Bake-Out Strategy Considering Energy Consumption for Improvement of Indoor Air Quality in Floor Heating Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
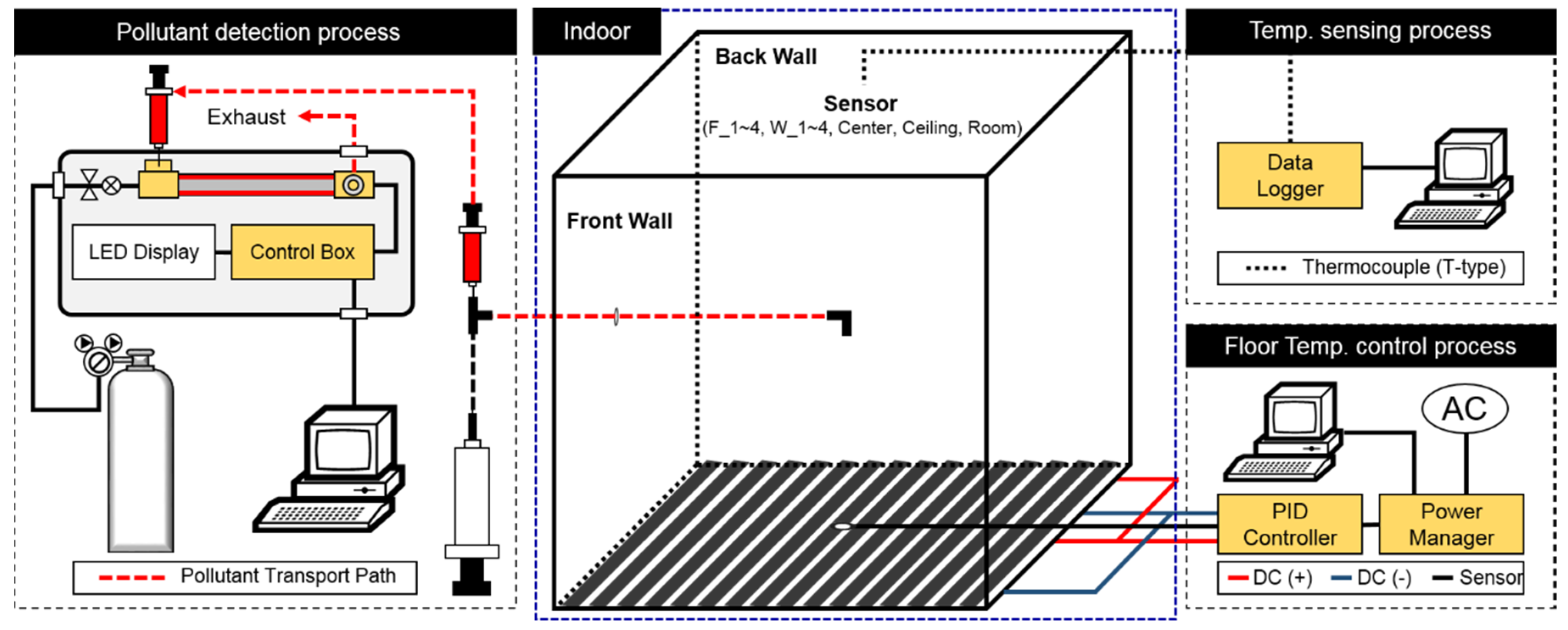
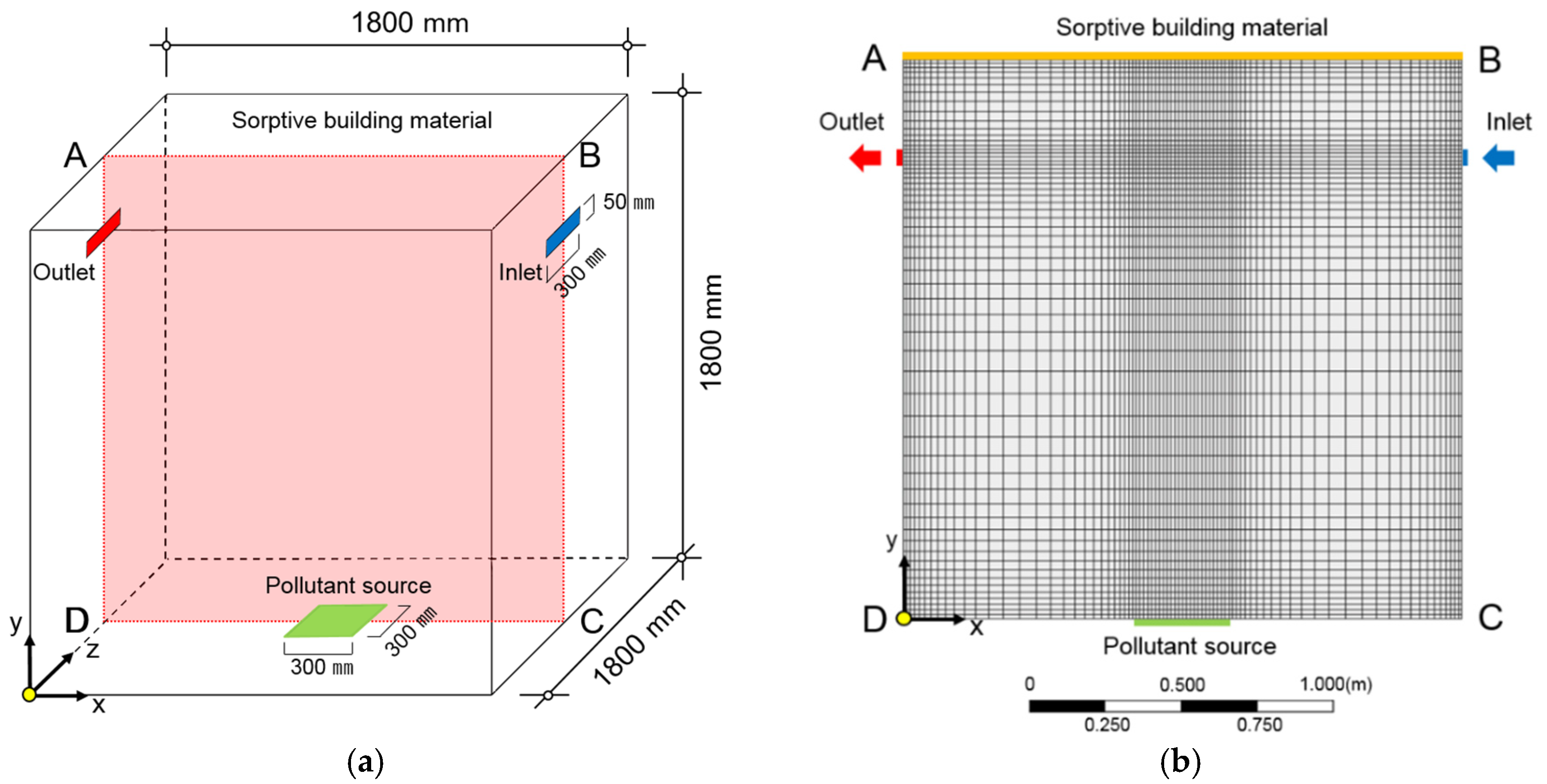
2.1.1. Target Space and Experiment Method
2.1.2. Floor Temperature Control
2.2. Numerical Model of CFD Analysis
2.2.1. Boundary Condition of CFD Analysis
2.2.2. Turbulence Model and Governing Equations for CFD Analysis
2.2.3. Pollutant Diffusion Model
2.2.4. Pollutant Adsorption Model
3. Results and Discussion
3.1. Floor Temperature Control and Energy Consumption
3.2. Quality Assurance of Pollutant Measurement and Analysis
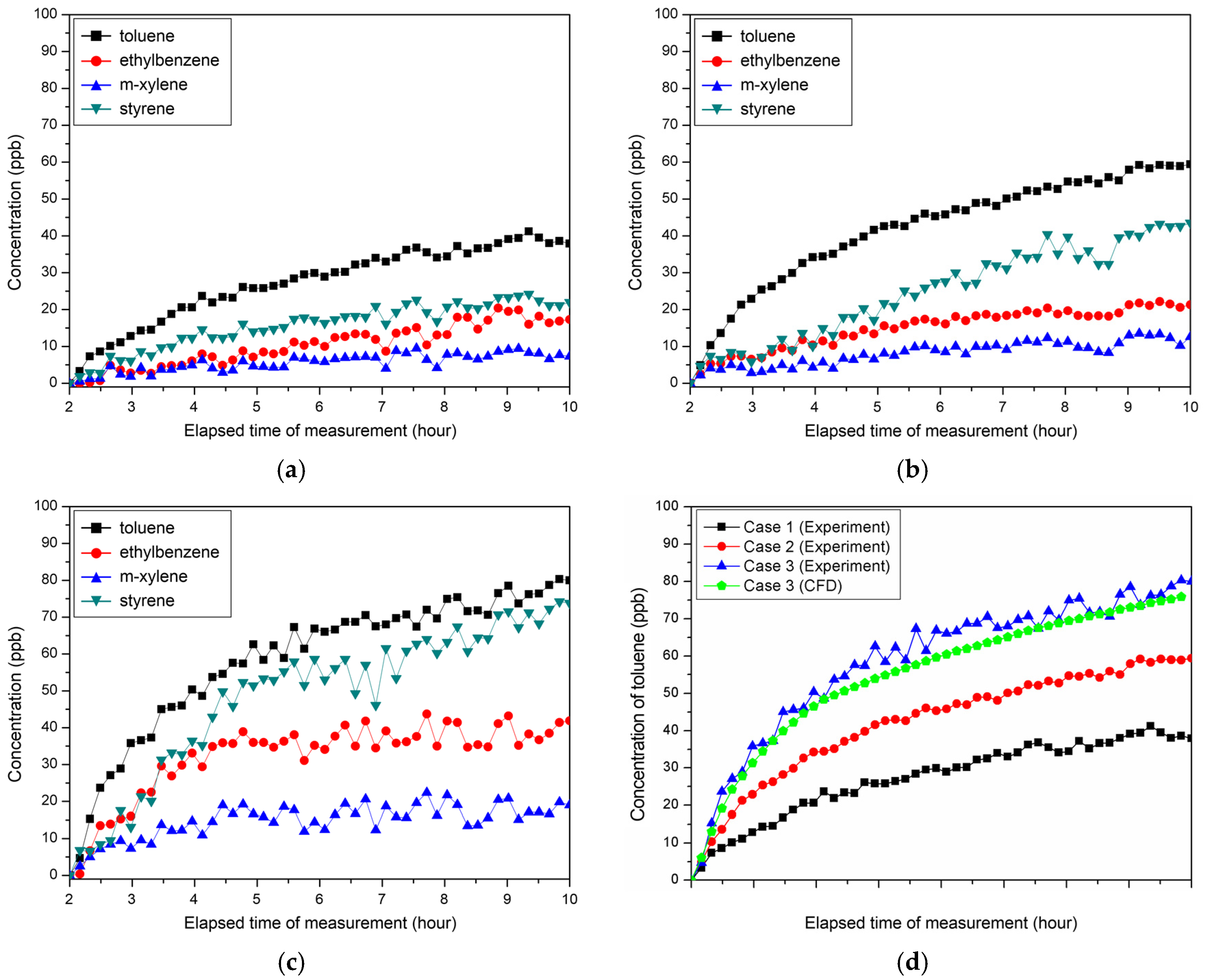
3.3. Change in Pollutant Concentration According to Floor Temperature (Bake Process)
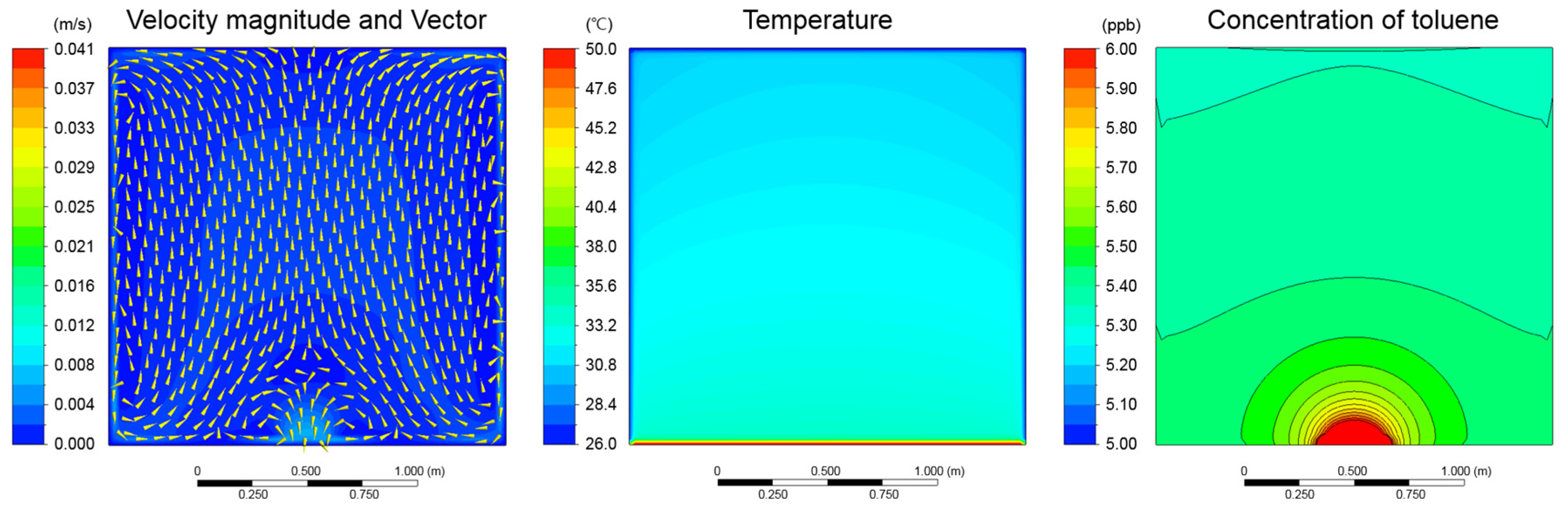
3.4. Evaluation of Contaminant Concentration Reduction through Use of Sorptive Building Material and Ventilation (Bake-out Process)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barabad, M.; Jung, W.; Versoza, M.; Kim, M.; Ko, S.; Park, D.; Lee, K. Emission Characteristics of Particulate Matter, Volatile Organic Compounds, and Trace Elements from the Combustion of Coals in Mongolia. Int. Environ. Res. Public Health 2018, 15, 1706. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef]
- Smith, M.T. Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Mo, J.; Zhang, Y. Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China. Environ. Int. 2014, 73, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Girman, J.R.; Alevantis, L.E.; Kulasingam, G.C.; Petreas, M.X.; Webber, L.M. The bake-out of an office building: A case study. Environ. Int. 1989, 15, 449–453. [Google Scholar] [CrossRef]
- Girman, J.R. Volatile organic compounds and building bake-out. Occup. Med. 1989, 4, 695–712. [Google Scholar] [PubMed]
- Lu, Y.; Liu, J.; Lu, B.-N.; Jiang, A.-X.; Zhang, J.-R.; Xie, B.; Li, J.; Zhang, L. Numerical value research on bake-out technology with dilution ventilation for building materials. Proc. Build. Simul. 2007, 2007, 907–911. [Google Scholar]
- Edwards, R.D.; Jurvelin, J.; Koistinen, K.; Saarela, K.; Jantunen, M. VOC source identification from personal and residential indoor, outdoor and workplace microenvironment samples in EXPOLIS-Helsinki, Finland. Atmos. Environ. 2001, 35, 4829–4841. [Google Scholar] [CrossRef]
- Kang, D.H.; Choi, D.H.; Lee, S.M.; Yeo, M.S.; Kim, K.W. Effect of bake-out on reducing VOC emissions and concentrations in a residential housing unit with a radiant floor heating system. Build. Environ. 2010, 45, 1816–1825. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Wei, S.; Wang, H. Experimental and simulation study on bake-out with dilution ventilation technology for building materials. J. Air Waste Manag. Assoc. 2016, 66, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Haghighat, F.; Ghaly, W. A study on VOC source and sink behavior in porous building materials-analytical model development and assessment. Indoor Air 2005, 15, 183–196. [Google Scholar] [CrossRef]
- Jiang, C.; Li, D.; Zhang, P.; Li, J.; Wang, J.; Yu, J. Formaldehyde and volatile organic compound (VOC) emissions from particleboard: Identification of odorous compounds and effects of heat treatment. Build. Environ. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Ataka, Y.; Kato, S.; Murakami, S.; Zhu, Q.; Ito, K.; Yokota, T. Study of effect of adsorptive building material on formaldehyde concentrations; development of measuring methods and modeling of adsorption phenomena. Indoor Air 2004, 14, 51–64. [Google Scholar] [CrossRef]
- Seo, J.; Kato, S.; Ataka, Y.; Chino, S. Performance test for evaluating the reduction of VOCs in rooms and evaluating the lifetime of sorptive building materials. Build. Environ. 2009, 44, 207–215. [Google Scholar] [CrossRef]
- Park, S.; Seo, J.; Kim, J.T. A study on the application of sorptive building materials to reduce the concentration and volume of contaminants inhaled by occupants in office areas. Energy Build. 2015, 98, 10–18. [Google Scholar] [CrossRef]
- Graf, M.; Barrettino, D.; Taschini, S.; Hagleitner, C.; Hierlemann, A.; Baltes, H. Metal oxide-based monolithic complementary metal oxide semiconductor gas sensor microsystem. Anal. Chem. 2004, 76, 4437–4445. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.-K.; Park, K.-W.; Kim, J.T. Test methods and reduction of organic pollutant compound emissions from wood-based building and furniture materials. Bioresour. Technol. 2010, 101, 6562–6568. [Google Scholar] [CrossRef]
- Yang, X. Study of Building Material Emissions and Indoor Air Quality. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1999. [Google Scholar]
- ANSYS 17.2. Fluent Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2011. [Google Scholar]
- Hussain, S.; Oosthuizen, P.H.; Kalendar, A. Evaluation of various turbulence models for the prediction of the airflow and temperature distributions in atria. Energy Build. 2012, 48, 18–28. [Google Scholar] [CrossRef]
- Abe, K.; Kondoh, T.; Nagano, Y. A new turbulence model for predicting fluid flow and heat transfer in separating and reattaching flows—I. Flow field calculations. Int. J. Heat Mass Transf. 1994, 37, 139–151. [Google Scholar] [CrossRef]
- Bulińska, A.; Popiołek, Z.; Buliński, Z. Experimentally validated CFD analysis on sampling region determination of average indoor carbon dioxide concentration in occupied space. Build. Environ. 2014, 72, 319–331. [Google Scholar] [CrossRef]
- The Society of Chemical Engineers. Handbook of Chemistry; The Society of Chemical Engineers: Tokyo, Japan, 1999. [Google Scholar]
- Seo, J.; Lee, S.; Kim, S.; Nam, Y. Control of emission rates of chemical compounds emitted by controlling their mass transfer coefficients on the surface of the tested building material. J. Adhes. Sci. Technol. 2013, 27, 610–619. [Google Scholar] [CrossRef]
- Lim, E.-S. Evaluation of Contaminant Concentration Reduction Effect of Sorptive Building Materials by New Ventilation Index-Net Escape Velocity. J. Archit. Inst. Korea Plan. Des. 2013, 29, 291–298. (In Korean) [Google Scholar] [CrossRef]
- ICH Expert Working Group. Validation of analytical procedures: Text and methodology Q2(R1). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 25–27 April 2005. [Google Scholar]
- ASHRAE. ASHRAE Standard 129—Measuring Air-Change Effectiveness; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2004. [Google Scholar]
- Piccolo, A.; Siclari, R.; Rando, F.; Cannistraro, M. Comparative performance of thermoacoustic heat exchangers with different pore geometries in oscillatory flow. Implementation of experimental techniques. Appl. Sci. 2017, 7, 784. [Google Scholar] [CrossRef]






| Case | Set Floor Temp. | Bake Process (Method) | Bake-out Process (Method) | |
|---|---|---|---|---|
| Case 1 | 30 °C | Floor heating (Experimental) | N/A | |
| Case 2 | 40 °C | |||
| Case 3 | 50 °C | Floor heating (Experimental and CFD analysis) | ALT 1 | Natural ventilation (CFD analysis) |
| ALT 2 | Mechanical ventilation (CFD analysis) | |||
| ALT 3 | Installation of SBM (CFD analysis) | |||
| Component | Parameter | Description |
|---|---|---|
| Room Condition | Cooling temp. | 25 °C |
| Test chamber | Ceiling height | 1800 mm |
| Floor area | 1800 mm × 1800 mm | |
| Material | Isopink (30 T) | |
| Experimental Equipment | Temp. sensor | Thermocouple (T-type) |
| Monitoring | Data Logger (GL 220) | |
| Heater | Heating film (PTC) | |
| Temp. control | PID controller (NX_1) | |
| Power consumption | Power manager (B200) | |
| Pollutant | Loctite 401 (20 g) | |
| Monitoring | Three notebooks | |
| Sampler | Sampling tube | |
| Sterile syringe (100 mL) | ||
| 1005TLL 5.0 mL syringe |
| Parameter | Description | |||
|---|---|---|---|---|
| Measurement principle | Gas chromatography using semiconductor gas sensor | |||
| Carrier gas | High purity cylinder air | |||
| Sampling method | Manual sampling with a syringe (5 cc) | |||
| Decomposition capability | 0.1 ppb | |||
| Measurement time | 8 min | |||
| Power supply | 100–240 V, 50/60 Hz | |||
| Measurement range | toluene | ethylbenzene | m-xylene | styrene |
| 5–1000 ppb | 5–1000 ppb | 5–1000 ppb | 5–1000 ppb | |
| Classification | ALT 1 | ALT 2 | ALT 3 |
|---|---|---|---|
| Bake-out method | Natural ventilation  | Mechanical ventilation  | Installation of SBM  |
| Inflow Boundary |  Umean = 0.054 m/s, Air change rate = 0.5 h−1 |  Umean = 0.324 m/s, Umax = 0.53 m/s, Air change rate = 3.0 h−1 | N/A |
| Outflow Boundary | Uout = outflow (Mass flow conservation) | Uout = outflow (Mass flow conservation) | |
| Installation of SBM | N/A | N/A | Ceiling (A = 3.24 m2) |
| Operating time | 2 h | 2 h | 2 h |
| Energy consumption | N/A | Fan energy = 50 W | N/A |
| Turbulent model | Low Reynolds number k-ε turbulence model | ||
| Number of meshes | Around 400,000 | ||
| Wall boundary | No-slip | ||
| Wall temperature | The experimental result was set as the value of each wall surface temp. (Figure 4) | ||
| Pollutant | Toluene (Molecular weight, 92.13 kg/kgmol) | ||
| Time step size | 30 s | ||
| Compound | Retention Time (s) | Signal Strength (mV) | Measured Concentration (ppb) | RSD (%) |
|---|---|---|---|---|
| Toluene | 92.16 ± 1.57 (1) | 154.42 ± 3.21 | 103.77 ± 2.96 | 2.07 |
| Ethylbenzene | 171.78 ± 1.44 | 132.03 ± 2.97 | 103.96 ± 3.01 | 2.25 |
| m-Xylene | 211.12 ± 0.95 | 135.80 ± 3.22 | 102.90 ± 3.39 | 2.37 |
| Styrene | 307.16 ± 1.78 | 72.92 ± 2.05 | 104.40 ± 3.99 | 2.82 |
| Compound | Injection Concentration (ppb) | Signal Strength (mV) | Measured Concentration (ppb) | Accuracy (%) | RSD (%) |
|---|---|---|---|---|---|
| Toluene | 10 | 53.49 ± 0.46 (1) | 10.67 ± 0.42 | 102.11–112.10 | 0.86 |
| 30 | 76.85 ± 0.82 | 32.22 ± 0.75 | 104.10–111.00 | 1.07 | |
| 100 | 158.95 ± 3.48 | 107.95 ± 3.21 | 104.80–111.20 | 2.19 | |
| 300 | 377.18 ± 3.66 | 309.25 ± 3.38 | 101.60–104.10 | 0.97 | |
| Ethylbenzene | 10 | 39.55 ± 0.30 | 10.37 ± 0.30 | 102.00–108.00 | 0.77 |
| 30 | 60.18 ± 0.79 | 31.25 ± 0.81 | 102.00–108.20 | 0.79 | |
| 100 | 133.10 ± 1.08 | 105.05 ± 1.09 | 103.60–105.90 | 0.10 | |
| 300 | 332.11 ± 2.84 | 306.45 ± 2.88 | 100.73–102.83 | 2.84 | |
| m-Xylene | 10 | 47.82 ± 0.32 | 10.25 ± 0.34 | 98.00–105.90 | 0.67 |
| 30 | 67.33 ± 0.34 | 30.80 ± 0.36 | 101.33–104.10 | 0.51 | |
| 100 | 139.70 ± 3.25 | 107.02 ± 3.42 | 102.40–110.00 | 2.32 | |
| 300 | 325.01 ± 1.65 | 302.15 ± 1.74 | 100.00–101.40 | 0.51 | |
| Styrene | 10 | 24.70 ± 0.08 | 10.82 ± 0.17 | 108.00–110.00 | 0.35 |
| 30 | 35.32 ± 0.43 | 31.42 ± 0.85 | 100.67–106.67 | 1.23 | |
| 100 | 74.96 ± 0.91 | 108.35 ± 1.78 | 106.80–110.90 | 1.22 | |
| 300 | 178.69 ± 1.46 | 309.65 ± 2.84 | 101.87–104.07 | 0.82 |
| Case | Set Temp. (°C) | Toluene (ppb) | Ethylbenzene (ppb) | m-Xylene (ppb) | o-Xylene (ppb) | Styrene (ppb) |
|---|---|---|---|---|---|---|
| Case 1 | 30 | 17.2 | 4.1 | 3.8 | - | 2.6 |
| Case 2 | 40 | 18.4 | 3.7 | 5.6 | - | 4.3 |
| Case 3 | 50 | 18.7 | 4.4 | 5.3 | - | 2.8 |
| Case | Et (Rate of Increase) | Em (Rate of Increase) | Maximum Concentration of Toluene (Rate of Increase) |
|---|---|---|---|
| Case 1 | 974 Wh (-) | 852 Wh (-) | 41.0 ppb (-) |
| Case 2 | 3634 Wh (273%) | 2755 Wh (223%) | 59.4 ppb (45%) |
| Case 3 | 6789 Wh (597%) | 5280 Wh (519%) | 80.3 ppb (96%) |
| ALT | Em with Fan Energy (Wh) | Age-of-Air (s) | Decreasing Rate of Concentration Per Energy Consumption (ppb/kWh) |
|---|---|---|---|
| ALT 1 | 5280 | 2156 | 10.68 |
| ALT 2 | 5380 | 1632 | 11.34 |
| ALT 3 | 5280 | 1936 | 11.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Seo, J. Bake-Out Strategy Considering Energy Consumption for Improvement of Indoor Air Quality in Floor Heating Environments. Int. J. Environ. Res. Public Health 2018, 15, 2720. https://doi.org/10.3390/ijerph15122720
Park S, Seo J. Bake-Out Strategy Considering Energy Consumption for Improvement of Indoor Air Quality in Floor Heating Environments. International Journal of Environmental Research and Public Health. 2018; 15(12):2720. https://doi.org/10.3390/ijerph15122720
Chicago/Turabian StylePark, Seonghyun, and Janghoo Seo. 2018. "Bake-Out Strategy Considering Energy Consumption for Improvement of Indoor Air Quality in Floor Heating Environments" International Journal of Environmental Research and Public Health 15, no. 12: 2720. https://doi.org/10.3390/ijerph15122720
APA StylePark, S., & Seo, J. (2018). Bake-Out Strategy Considering Energy Consumption for Improvement of Indoor Air Quality in Floor Heating Environments. International Journal of Environmental Research and Public Health, 15(12), 2720. https://doi.org/10.3390/ijerph15122720





