Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease
Abstract
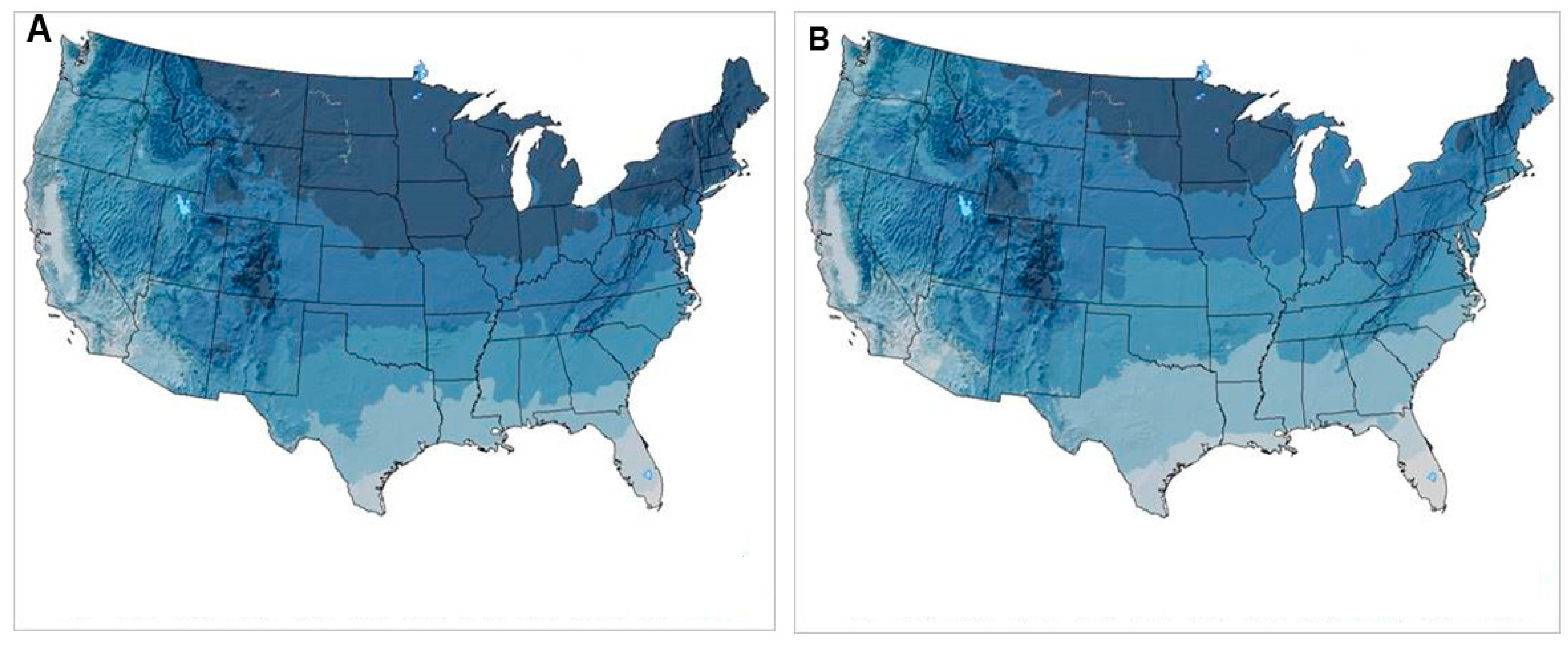
:1. Introduction
2. Impediments to Range Expansion
3. Selected North American Ticks and Range Expansion
3.1. American Dog Tick, Dermacentor variabilis
3.2. Lone Star Tick, Amblyomma americanum
3.3. Gulf Coast Tick, Amblyomma maculatum
3.4. Black-Legged Tick, Ixodes scapularis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C.; Murrell, A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology 2004, 129 (Suppl. 1), S15–S36. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Guglielmone, A.A.; Mangold, A.J. An overview of systematics and evolution of ticks. Front. Biosci. 2009, 14, 2857–2877. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M. (Eds.) Overview: Ticks, People and Animals. In Biology of Ticks, 2nd ed.; Oxford University Press: New York, NY, USA, 2014; pp. 3–16. [Google Scholar]
- Randolph, S.E. Ecology of non-nidicolous ticks. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; pp. 3–16. [Google Scholar]
- Lighton, J.R.B.; Fielden, L.J. Mass scaling of standard metabolism in ticks: A valid case of low metabolic rates in sit-and-wait strategists. Physiol. Zool. 1994, 68, 43–62. [Google Scholar] [CrossRef]
- Clow, K.M.; Leighton, P.A.; Ogden, N.H.; Lindsay, L.R.; Miche, P.; Pearl, D.L.; Jardine, C.M. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE 2017, 12, e0189393. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi and Lyme disease in North America. J. Med. Entomol. 2016, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Jarnevich, C.S.; Monaghan, A.J.; Eisen, R.J. Modeling the Geographic Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Contiguous United States. J. Med. Entomol. 2016, 53, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and Evolutionary Genomics of Amblyomma americanum, an Expanding Arthropod Disease Vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Siroký, P.; Kubelová, M.; Bednář, M.; Moddy, D.; Hub Alek, Z.; Tkadlec, E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet. Parasitol. 2011, 183, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.; Dillon, L.; Patel, S.N.; Ralevski, F. Prevalence of rickettsia species on Dermacentor variabilis from Ontario, Canada. Ticks Tick Borne Dis. 2016, 7, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Burdett, C.; McCool, M.J.; Fox, A.; Riggs, P. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the USA. Med. Vet. Entomol. 2015, 29, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Biology of Ticks, 1st ed.; Oxford University Press: New York, NY, USA, 1991; Volume 2. [Google Scholar]
- Garvie, M.A.; McKiel, J.A.; Sonenshine, D.E.; Campbell, A. Seasonal dynamics of American dog tick Dermacentor variabilis (Say) populations in southwestern Nova Scotia. Can. J. Zool. 1978, 56, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, R.; Krebs, C.J. Population dynamics of red-backed voles (Myodes) in North America. Oecologia 2012, 168, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Yunik, M.E.; Galloway, T.D.; Lindsay, L.R. Ability of unfed Dermacentor variabilis (Acari: Ixodidae) to survive a second winter as adults in Manitoba, Canada, near the northern limit of their range. J. Med. Entomol. 2015, 52, 138–142. [Google Scholar] [CrossRef] [PubMed]
- McEnroe, W.D. The effect of mean winter temperature around 0 °C on the population size of the American dog tick Dermacentor variabilis (Say) (Acarina: Ixodidae). Acarologia 1975, 17, 651–663. [Google Scholar]
- Minigan, J.N.; Hager, H.A.; Peregrine, A.S.; Newman, J.A. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis. 2017, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Springer, Y.P.; Jarnevich, C.S.; Barnett, D.T.; Monaghan, A.J.; Eisen, R.J. Modeling the present and future geographic distribution of the Lone Star Tick, Amblyomma americanum (Ixodida: Ixodidae), in the Continental United States. Am. J. Trop. Med. Hyg. 2015, 93, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Influence of meso- and microscale habitat structure on focal distribution of sympatric Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Sonenshine, D.E.; Gaff, H.D.; Hynes, W.L. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J. Med. Entomol. 2015, 52, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Goddard, J. The evolving medical and veterinary importance of the Gulf Coast Tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Teel, P.D.; Ketchum, H.R.; Mock, D.E.; Wright, R.E.; Strey, O.F. The Gulf Coast tick: A review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010, 47, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Allerdice, M.E.J.; Beati, L.; Yaglom, H.; Lash, R.R.; Delgado-de la Mora, J.; Licona-Enriquez, J.D.; Delgado-de la Mora, D.; Paddock, C.D. Rickettsia parkeri (Rickettsiales: Rickettsiaceae) detected in ticks of the Amblyomma maculatum (Acari: Ixodidae) group collected from multiple locations in southern Arizona. J. Med. Entomol. 2017, 54, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Florin, D.; Jiang, J.; Robbins, R.G.; Richards, A.L. Infection of the Gulf Coast tick, Amblyomma maculatum with Rickettsia parkeri: First report from the state of Delaware. Syst. Appl. Acarol. 2013, 18, 27–29. [Google Scholar] [CrossRef]
- Nadolny, R.M.; Gaff, H.D. Natural history of Amblyomma maculatum in Virginia. Ticks Tick Borne Dis. 2018, 9, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Araya-Anchetta, A.; Busch, J.D.; Scoles, G.A.; Wagner, D.M. Thirty years of tick population genetics: A comprehensive review. Infect. Genet. Evol. 2015, 29, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.A.; Goddard, J.; Caprio, M.; Paddock, C.D.; Mixson-Hayden, T.; Varela-Stokes, A.S. Population analyses of Amblyomma maculatum ticks and Rickettsia parkeri using single-strand conformation polymorphism. Ticks Tick Borne Dis. 2013, 4, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Nadolny, R.; Gaff, H.; Carlsson, J.; Gauthier, D. Comparative population genetics of two invading ticks: Evidence of the ecological mechanisms underlying tick range expansions. Infect. Genet. Evol. 2015, 35, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bishop, F.; Trembley, H.L. Distribution and hosts of certain North American ticks. J. Parasitol. 1945, 31, 1–54. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, S.; Ogden, N.H.; Leighton, P.A.; Lindsay, L.R.; Thivierge, K. Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: Increasing risk of Lyme disease. Ticks Tick Borne Dis. 2016, 7, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Bennett, S.W.; Beati, L.; Bartholomay, L.C. Range Expansion and Increasing Borrelia burgdorferi Infection of the Tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J. Med. Entomol. 2017, 54, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Gabriele-Rivet, V.; Koffi, J.K.; Pelcat, Y.; Arsenault, J.; Cheng, A.; Lindsay, L.R.; Lysyk, T.J.; Rochon, K.; Ogden, N.H. A risk model for the Lyme disease vector Ixodes scapularis (Acari: Ixodidae) in the prairie provinces of Canada. J. Med. Entomol. 2017, 54, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.T.; Nekomoto, T.S.; Victor, J.C.; Paul, W.S.; Piesman, J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 1998, 35, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Bjork, J.K.; Neitzel, D.F.; Dorr, F.M.; Schiffman, E.K.; Eisen, R.J. Habitat Suitability Model for the Distribution of Ixodes scapularis (Acari: Ixodidae) in Minnesota. J. Med. Entomol. 2016, 53, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Burks, C.S.; Stewart, R.J.; Needham, G.R.; Lee, R.E. Cold hardiness in the ixodid ticks (Ixodidae). In Acarology IX; Mitchell, R., Horn, D.J., Needham, G.R., Welbourn, W.C., Eds.; Ohio Biol. Survey: Columbus, OH, USA, 1996; Volume 1, pp. 85–87. [Google Scholar]
- Neelakanta, G.; Sultana, H.; Fish, D.; Anderson, J.F.; Fikrig, E. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Investig. 2010, 120, 3179–3190. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Rost, G.; Zou, X. Impact of spring bird migration on the range expansion of Ixodes scapularis tick populations. Bull. Math. Biol. 2016, 78, 138–168. [Google Scholar] [CrossRef] [PubMed]
- Woodward, F.L.; Lomas, M.R.; Kelly, C.K. Global climate and the distribution of plant biomes. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1465–1476. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. https://doi.org/10.3390/ijerph15030478
Sonenshine DE. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. International Journal of Environmental Research and Public Health. 2018; 15(3):478. https://doi.org/10.3390/ijerph15030478
Chicago/Turabian StyleSonenshine, Daniel E. 2018. "Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease" International Journal of Environmental Research and Public Health 15, no. 3: 478. https://doi.org/10.3390/ijerph15030478
APA StyleSonenshine, D. E. (2018). Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. International Journal of Environmental Research and Public Health, 15(3), 478. https://doi.org/10.3390/ijerph15030478



