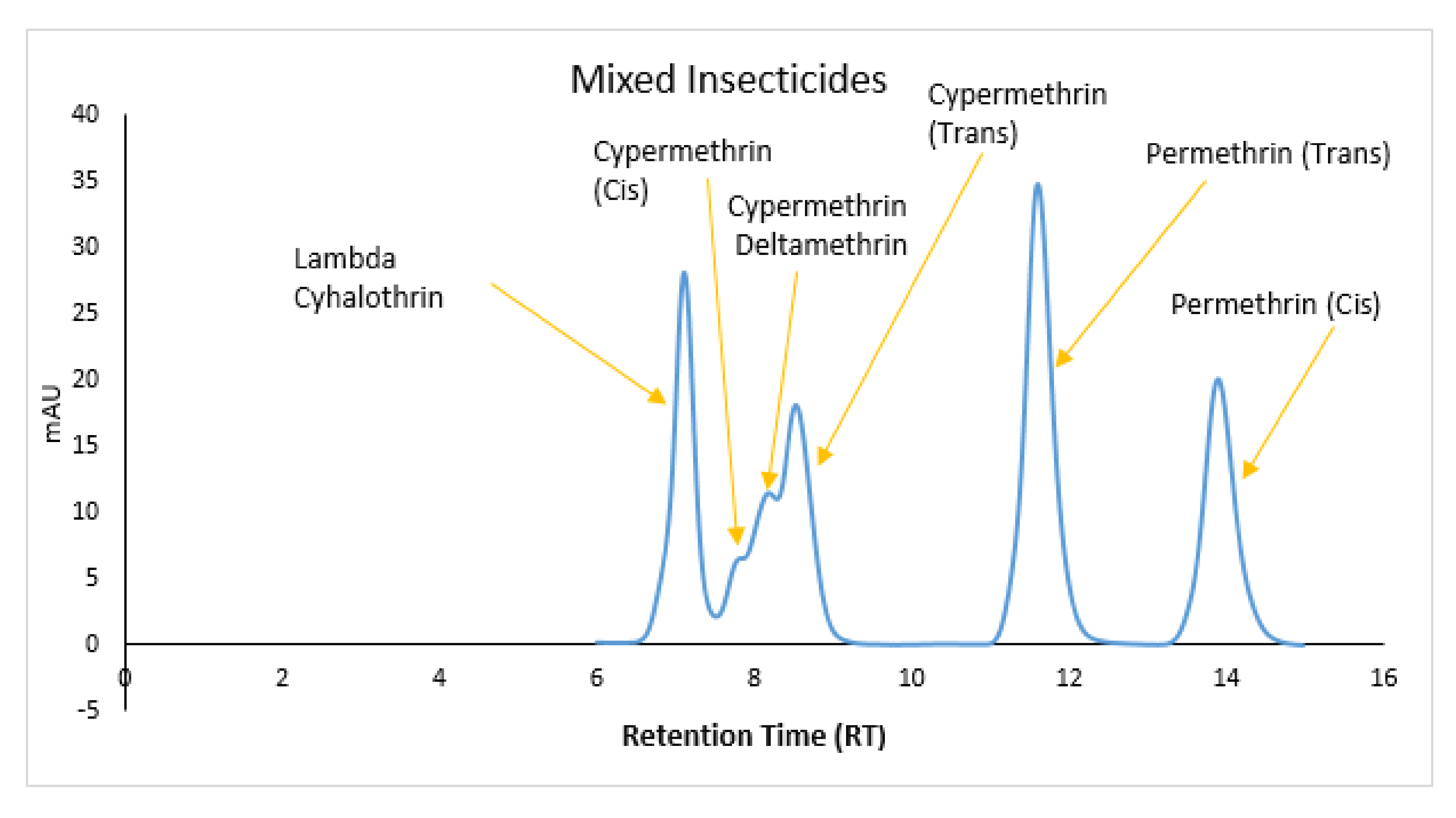
| Sample Name | Retention Time |
|---|---|
| Lambda-Cyhalothrin | 7.14 |
| Cypermethrin (Cis) | 8.09 |
| Cypermethrin (Trans) | 8.66 |
| Deltamethrin | 8.51 |
| Permethrin (Trans) | 11.63 |
| Permethrin (Cis) | 13.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togola, A.; Meseka, S.; Menkir, A.; Badu-Apraku, B.; Boukar, O.; Tamò, M.; Djouaka, R. Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 849. https://doi.org/10.3390/ijerph15050849
Togola A, Meseka S, Menkir A, Badu-Apraku B, Boukar O, Tamò M, Djouaka R. Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria. International Journal of Environmental Research and Public Health. 2018; 15(5):849. https://doi.org/10.3390/ijerph15050849
Chicago/Turabian StyleTogola, Abou, Silvestro Meseka, Abebe Menkir, Baffour Badu-Apraku, Ousmane Boukar, Manuele Tamò, and Rousseau Djouaka. 2018. "Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria" International Journal of Environmental Research and Public Health 15, no. 5: 849. https://doi.org/10.3390/ijerph15050849






