Exploration of Rice Husk Compost as an Alternate Organic Manure to Enhance the Productivity of Blackgram in Typic Haplustalf and Typic Rhodustalf
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Experiment
2.1.1. Microorganisms, Earth Worm and Source of Plant Materials
2.1.2. Compost of Rice Husk
2.1.3. Changes in Physico-Chemical Properties during Composting of Rice Husk and Characterisation
2.2. Pot Experiment
2.2.1. Experimental Design
2.2.2. Blackgram Growth and Nutrient Status Analyses
2.3. Statistical Analysis
3. Results and Discussion
3.1. Composting of Raw Rice Husk
3.1.1. Changes of Physico-Chemical Properties during Composting of Rice Husk
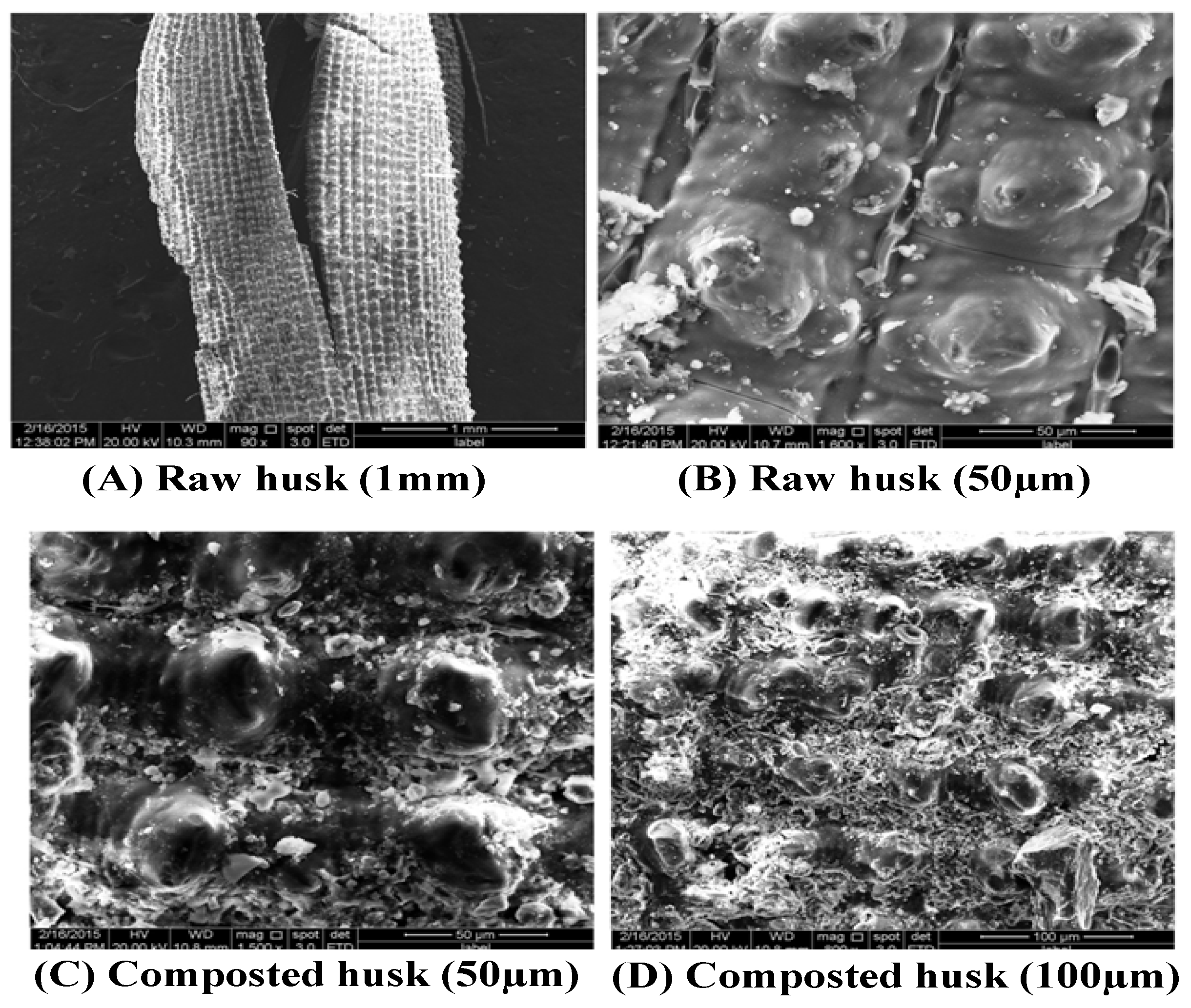
3.1.2. Structural Changes of Rice Husks during Composting Process (SEM)
3.2. Effect of CRH on Blackgram Growth Promotion and Soil Properties
3.2.1. Soil Properties
3.2.2. Growth Attributes
3.2.3. Yield Attributes
3.2.4. Grain and Haulm Yield
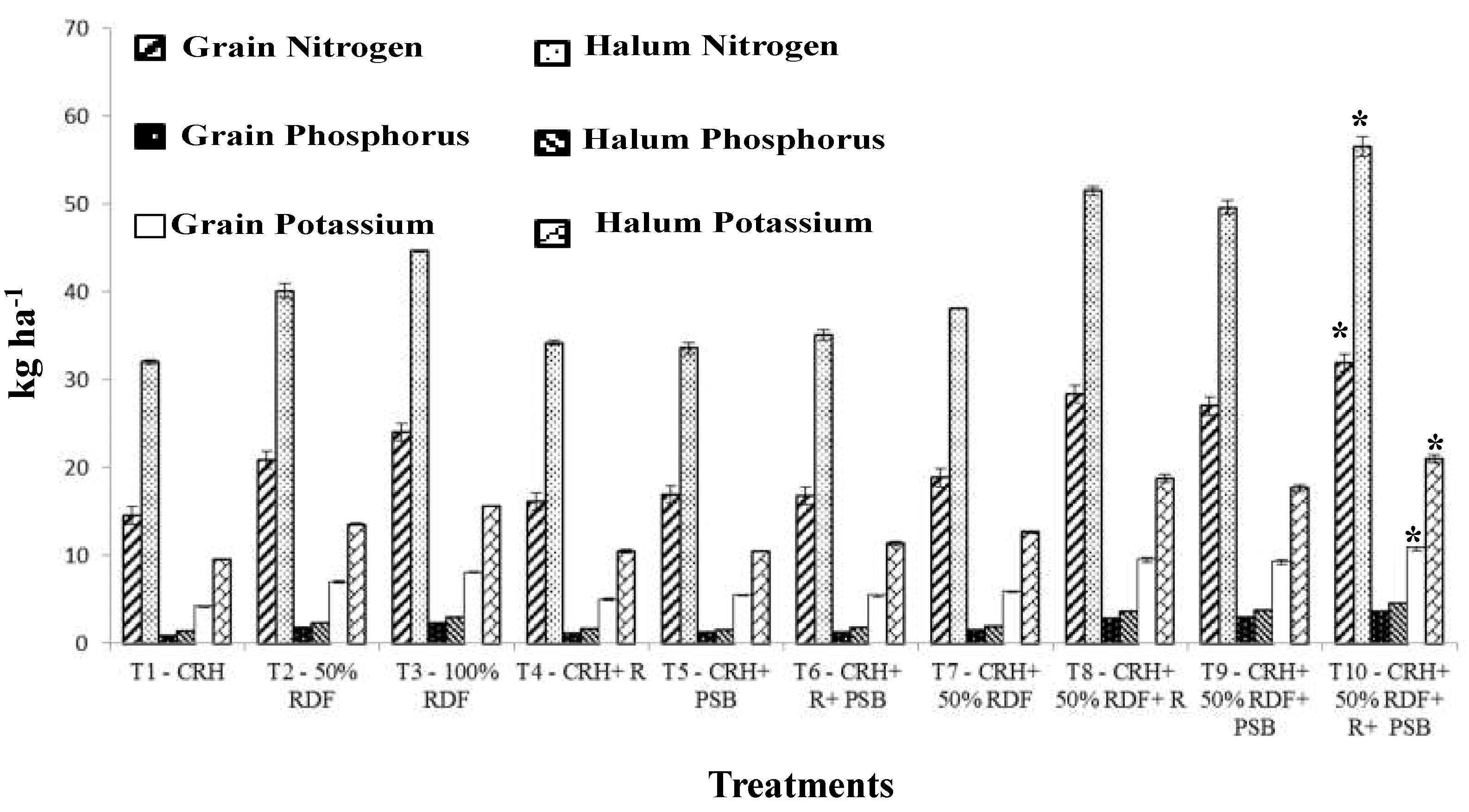
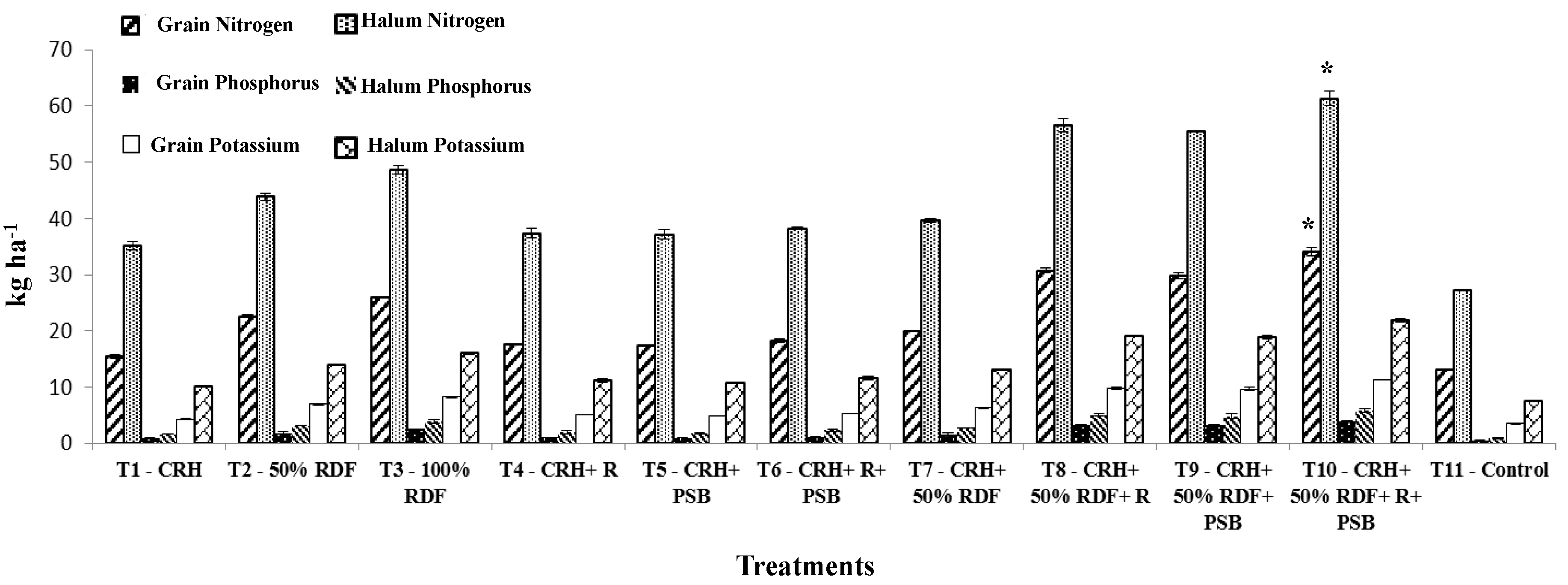
3.2.5. Protein and Nutrient Content
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Subramniyan, S.; Prema, P. Cellulase-free Xylanases from Bacillus and Other Microorganisms. FEMS Microbiol. Lett. 2000, 183, 1–7. [Google Scholar] [CrossRef]
- Premalatha, N.; Gopal, N.O.; Arul Jose, P.; Anandham, R.; Kwon, S.W. Optimization of cellulase production by Enhydrobacter sp. ACCA2 and its application in biomass saccharification. Front. Microbiol. 2015, 6, 1046. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.B.; Tamraka, D.K.; Tamrakar, M.P.; Thakur, K.; Tedia, A.T.; Keshry, P.K. Effect of crop beneficial microbes on decomposition rate of different crop residues. J. Oil Crop. 2004, 14, 1–4. [Google Scholar]
- Badarinath, K.V.S.; Kiran Chand, T.R.; Krishna Prasad, V. Agriculture Crop Residue Burning in The Indo-Gangetic Plains: A Study Using IRS-P6 AWiFS Satellite Data. Curr. Sci. 2006, 91, 1085–1089. [Google Scholar]
- Gaind, S.; Pandey, A.P. Bioconversion of Crop Residues of Barley (Hordeum vulgare) and Rice (Oryza sativa) by Gliocladium virens and Trichoderma reesei QM 9414 under solid-state-fermentation conditions. J. Indian Bot. Soc. 2006, 77, 205–212. [Google Scholar]
- Prasad, C.S.; Maiti, K.N.; Venugopal, R. Effect of RHA in White Ware Composition. Ceram. Int. 2000, 27, 629–635. [Google Scholar] [CrossRef]
- Maki, M.L.; Idrees, A.; Leung, K.T.; Qin, W. Newly Isolated and Characterized Bacteria with Great Application Potential for Decomposition of Lignocellulosic Biomass. Mol. Microbiol. Biotechnol. 2012, 22, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.B. Isolation of cellulolytic bacteria, including actinomycetes, from coffee exocarps in coffee-producing areas in Vietnam. Int. J. Recycl. Org. Waste Agric. 2014, 3, 48. [Google Scholar] [CrossRef]
- Kausar, H.; Sariah, M.; Mohd Saud, H.; Zahangir Alam, M.; Razi Ismail, M. Development of Compatible Lignocellulolytic Fungal Consortium for Rapid Composting of Rice Straw. Int. Biodeterior. Biodegrad. 2010, 64, 594–600. [Google Scholar] [CrossRef]
- Mahesh, K.; Hosmani, S.P. Morphological Changes and Nutrient Uptake in Some Cultivars of Rice Treated with Bavistin. J. Ecotoxicol. Environ. Monit. 2004, 12, 38–39. [Google Scholar]
- Sequi, P.; De Nobili, M.; Leita, L.; Cercignani, G. A new index of humification. Agrochemica 1986, 30, 175–179. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Wantanable, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soil by Extraction with Sodium Bicarbonate; United State Department of Agriculture CIRC: Washinton, DC, USA, 1954; p. 939. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967. [Google Scholar]
- Kumar, A.; Gaind, S.; Nain, L. Evaluation of Thermophilic Fungal Consortium for Paddy Straw Composting. Biodegradation 2008, 19, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal Bioconversion of Lignocellulosic Residues; Opportunities and Perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Sindhu, S.S. Composting of Rice Straw Using Different Inocula and Analysis of Compost Quality. Microb. J. 2011, 4, 126–138. [Google Scholar] [CrossRef]
- Suthar, S. Nutrient Changes and Biodynamics of Epigeic Earthworm Perionyx Excavates (Perrier) during recycling of some agriculture wastes. Bioresour. Technol. 2007, 98, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Eiland, F.M.; Klamer, A.; Lind, N.; Leth, M.; Baath, E. Influence of Initial C:N Ratio on Chemical and Microbial Composition during Long Term Composting of Straw. Microb. Ecol. 2004, 41, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, B.; Rajannan, G.; Jothimani, P. Compost Maturity Test for Empty Fruit Bunch of Palm Oil Industry Solid Waste. Sch. Acad. J. Biosci. 2013, 1, 98–101. [Google Scholar]
- Baharuddin, A.S.; Abdul Rahman, N.A.; Shah, U.K.; Hassan, M.A.; Wakisaka, M.; Shirai, Y. Evaluation of Pressed Shredded Empty Fruit Bunch (EFB)-palm Oil Mill Effluent (POME) Anaerobic Sludge Based Compost Using Fourier Transform Infrared (FTIR) and Nuclear Magnetic Resonance (NMR) Analysis. Afr. J. Biotechnol. 2011, 10, 8082–8089. [Google Scholar]
- Senthil Kumar, P.; Satees Kumar, N.M.; Rajendran, V.; Uthaya Kumar, V.; Anbuganapathi, G. Evaluation of vermicompost Maturity using Scanning Electron Microscopy and Paper Chromatography analysis. J. Agric. Food Chem. 2014, 62, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A. Foliar Application of Effective Microorganisms on Pea as an Alternative Fertilizer. Agron. Sustain. Dev. 2006, 26, 257–262. [Google Scholar] [CrossRef]
- Malik, M.M.R.; Akhtar, M.J.; Ahmad, I.; Khalid, M. Synergistic Use of Rhizobium, Compost and Nitrogen to Improve Growth and Yield of Mungbean (Vigna radiata). Pak. J. Agric. Sci. 2014, 51, 393–398. [Google Scholar]
- Shivram, D.R.; Ahlawat, I.P.S. Effect of cropping systems and fertilizers on pigeonpea and wheat sequence. Indian J. Agron. 2000, 45, 669–676. [Google Scholar]
- Sheikh, T.K.; Ishfaq Akbar, P.; Raies Bhat, A.; Khan, I.M. Response to Biological and Inorganic Nutritional Applications in Black Gram (Vigna mungo L.) CV-t9. World J. Agric. Sci. 2012, 8, 479–480. [Google Scholar]
- Javaid, A.; Bajwa, R.; Anjum, T. Effect of Heat Sterilization and EM (Effective Microorganisms) Application of Wheat (Triticum aestivum L.) Grown in Organic Matter Amended Soils. Cereal Res. Commun. 2009, 36, 489–499. [Google Scholar] [CrossRef]
- Davari, M.; Niwas Sharma, S.; Mizakhani, M. Residual Influence of Organic Materials, Crop Residues and Biofertilizers on Performance of Succeeding Mung Bean in an Organic Rice Based Cropping System. Int. J. Recycl. Org. Waste Agric. 2012, 1, 14. [Google Scholar] [CrossRef]
- Sangeetha, S.P.; Balakrishnan, A.; Devasenapathy, P. Influence of Organic Manures on Yield and Quality of Rice (Oryza sativa L.) and Blackgram (Vigna mungo L.) in Rice-Blackgram Cropping Sequence. Am. J. Plant Sci. 2013, 4, 1151–1157. [Google Scholar] [CrossRef]
- Selvakumar, G.; Reetha, S.; Thamizhiniyan, P. Response of Biofertilizers on Growth, Yield Attributes and Associated Protein Profiling Changes of Blackgram (Vigna mungo L. Hepper). World Appl. Sci. J. 2012, 16, 1368–1374. [Google Scholar]
- Uyanoz, R. The Effects of Different Bio-organic, Chemical Fertilizers and their Combination on Yield, Macro and Micro Nutrition Content of Dry Bean (Phaseolus vulgaris L.). Int. J. Agric. Res. 2007, 2, 115–125. [Google Scholar]
- Gamma, A.M.; Mohamed, M.H. Application of Bio-organic Agriculture and its Effect on Guar (Cyamopsis tetragonoloba L.) root nodules, forage, seed yield and yield quality. World J. Agric. Sci. 2007, 3, 91–96. [Google Scholar]
- Alam, F.; Bhuiyan, M.A.H.; Alam, S.S.; Waghmode, T.R.; Kim, P.J.; Lee, Y.B. Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci. Biotechnol. Biochem. 2015, 79, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.P.S.; Sharma, G.L.; Chahar, M.S. Effect of phosphorus and biofertilizers on the growth and Productivity of Black Gram. Ann. Agric. Res. 2002, 23, 491–493. [Google Scholar]
| Treatments | Organic Carbon | Total Nitrogen | Total Phosphorus | Total Potassium | C:N Ratio | C:P Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Ratio | |||||||||||
| Day 15 | Day 90 | Day 15 | Day 90 | Day 15 | Day 90 | Day 15 | Day 90 | Day 15 | Day 90 | Day 15 | Day 90 | |
| T1 | 25.80 ± 0.63 | 22.78 ± 0.15 | 0.31 ± 0.01 e | 0.63 ± 0.01 g | 0.20 ± 0.00 f | 0.61 ± 0.01 f | 0.29 ± 0.00 g | 0.78 ± 0.02 h | 83:1 | 36:1 | 129:1 | 37:1 |
| T2 | 25.65 ± 0.11 | 20.76 ± 0.52 | 0.34 ± 0.00 b,c | 0.87 ± 0.01 e | 0.26 ± 0.00 d | 0.78 ± 0.01 d | 0.35 ± 0.01 e | 0.99 ± 0.02 f | 75:1 | 24:1 | 99:1 | 27:1 |
| T3 | 24.60 ± 0.51 | 20.16 ± 0.14 | 0.39 ± 0.00 a | 0.92 ± 0.02 d | 0.32 ± 0.01 b | 0.82 ± 0.00 c,d | 0.43 ± 0.00 c | 1.09 ± 0.03 d | 63:1 | 22:1 | 77:1 | 25:1 |
| T4 | 24.82 ± 0.61 | 19.86 ± 0.37 | 0.36 ± 0.01 b | 0.95 ± 0.01 c | 0.33 ± 0.01 b | 0.86 ± 0.02 c | 0.46 ± 0.01 b | 1.14 ± 0.02 c | 69:1 | 21:1 | 75:1 | 23:1 |
| T5 | 25.62 ± 0.01 | 16.01 ± 0.17 | 0.34 ± 0.00 b,c | 1.10 ± 0.00 a | 0.29 ± 0.00 c | 0.96 ± 0.00 a | 0.44 ± 0.00 b,c | 1.24 ± 0.03 a | 75:1 | 14:1 | 88:1 | 17:1 |
| T6 | 24.72 ± 0.22 | 17.76 ± 0.45 | 0.36 ± 0.01 b | 1.03 ± 0.00 b | 0.36 ± 0.00 a | 0.91 ± 0.01 b | 0.49 ± 0.00 a | 1.19 ± 0.01 b | 69:1 | 17:1 | 69:1 | 20:1 |
| T7 | 25.59 ± 0.36 | 21.77 ± 0.32 | 0.32 ± 0.01 d,e | 0.69 ± 0.01 f | 0.21 ± 0.00 e | 0.69 ± 0.00 e | 0.32 ± 0.00 f | 0.82 ± 0.01 g | 80:1 | 31:1 | 122:1 | 32:1 |
| T8 | 24.78 ± 0.61 | 21.26 ± 0.39 | 0.33 ± 0.01 d,e | 0.70 ± 0.01 f | 0.24 ± 0.00 d | 0.73 ± 0.00 e | 0.38 ± 0.01 d | 1.04 ± 0.00 e | 75:1 | 30:1 | 103:1 | 29:1 |
| LSD (p ≤ 0.05) | 0.60 | 0.47 | 0.02 | 0.02 | 0.02 | 0.04 | 0.02 | 0.04 | - | - | - | - |
| Treatments | Typic Haplustalf | Typic Rhodustalf | ||||||
|---|---|---|---|---|---|---|---|---|
| Branches Per Plant | Pods Per Branch | Grains Per Pod | 100 Grain Weight (g) | Branches Per Plant | Pods Per Branch | Grains Per Pod | 100 Grain Weight (g) | |
| T1 | 1.98 ± 0.05 f | 2.11 ± 0.03 e | 3.01 ± 0.03 e | 3.11 ± 0.02 g | 5.44 ± 0.04 f | 5.60 ± 0.02 i | 4.17 ± 0.01 | 4.15 ± 0.02 |
| T2 | 2.29 ± 0.01 d | 2.45 ± 0.03 c | 3.20 ± 0.05 d | 3.38 ± 0.06 d | 5.81 ± 0.10 d | 6.42 ± 0.06 e | 4.40 ± 0.04 | 4.33 ± 0.02 |
| T3 | 2.41 ±0.05 c | 2.58 ± 0.04 b | 3.33 ± 0.07 c | 3.50 ± 0.04 c | 5.93 ± 0.09 c | 6.57 ± 0.05 d | 4.52 ± 0.01 | 4.52 ± 0.07 |
| T4 | 2.12 ± 0.04 e | 2.25 ± 0.02 d | 3.02 ± 0.03 e | 3.19 ± 0.02 f,g | 5.59 ± 0.14 e | 6.03 ± 0.08 h | 4.20 ± 0.07 | 4.20 ± 0.05 |
| T5 | 2.11 ± 0.05 e | 2.23 ± 0.00 d | 3.02 ± 0.06 e | 3.22 ± 0.04 f,g | 5.56 ± 0.14 e | 5.97 ± 0.08 h | 4.18 ± 0.09 | 4.18 ± 0.02 |
| T6 | 2.14 ± 0.03 e | 2.29 ± 0.01 d | 3.03 ± 0.02 e | 3.26 ± 0.06 e,f | 5.63 ± 0.12 e | 6.16 ± 0.07 g | 4.25 ± 0.03 | 4.25 ± 0.03 |
| T7 | 2.26 ± 0.05 d | 2.41 ± 0.02 c | 3.17 ± 0.06 d | 3.36 ± 0.01 d,e | 5.74 ± 0.04 d | 6.29 ± 0.02 f | 4.38 ± 0.02 | 4.38 ± 0.07 |
| T8 | 2.56 ± 0.04 b | 2.65 ± 0.02 b | 3.56 ± 0.09 b | 3.71 ± 0.03 b | 6.25 ± 0.03 b | 6.89 ± 0.02 b | 4.64 ± 0.05 | 4.64 ± 0.09 |
| T9 | 2.53 ± 0.04 b | 2.63 ± 0.06 b | 3.43 ± 0.08 c | 3.68 ± 0.07 b | 6.12 ± 0.03 b | 6.71 ± 0.01 c | 4.55 ± 0.11 | 4.55 ± 0.11 |
| T10 | 2.68 ± 0.03 a | 3.78 ± 0.03 a | 3.76 ± 0.04 a | 3.94 ± 0.01 a | 7.16 ± 0.18 a | 7.45 ± 0.10 a | 4.70 ± 0.02 | 4.80 ± 0.05 |
| T11 | 1.85 ± 0.04 g | 1.98 ± 0.03 f | 2.99 ± 0.07 f | 3.03 ± 0.06 h | 5.33 ± 0.12 g | 5.41 ± 0.07 j | 4.15 ± 0.03 | 4.12 ± 0.08 |
| LSD (p ≤ 0.05) | 0.10 | 0.11 | 0.12 | 0.11 | 0.10 | 0.12 | NS | NS |
| Treatments | Typic Haplustalf | Typic Rhodustalf | ||||
|---|---|---|---|---|---|---|
| Grain Yield Per kg/ha | Haulm Yield Per kg/ha | Protein Content % | Grain Yield Per kg/ ha | Haulm Yield Per kg/ha | Grain Crude Protein Content % | |
| T1 | 585 ± 9.89 g | 1265 ± 14.55 f | 15.50 ± 0.34 | 579 ± 8.20 g | 1284 ± 20.18 g | 16.75 ± 0.20 e |
| T2 | 779 ± 18.65 d | 1475 ± 16.12 c | 16.75 ± 0.11 | 783 ± 19.15 d | 1477 ± 11.53 d | 18.06 ± 0.07 c,d |
| T3 | 848 ± 11.92 c | 1550 ± 39.53 c | 17.69 ± 0.07 | 849 ± 21.65 c | 1555 ± 2.43 c | 19.06 ± 0.04 c |
| T4 | 649 ± 15.87 f | 1346 ± 1.40 e | 15.56 ± 0.12 | 655 ± 16.02 f | 1353 ± 13.38 f | 16.88 ± 0.07 e |
| T5 | 675 ± 5.27 e,f | 1318 ± 4.80 e | 15.63 ± 0.19 | 640 ± 7.99 f | 1337 ± 10.44 f | 17.00 ± 0.11 e |
| T6 | 670 ± 15.69 e,f | 1366 ± 14.22 d,e | 15.69 ± 0.17 | 666 ± 9.71 f | 1370 ± 4.28 e,f | 17.06 ± 0.10 d,e |
| T7 | 711 ± 14.06 e | 1409 ± 33.00 d | 16.56 ± 0.19 | 725 ± 8.30 e | 1417 ± 16.96 e | 17.13 ± 0.11 d,e |
| T8 | 921 ± 7.19 b | 1644 ± 22.25 b | 19.25 ± 0.21 | 937 ± 8.29 b | 1692 ± 1.76 b | 20.30 ± 0.12 b |
| T9 | 912 ± 8.07 b | 1621 ± 30.37 b | 18.50 ± 0.25 | 932 ± 0.49 b | 1688 ± 36.90 b | 20.00 ± 0.14 b |
| T10 | 988 ± 23.65 a | 1712 ± 17.82 a | 20.19 ± 0.36 | 994 ± 12.42 a | 1752 ± 12.77 a | 21.44 ± 0.21 a |
| T11 | 510 ± 9.02 h | 1006 ± 10.99 g | 13.63 ± 0.28 | 522 ± 2.17 h | 1056 ± 7.69 h | 15.63 ± 0.16 f |
| LSD (p ≤ 0.05) | 58.78 | 66.10 | 1.15 | 53.51 | 56.02 | 1.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiyageshwari, S.; Gayathri, P.; Krishnamoorthy, R.; Anandham, R.; Paul, D. Exploration of Rice Husk Compost as an Alternate Organic Manure to Enhance the Productivity of Blackgram in Typic Haplustalf and Typic Rhodustalf. Int. J. Environ. Res. Public Health 2018, 15, 358. https://doi.org/10.3390/ijerph15020358
Thiyageshwari S, Gayathri P, Krishnamoorthy R, Anandham R, Paul D. Exploration of Rice Husk Compost as an Alternate Organic Manure to Enhance the Productivity of Blackgram in Typic Haplustalf and Typic Rhodustalf. International Journal of Environmental Research and Public Health. 2018; 15(2):358. https://doi.org/10.3390/ijerph15020358
Chicago/Turabian StyleThiyageshwari, Subramanium, Pandurangan Gayathri, Ramasamy Krishnamoorthy, Rangasamy Anandham, and Diby Paul. 2018. "Exploration of Rice Husk Compost as an Alternate Organic Manure to Enhance the Productivity of Blackgram in Typic Haplustalf and Typic Rhodustalf" International Journal of Environmental Research and Public Health 15, no. 2: 358. https://doi.org/10.3390/ijerph15020358
APA StyleThiyageshwari, S., Gayathri, P., Krishnamoorthy, R., Anandham, R., & Paul, D. (2018). Exploration of Rice Husk Compost as an Alternate Organic Manure to Enhance the Productivity of Blackgram in Typic Haplustalf and Typic Rhodustalf. International Journal of Environmental Research and Public Health, 15(2), 358. https://doi.org/10.3390/ijerph15020358







