Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cereal Materials
2.2. Instrumentation
2.3. Chemicals and Reagents
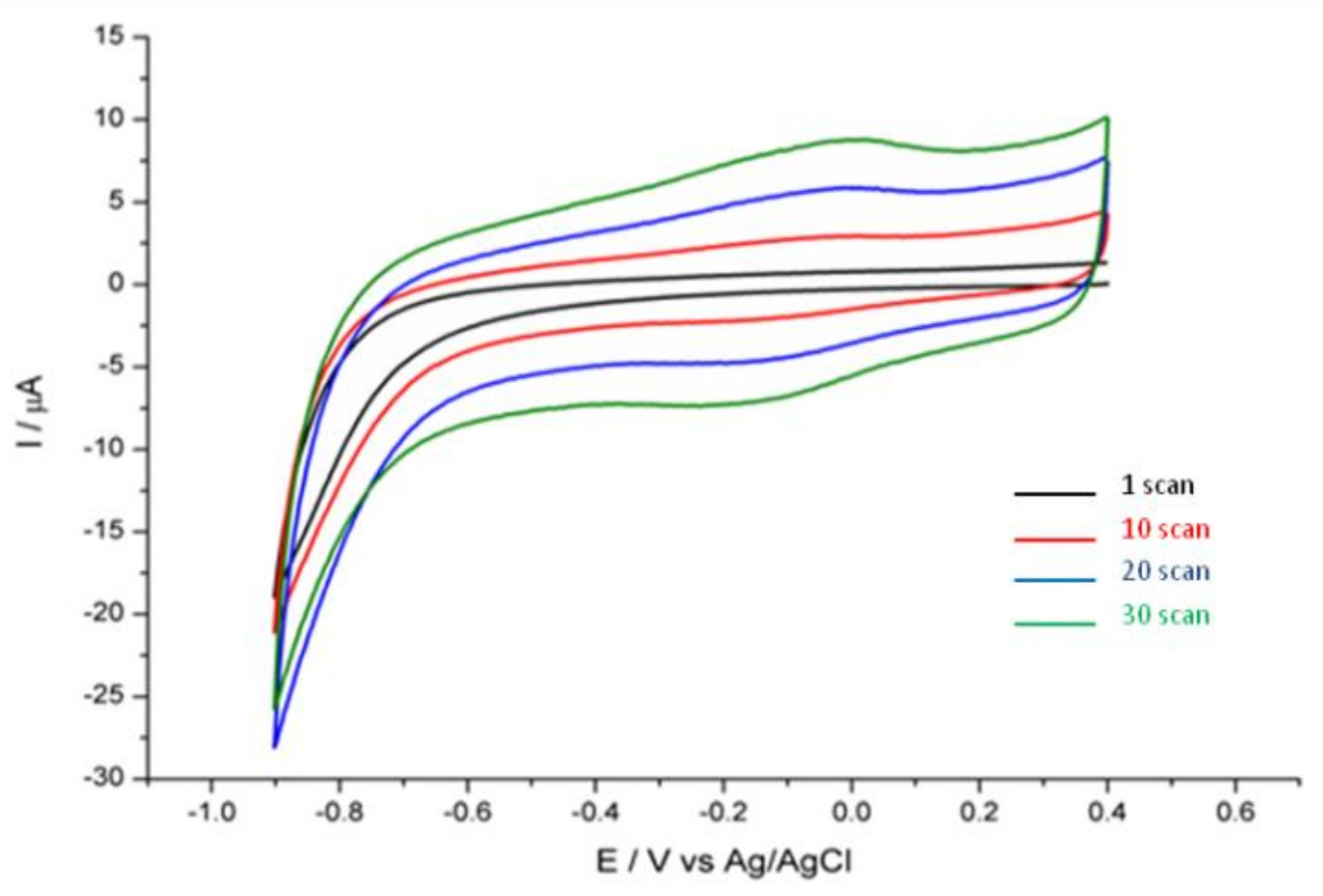
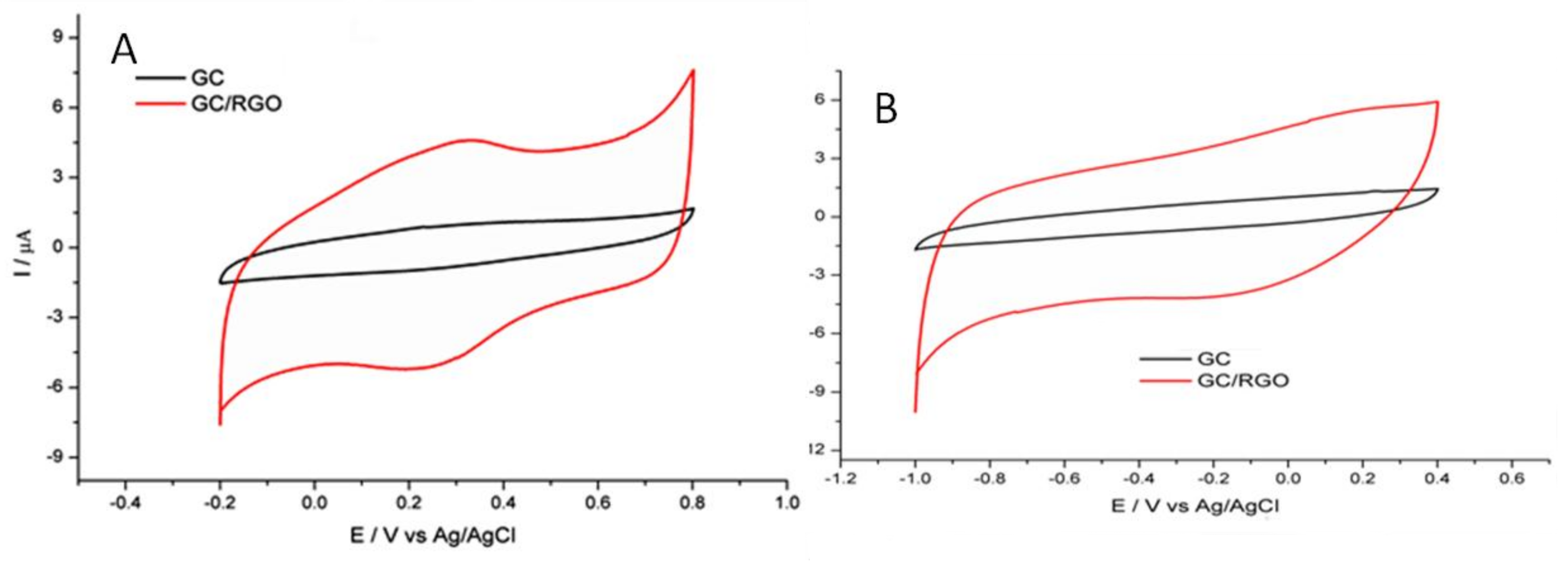
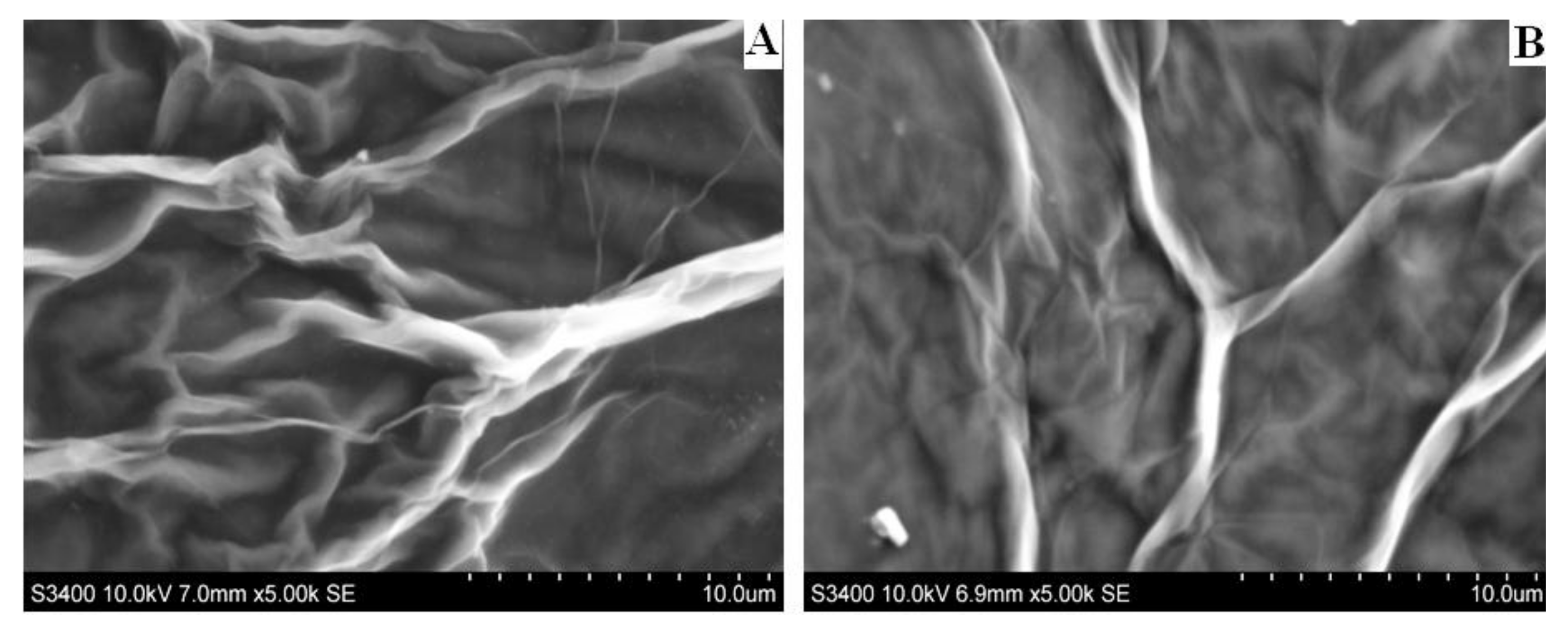
2.4. Preparation of Glassy Carbon/Reduced Graphene Oxide Modified Electrode (GC/RGO)
2.5. Thallium Determination by Differential Pulse Anodic Stripping Voltammetry (DPASV) at GC/RGO Electrode
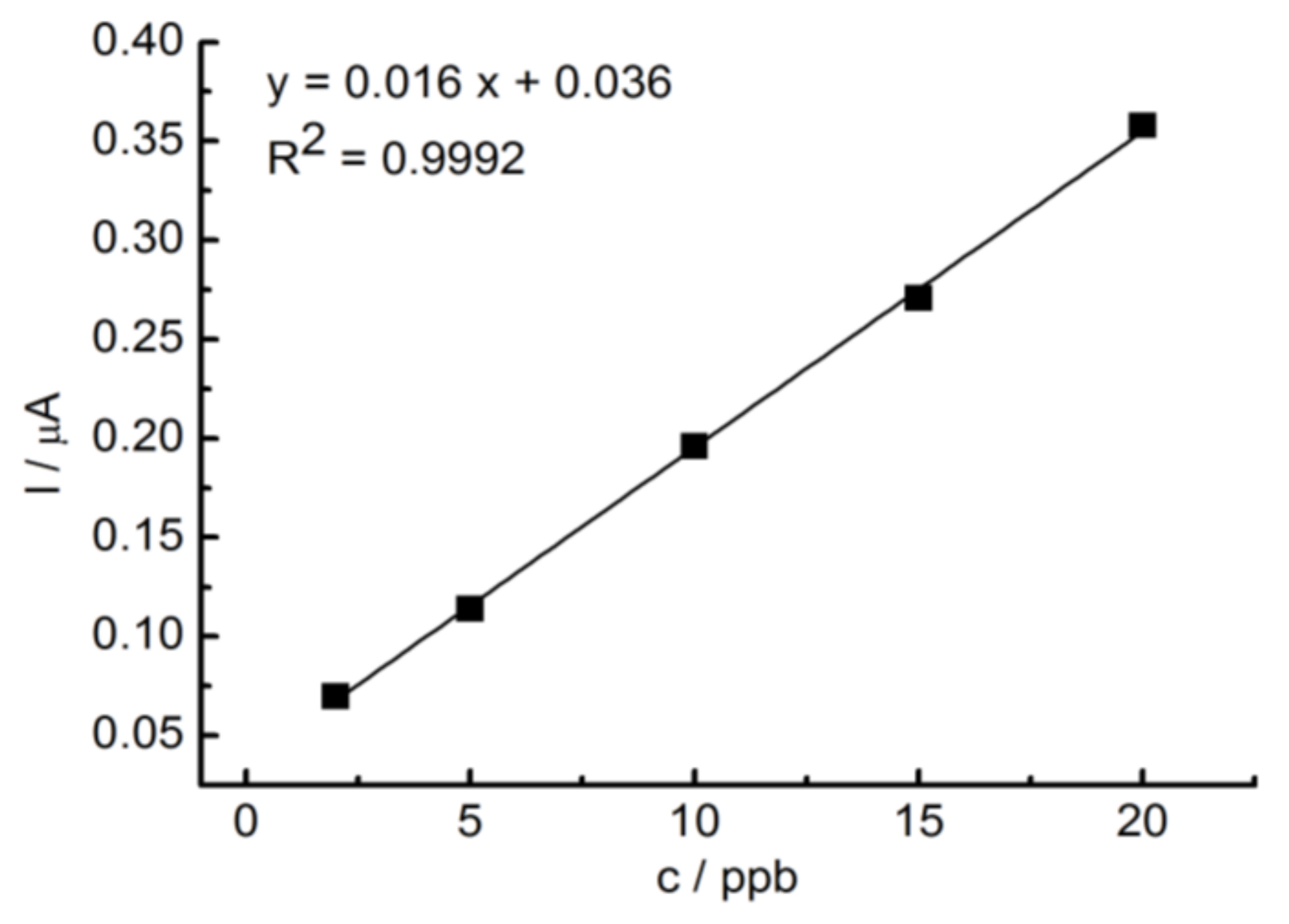
3. Results and Discussion
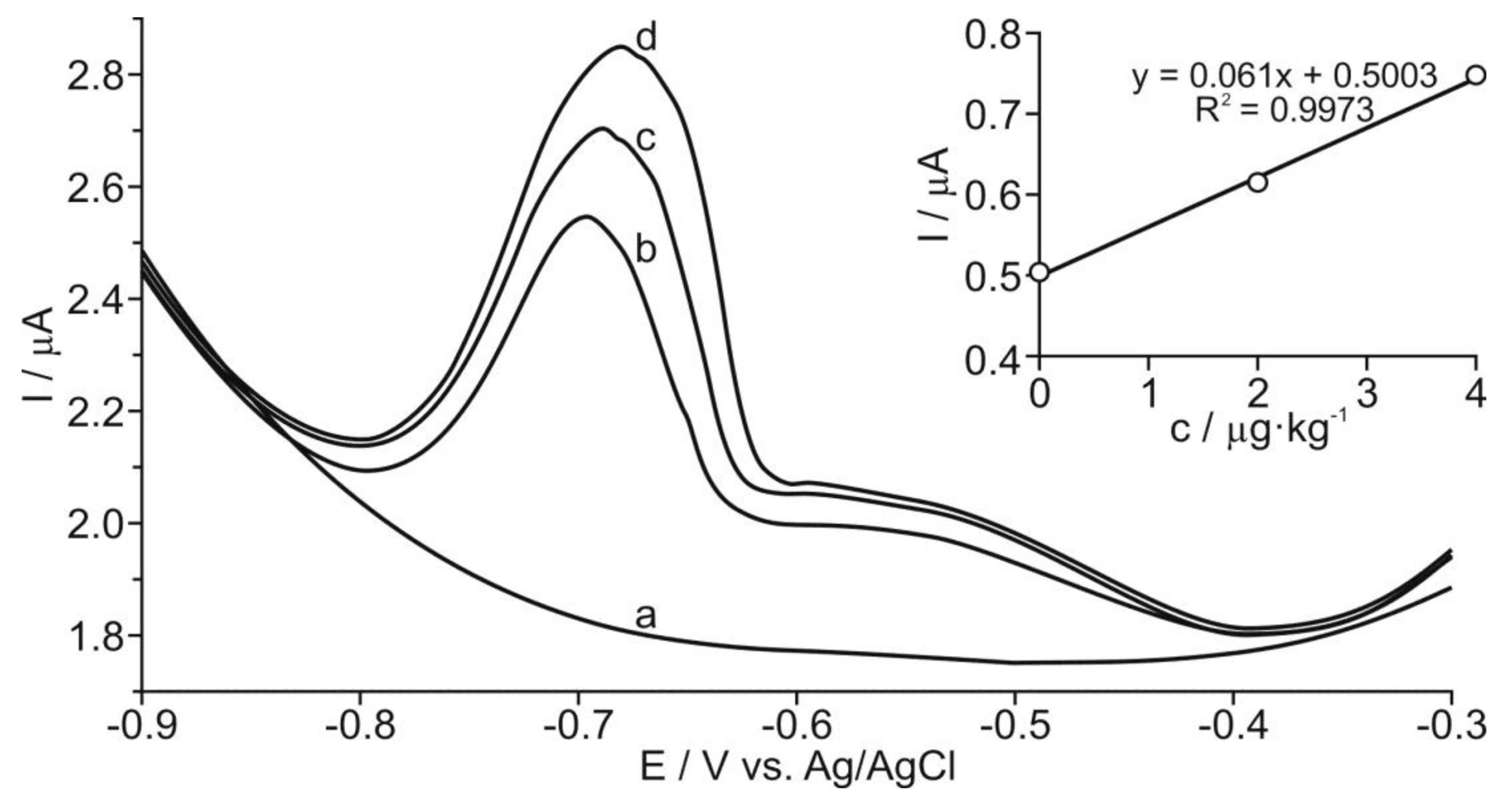
Determination of Thallium at GC/RGO Modified Electrode by DPASV
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zembrzuska, J.; Karbowska, B. Identification of complexes involving thallium (I) and thallium (III) with EDTA and DTPA ligands by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectr. 2017, 31, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Groesslova, Z.; Vanek, A.; Mihaljevic, M.; Ettler, V.; Hojdovác, M.; Zádorováa, T.; Pavlů, L.; Penížek, V.; Vaněčková, B.; Komárek, M.; et al. Bioaccumulation of thallium in a neutral soil as affectedby solid-phase association. J. Geochem. Explor. 2015, 159, 208–212. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A. Arsen i tal w glebach i roślinach rejonu Bukowna. Ochrona Środowiska i Zasobów Naturalnych 2009, 40, 199–208. (In Polish) [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; PWN: Warszawa, Poland, 1999. (In Polish) [Google Scholar]
- Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.H. Applications of stripping voltammetric techniques in food analysis. Arab. J. Chem. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Pałdyna, J.; Wawrzyńska, M.; Stryjewska, E. Indirect anodic stripping voltammetric determination of Tl (I) and Tl (III) in the Baltic sea water samples enriched in thallium species. Electroanalysis 2011, 23, 605–610. [Google Scholar]
- Bonfil, Y.; Brand, M.; Kirowa-Eisner, E. Characteristics of subtractive anodic stripping voltammetry of Pb and Cd at silver and gold electrodes. Anal. Chim. Acta 2002, 464, 99–114. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Pałdyna, J.; Golimowski, J. Determination of Thallium at A Silver-Gold Alloy Electrode by Voltammetric Methods in Plant Material and Bottom Sediment Containing Cd and Pb. Electroanalysis 2007, 19, 620–622. [Google Scholar] [CrossRef]
- Korolczuk, M.; Surmacz, W.; Tyszczuk, K. Determination of thallium in a flow system by anodic stripping voltammetry at a bismuth film electrode. Electroanalysis 2007, 19, 2217–2221. [Google Scholar] [CrossRef]
- Hočevar, S.B.; Švancara, I.; Ogorevc, B.; Vytřas, K. Antimony film electrode for electrochemical stripping analysis. Anal. Chem. 2007, 79, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Kirgoz, U.A.; Hocevar, S.B.; Ogorevc, B. Insights into the anodic stripping voltammetric behavior of bismuth film electrodes. Anal. Chim. Acta 2001, 434, 29–34. [Google Scholar] [CrossRef]
- Tarasova, V.A. Voltammetric determination of thallium (I) at a mechanically renewed Bi-graphite electrode. J. Anal. Chem. 2007, 62, 157–160. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Piekarska, J. Determination of lead and cadmium at silver electrode by subtractive anodic stripping voltammetry in plant materials containing Tl. Electroanalysis 2005, 17, 815–818. [Google Scholar] [CrossRef]
- Karbowska, B.; Zembrzuski, W. Fractionation and mobility of thallium in volcanic ashes after eruption of Eyjafjallajökull (2010) in Iceland. Bull. Environ. Contam. Toxicol. 2016, 97, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Batchelor-McAuley, C.; Wildgoose, G.C.; Compton, R.G. The contrasting behaviour of polycrystalline bulk gold and gold nanoparticle modified electrodes towards the underpotential deposition of thallium. New J. Chem. 2008, 32, 941–946. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Rezaei, M.; Sabounchei, S.J.; Sarlakifar, M. Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode. Anal. Chim. Acta 2015, 870, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Istrate, O.M.; Rotariu, L.; Bala, C. Electrochemical determination of NADH using screen printed carbon electrodes modified with reduced graphene oxide and poly(allylamine hydrochloride). Michrochim. Acta 2016, 183, 57–65. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Kryjewski, M.; Popenda, L.; Lijewski, S.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. An enhanced electrochemical nanohybrid sensing platform consisting of reduced graphene oxide and sulfanyl metalloporphyrazines for sensitive determination of hydrogen peroxide and L-cysteine. Dyes Pigments 2017, 138, 190–203. [Google Scholar] [CrossRef]
- Liu, D.; Long, Y.T. Superior Catalytic Activity of Electrochemically Reduced Graphene Oxide Supported Iron Phthalocyanines toward Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 24063–24068. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, B.; Zembrzuski, W. Determining Thallium in a Commercial Tobacco Brand Available in Poland. Pol. J. Environ. Stud. 2016, 25, 2217–2220. [Google Scholar] [CrossRef]
- Karbowska, B.; Rębiś, T.; Milczarek, G. Mercury-modified Lignosulfonate-stabilized Gold Nanoparticles as an Alternative Material for Anodic Stripping Voltammetry of Thallium. Electroanalysis 2017, 29, 2090–2097. [Google Scholar] [CrossRef]
- Österholm, A.; Lindfors, T.; Kauppila, J.; Damlin, P.; Kvarnström, C. Electrochemical incorporation of graphene oxide into conducting polymer films. Electrochimica Acta 2012, 83, 463–470. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Wallace, J.M. Kale and Thallium: Insights from Your Nutrition Team. Permaculture Solutions for Healing. Available online: https://poshretreat.org/2015/10/20/kale-and-thallium-insights-from-a-permaculturegardenernutrition-pro/ (accessed on 1 April 2018).
- Lansdown, A.B.G. The Carcinogenicity of Metals: Human Risk through Occupational and Environmental Exposure; RSCP Publishing: Cambridge, UK, 2014. [Google Scholar]
- LaCoste, C.; Robinson, B.; Brooks, R. Uptake of thallium by vegetables: Its significance for human health, phytoremediation, and phytomining. J. Plant Nutr. 2001, 24, 1205–1215. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Repetto, M. Human Thallium Toxicity. In Thallium in the Environment; Nriagu, J.O., Ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Wojtkowiak, T.; Karbowska, B.; Zembrzuski, W.; Siepak, M.; Lukaszewski, Z. Miocene colored waters: A new significant source of thallium in the environment. J. Geochem. Explor. 2016, 161, 42–48. [Google Scholar] [CrossRef]
| Cereal Products | Arthmetic Mean (mg/kg) | Minimum (mg/kg) | Maximum (mg/kg) | Median (mg/kg) | Standard DeviationSD (mg/kg) |
|---|---|---|---|---|---|
| Buctwheat groats | 0.0587 | 0.0449 | 0.0664 | 0.0648 | 0.0119 |
| Oatmeal porridge | 0.0334 | 0.0225 | 0.0419 | 0.0358 | 0.0099 |
| White rice | 0.0268 | 0.0205 | 0.0317 | 0.0282 | 0.0057 |
| Spagetti | 0.0798 | 0.0762 | 0.0845 | 0.0788 | 0.0042 |
| Rye bread | 0.0486 | 0.0363 | 0.0654 | 0.0441 | 0.0151 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbowska, B.; Rębiś, T.; Milczarek, G. Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products. Int. J. Environ. Res. Public Health 2018, 15, 653. https://doi.org/10.3390/ijerph15040653
Karbowska B, Rębiś T, Milczarek G. Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products. International Journal of Environmental Research and Public Health. 2018; 15(4):653. https://doi.org/10.3390/ijerph15040653
Chicago/Turabian StyleKarbowska, Bozena, Tomasz Rębiś, and Grzegorz Milczarek. 2018. "Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products" International Journal of Environmental Research and Public Health 15, no. 4: 653. https://doi.org/10.3390/ijerph15040653




