The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data
Abstract
1. Background
2. Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics with All Types Trauma
3.2. Characteristics of Patients with Head Injury
3.3. Characteristics of Patients without Head Injury
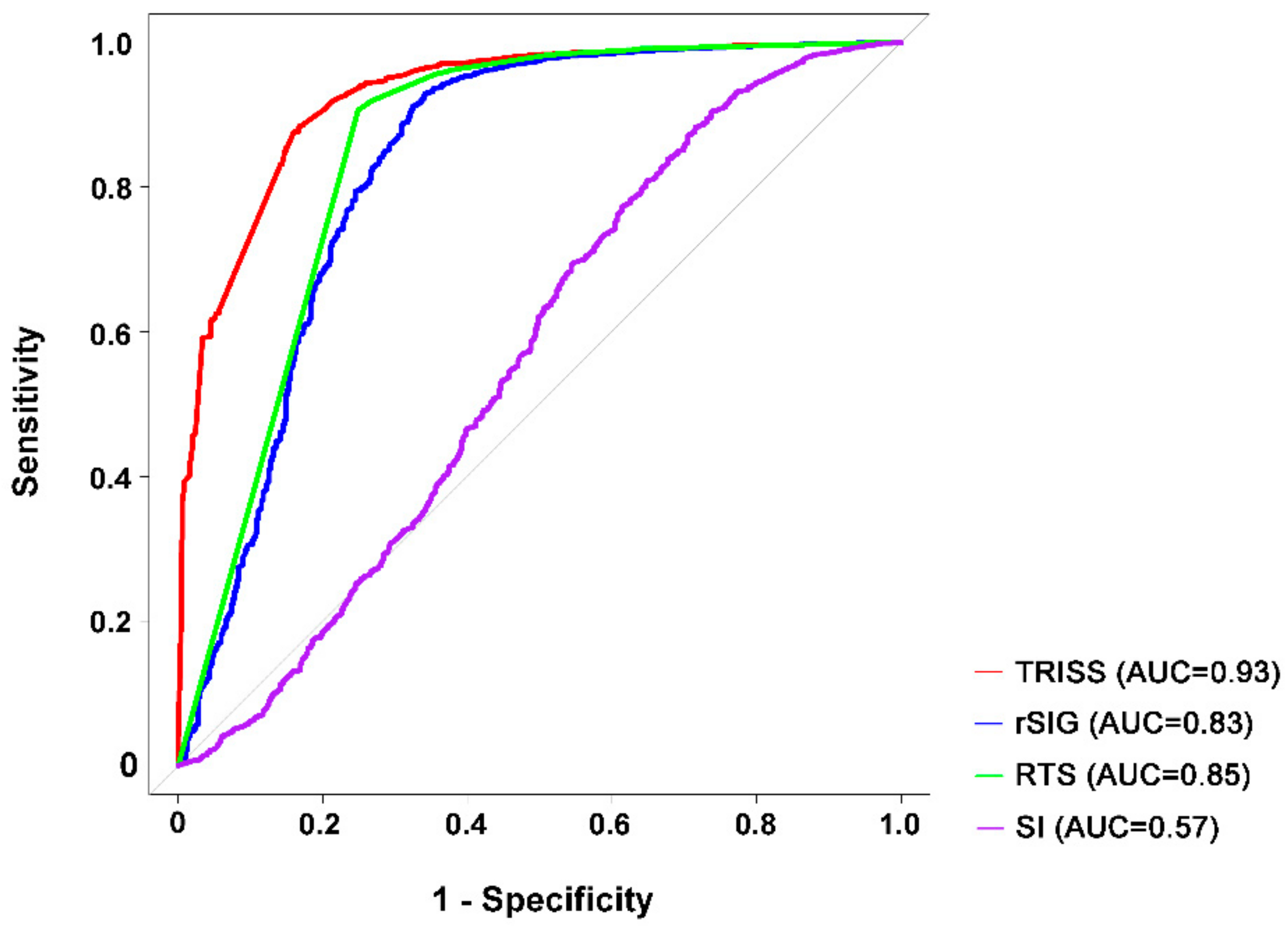
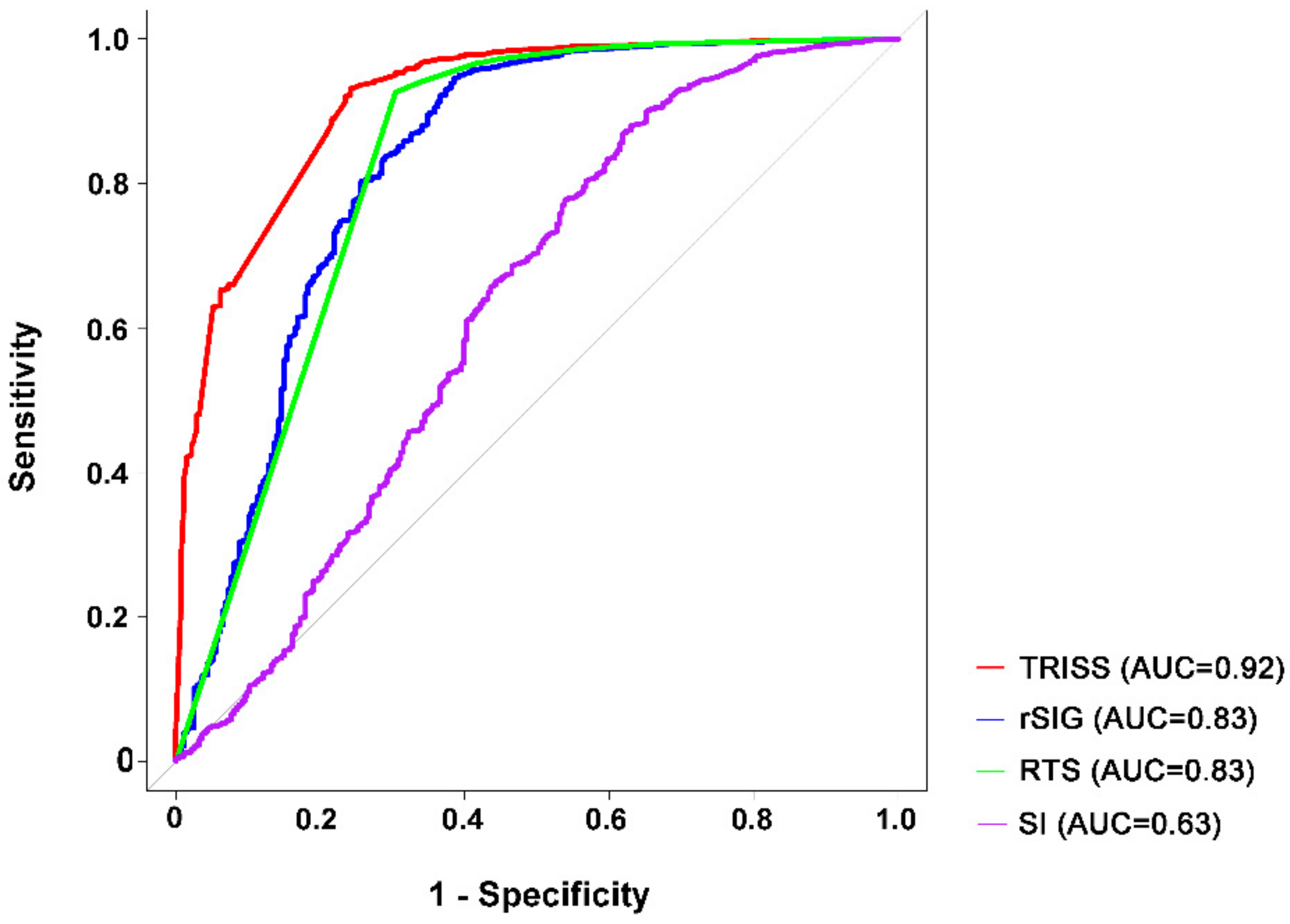
3.4. Predictive Accuracy for Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabbe, B.J.; Cameron, P.A.; Wolfe, R. TRISS: Does it get better than this? Acad. Emerg. Med. 2004, 11, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Glance, L.G.; Osler, T. Beyond the major trauma outcome study: Benchmarking performance using a national contemporary, population-based trauma registry. J. Trauma 2001, 51, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971, 215, 277–280. [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A revision of the Trauma Score. J. Trauma 1989, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
- De Munter, L.; Polinder, S.; Lansink, K.W.; Cnossen, M.C.; Steyerberg, E.W.; de Jongh, M.A. Mortality prediction models in the general trauma population: A systematic review. Injury 2017, 48, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tohira, H.; Jacobs, I.; Mountain, D.; Gibson, N.; Yeo, A. Systematic review of predictive performance of injury severity scoring tools. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Allgower, M.; Burri, C. [“Shock index”]. Dtsch. Med. Wochenschr. 1967, 92, 1947–1950. [Google Scholar] [PubMed]
- Mutschler, M.; Nienaber, U.; Munzberg, M.; Wolfl, C.; Schoechl, H.; Paffrath, T.; Bouillon, B.; Maegele, M. The Shock Index revisited—A fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit. Care 2013, 17, R172. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.Y.; Nightingale, P.; Little, R.A.; Edwards, J.D. Shock index: A re-evaluation in acute circulatory failure. Resuscitation 1992, 23, 227–234. [Google Scholar] [CrossRef]
- Nakasone, Y.; Ikeda, O.; Yamashita, Y.; Kudoh, K.; Shigematsu, Y.; Harada, K. Shock index correlates with extravasation on angiographs of gastrointestinal hemorrhage: A logistics regression analysis. Cardiovasc. Interv. Radiol. 2007, 30, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.P.; Koenigsberg, M.; Clark, J.M.; Weir, W.B.; Philbin, N. Shock index and prediction of traumatic hemorrhagic shock 28-day mortality: Data from the DCLHb resuscitation clinical trials. West. J. Emerg. Med. 2014, 15, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Fitzgerald, M.; Chan, J. The utility of a shock index ≥1 as an indication for pre-hospital oxygen carrier administration in major trauma. Injury 2014, 45, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.F.; Rau, C.S.; Wu, S.C.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C.; Hsieh, C.H. Use of the reverse shock index for identifying high-risk patients in a five-level triage system. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Kuo, P.J.; Hsu, S.Y.; Rau, C.S.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H. The use of the reverse shock index to identify high-risk trauma patients in addition to the criteria for trauma team activation: A cross-sectional study based on a trauma registry system. BMJ Open 2016, 6, e011072. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.H.; Rau, C.S.; Hsu, S.Y.; Wu, S.C.; Kuo, P.J.; Hsieh, H.Y.; Chen, Y.C.; Hsieh, C.H. Using the Reverse Shock Index at the Injury Scene and in the Emergency Department to Identify High-Risk Patients: A Cross-Sectional Retrospective Study. Int. J. Environ. Res. Public Health 2016, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.H.; Wu, S.C.; Rau, C.S.; Kuo, P.J.; Hsu, S.Y.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H. Systolic Blood Pressure Lower than Heart Rate upon Arrival at and Departure from the Emergency Department Indicates a Poor Outcome for Adult Trauma Patients. Int. J. Environ. Res. Public Health 2016, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Current management of massive hemorrhage in trauma. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Shere-Wolfe, R.F.; Galvagno, S.M., Jr.; Grissom, T.E. Critical care considerations in the management of the trauma patient following initial resuscitation. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yin, P.; Ning, P.; Wang, L.; Cheng, X.; Liu, Y.; Schwebel, D.C.; Liu, J.; Qi, J.; Hu, G.; et al. Trends in traumatic brain injury mortality in China, 2006–2013: A population-based longitudinal study. PLoS Med. 2017, 14, e1002332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR. Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Emami, P.; Czorlich, P.; Fritzsche, F.S.; Westphal, M.; Rueger, J.M.; Lefering, R.; Hoffmann, M. Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: A retrospective, multicenter cohort study. J. Neurosurg. 2017, 126, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Nik, A.; Sheikh Andalibi, M.S.; Ehsaei, M.R.; Zarifian, A.; Ghayoor Karimiani, E.; Bahadoorkhan, G. The Efficacy of Glasgow Coma Scale (GCS) Score and Acute Physiology and Chronic Health Evaluation (APACHE) II for Predicting Hospital Mortality of ICU Patients with Acute Traumatic Brain Injury. Bull. Emerg. Trauma 2018, 6, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Tanaka, N. Reverse shock index multiplied by Glasgow Coma Scale score (rSIG) is a simple measure with high discriminant ability for mortality risk in trauma patients: An analysis of the Japan Trauma Data Bank. Crit. Care 2018, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, B.; Givon, A.; Rozenfeld, M.; Radomislensky, I.; Peleg, K. Traumatic brain injury: It is all about definition. Brain Inj. 2016, 30, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma 1987, 27, 370–378. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA 1996, 275, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Zarzaur, B.L.; Croce, M.A.; Magnotti, L.J.; Fabian, T.C. Identifying life-threatening shock in the older injured patient: An analysis of the National Trauma Data Bank. J. Trauma 2010, 68, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.C.; Magana, J.N.; Kaczor, K.; Lorenz, D.J.; Meyers, G.; Bennett, B.L.; Kanegaye, J.T. The Prevalence of Bruising among Infants in Pediatric Emergency Departments. Ann. Emerg. Med. 2016, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.; Reines, H.D.; Wulf-Gutierrez, M.E. Clinical review: Hemorrhagic shock. Crit. Care 2004, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Abe, T.; Kohshi, K.; Tokuda, Y.; Cook, E.F.; Kukita, I. Revised trauma scoring system to predict in-hospital mortality in the emergency department: Glasgow Coma Scale, Age, and Systolic Blood Pressure score. Crit. Care 2011, 15, R191. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total | Survival | p-Value | ||
|---|---|---|---|---|---|
| (n = 18,750) | No (n = 502) | Yes (n = 18,248) | |||
| Age (years) | 55 (38, 69) | 65 (48, 77) | 54 (37, 68) | <0.001 | |
| ISS | 9 (4, 11) | 25 (16, 29) | 9 (4, 10) | <0.001 | |
| GCS | 15 (15, 15) | 6 (3, 14) | 15 (15, 15) | <0.001 | |
| SBP (mmHg) | 147 (127, 170) | 153 (121, 184) | 147 (127, 169) | 0.122 | |
| HR (times/min) | 85 (74, 97) | 94 (77, 114) | 84 (74, 96) | <0.001 | |
| RR (times/min) | 18 (18, 20) | 19 (18, 20) | 18 (18, 20) | <0.001 | |
| SI | 0.57 (0.47, 0.69) | 0.62 (0.48, 0.84) | 0.57 (0.47, 0.69) | <0.001 | |
| rSI | 1.75 (1.44, 2.11) | 1.60 (1.19, 2.10) | 1.75 (1.45, 2.11) | <0.001 | |
| rSIG | 25.46 (20.57, 30.98) | 9.39 (5.25, 19.79) | 25.64 (20.85, 31.09) | <0.001 | |
| RTS | 7.84 (7.84, 7.84) | 5.97 (4.09, 7.44) | 7.84 (7.84, 7.84) | <0.001 | |
| TRISS | 0.98 (0.97, 0.99) | 0.70 (0.37, 0.92) | 0.98 (0.97, 0.99) | <0.001 | |
| Sex, n (%) | Female | 8150 (43.5%) | 203 (35.3%) | 7975 (43.7%) | <0.001 |
| Male | 10,600 (56.5%) | 325 (64.7%) | 10,275 (56.3%) | ||
| AIS (Head), n (%) | 0 | 13,407 (71.5%) | 87 (17.3%) | 13,320 (73.0%) | <0.001 |
| 1 | 1006 (5.4%) | 12 (2.4%) | 994 (5.5%) | ||
| 2 | 388 (2.1%) | 6 (1.2%) | 382 (2.1%) | ||
| 3 | 1280 (6.8%) | 27 (5.4%) | 1253 (6.9%) | ||
| 4 | 2129 (11.4%) | 122 (24.3%) | 2007 (11.0%) | ||
| 5 | 525 (2.8%) | 235 (46.8%) | 290 (1.6%) | ||
| 6 | 15 (0.1%) | 13 (2.6%) | 2 (0.01%) | ||
| AIS (Face), n (%) | 0 | 16,003 (85.4%) | 438 (87.3%) | 15,565 (85.3%) | 0.002 |
| 1 | 873 (4.7%) | 10 (2.0%) | 863 (4.7%) | ||
| 2 | 1829 (9.8%) | 50 (10.0%) | 1779 (9.8%) | ||
| 3 | 45 (0.2%) | 4 (0.8%) | 41 (0.2%) | ||
| AIS (Thorax), n (%) | 0 | 16,376 (87.3%) | 375 (74.7%) | 16,001 (87.7%) | <0.001 |
| 1 | 389 (2.1%) | 11 (2.2%) | 378 (2.1%) | ||
| 2 | 592 (3.2%) | 14 (2.89%) | 578 (3.2%) | ||
| 3 | 948 (5.1%) | 51 (10.2%) | 897 (4.9%) | ||
| 4 | 419 (2.2%) | 43 (8.6%) | 376 (2.1%) | ||
| 5 | 25 (0.1%) | 7 (1.4%) | 18 (0.1%) | ||
| 6 | 1 (0.01%) | 1 (0.2%) | 0 (0.0%) | ||
| AIS (Abdomen), n (%) | 0 | 17,483 (93.2%) | 436 (86.9%) | 17,047 (93.4%) | <0.001 |
| 1 | 86 (0.5%) | 2 (0.4%) | 84 (0.5%) | ||
| 2 | 651 (3.5%) | 27 (5.4%) | 624 (3.4%) | ||
| 3 | 366 (2.0%) | 14 (2.8%) | 352 (1.9%) | ||
| 4 | 129 (0.7%) | 19 (3.8%) | 110 (0.6%) | ||
| 5 | 35 (0.2%) | 4 (0.8%) | 31 (0.2%) | ||
| AIS (Extremity), n (%) | 0 | 5040 (26.9%) | 316 (63.0%) | 4724 (25.9%) | <0.001 |
| 1 | 1163 (6.2%) | 9 (1.8%) | 1154 (6.3%) | ||
| 2 | 7139 (38.1%) | 89 (17.7%) | 7050 (38.6%) | ||
| 3 | 5358 (28.6%) | 73 (14.5%) | 5285 (29.0%) | ||
| 4 | 43 (0.2%) | 12 (2.4%) | 31 (0.2%) | ||
| 5 | 7 (0.04%) | 3 (0.6%) | 4 (0.02%) | ||
| AIS (External), n (%) | 0 | 17,027 (90.8%) | 465 (92.6%) | 16,562 (90.8%) | <0.001 |
| 1 | 1613 (8.6%) | 27 (5.4%) | 1586 (8.7%) | ||
| 2 | 85 (0.5%) | 2 (0.4%) | 83 (0.5%) | ||
| 3 | 12 (0.06%) | 0 (0.0%) | 12 (0.1%) | ||
| 4 | 3 (0.02%) | 0 (0.0%) | 3 (0.02%) | ||
| 5 | 5 (0.03%) | 4 (0.8%) | 1 (0.01%) | ||
| 6 | 5 (0.03%) | 4 (0.8%) | 1 (0.01%) | ||
| Variables | Total | Survival | p-Value | ||
|---|---|---|---|---|---|
| (n = 2438) | No (n = 229) | Yes (n = 2209) | |||
| Age (years) | 61 (45, 74) | 68 (54, 78) | 60 (44, 74) | <0.001 | |
| ISS | 16 (9, 16) | 25 (16, 25) | 16 (9, 16) | <0.001 | |
| GCS | 15 (11, 15) | 4 (3, 9) | 15 (13, 15) | <0.001 | |
| SBP (mmHg) | 154 (134, 180) | 158 (133, 197) | 154 (135, 178) | 0.01 | |
| HR (times/min) | 84 (74, 97) | 92 (75, 109) | 84 (74, 96) | <0.001 | |
| RR (times/min) | 18 (18, 20) | 19 (17, 20) | 18 (18, 20) | 0.993 | |
| SI | 0.55 (0.45, 0.66) | 0.56 (0.45, 0.73) | 0.55 (0.45, 0.66) | 0.111 | |
| rSI | 1.83 (1.51, 2.24) | 1.77 (1.37, 2.23) | 1.83 (1.52, 2.24) | 0.111 | |
| rSIG | 24.62 (17.75, 30.79) | 8.76 (5.56, 18.20) | 25.38 (19.22, 31.23) | <0.001 | |
| RTS | 7.84 (6.90, 7.84) | 5.03 (4.09, 6.90) | 7.84 (7.84, 7.84) | <0.001 | |
| TRISS | 0.97 (0.94, 0.99) | 0.68 (0.45, 0.89) | 0.97 (0.94, 0.99) | <0.001 | |
| Sex, n (%) | Female | 906 (37.2%) | 380 (35.4%) | 16,866 (37.4%) | 0.605 |
| Male | 1532 (62.8%) | 148 (64.6%) | 1384 (62.7%) | ||
| AIS (Head), n (%) | 2 | 170 (7.0%) | 2 (0.9%) | 168 (7.6%) | <0.001 |
| 3 | 693 (28.4%) | 15 (6.6%) | 678 (30.7%) | ||
| 4 | 1253 (51.4%) | 68 (29.7%) | 1185 (53.6%) | ||
| 5 | 310 (12.7%) | 134 (58.5%) | 176 (8.0%) | ||
| 6 | 12 (0.5%) | 10 (4.4%) | 2 (0.1%) | ||
| Variables | Total | Survival | p-Value | ||
|---|---|---|---|---|---|
| (n = 16,312) | No (n = 273) | Yes (n = 16,039) | |||
| Age (years) | 54 (37, 68) | 61 (42, 77) | 54 (37, 67( | <0.001 | |
| ISS | 9 (4, 9) | 29 (18, 34) | 8 (4, 9) | <0.001 | |
| GCS | 15 (15, 15) | 7 (3, 15) | 15 (15, 15) | <0.001 | |
| SBP (mmHg) | 146 (126, 168) | 146 (107, 176) | 146 (126, 168) | 0.023 | |
| HR (times/min) | 85 (75, 97) | 96 (78, 117) | 85 (75, 96) | <0.001 | |
| RR (times/min) | 18 (18, 20) | 20 (18, 20) | 18 (18, 20) | <0.001 | |
| SI | 0.58 (0.48, 0.70) | 0.67 (0.51, 0.93) | 0.58 (0.48, 0.69) | <0.001 | |
| rSI | 1.73 (1.43, 2.09) | 1.50 (1.07, 1.98) | 1.73 (1.44, 2.09) | <0.001 | |
| rSIG | 25.56 (20.83, 31.00) | 10.69 (5.07, 20.43) | 25.67 (21.00, 31.09) | <0.001 | |
| RTS | 7.84 (7.84, 7.84) | 5.97 (4.09, 7.84) | 7.84 (7.84, 7.84) | <0.001 | |
| TRISS | 0.98 (0.97, 1.00) | 0.72 (0.36, 0.93) | 0.98 (0.97, 1.00) | <0.001 | |
| Sex, n (%) | Female | 7244 (44.4%) | 351 (35.2%) | 9359 (44.6%) | 0.002 |
| Male | 9068 (55.6%) | 177 (64.8%) | 8891 (55.4%) | ||
| AIS (Head), n (%) | 0 | 13,407 (82.2%) | 87 (31.97%) | 13,320 (83.1%) | <0.001 |
| 1 | 1006 (6.2%) | 12 (4.4%) | 994 (6.2%) | ||
| AIS (Face), n (%) | 0 | 13,721 (84.1%) | 214 (78.4%) | 13,507 (84.2%) | <0.001 |
| 1 | 717 (4.4%) | 5 (1.8%) | 712 (4.4%) | ||
| 2 | 1829 (11.2%) | 50 (18.3%) | 1779 (11.1%) | ||
| 3 | 45 (0.3%) | 4 (1.5%) | 41 (0.3%) | ||
| AIS (Thorax), n (%) | 0 | 13,992 (85.8%) | 152 (55.7%) | 13,840 (86.3%) | <0.001 |
| 1 | 335 (2.1%) | 5 (1.8%) | 330 (2.1%) | ||
| 2 | 592 (3.6%) | 14 (5.1%) | 578 (3.6%) | ||
| 3 | 948 (5.8%) | 51 (18.7%) | 897 (5.6%) | ||
| 4 | 419 (2.6%) | 43 (15.8%) | 376 (2.3%) | ||
| 5 | 25 (0.2%) | 7 (2.6%) | 18 (0.1%) | ||
| 6 | 1 (0.01%) | 1 (0.4%) | 0 (0.0%) | ||
| AIS (Abdomen), n (%) | 0 | 15,055 (92.3%) | 208 (76.2%) | 14,847 (92.6%) | <0.001 |
| 1 | 76 (0.5%) | 1 (0.4%) | 75 (0.5%) | ||
| 2 | 651 (4.0%) | 27 (9.9%) | 624 (3.9%) | ||
| 3 | 366 (2.2%) | 14 (5.1%) | 352 (2.2%) | ||
| 4 | 129 (0.8%) | 19 (7.0%) | 110 (0.7%) | ||
| 5 | 35 (0.2%) | 4 (1.5%) | 31 (0.2%) | ||
| AIS (Extremity), n (%) | 0 | 2743 (16.8%) | 94 (34.4%) | 2649 (16.5%) | <0.001 |
| 1 | 1022 (6.3%) | 2 (0.7%) | 1020 (6.4%) | ||
| 2 | 7139 (43.8%) | 89 (32.6%) | 7050 (44.0%) | ||
| 3 | 5358 (32.9%) | 73 (26.7%) | 5285 (33.0%) | ||
| 4 | 43 (0.3%) | 12 (4.4%) | 31 (0.2%) | ||
| 5 | 7 (0.04%) | 3 (1.1%) | 4 (0.02%) | ||
| AIS (External), n (%) | 0 | 14,818 (90.8%) | 246 (90.1%) | 14,572 (90.95%) | <0.001 |
| 1 | 1384 (8.5%) | 17 (6.2%) | 1367 (8.5%) | ||
| 2 | 85 (0.5%) | 2 (0.7%) | 83 (0.5%) | ||
| 3 | 12 (0.1%) | 0 (0.0%) | 12 (0.1%) | ||
| 4 | 3 (0.02%) | 0 (0.0%) | 3 (0.02%) | ||
| 5 | 5 (0.03%) | 4 (1.5%) | 1 (0.01%) | ||
| 6 | 5 (0.03%) | 4 (1.5%) | 1 (0.01%) | ||
| Variables | Best Cutoff Point | Sensitivity (%) | Specificity (%) | AUC | |
|---|---|---|---|---|---|
| TRISS | All patients | 1 | 88.4 | 83.3 | 0.93 |
| Head injury | 0.9 | 84.2 | 81.7 | 0.89 | |
| No head injury | 0.9 | 93.1 | 75.8 | 0.92 | |
| rSIG | All patients | 14.8 | 92.9 | 65.9 | 0.83 |
| Head injury | 14.8 | 86.8 | 70.7 | 0.82 | |
| No head injury | 14 | 94.5 | 61.5 | 0.83 | |
| RTS | All patients | 7.7 | 90.7 | 75.1 | 0.85 |
| Head | 6.5 | 88.5 | 71.6 | 0.85 | |
| No Head | 7.7 | 92.7 | 69.6 | 0.83 | |
| SI | All patients | 0.8 | 88.3 | 28.5 | 0.57 |
| Head injury | 0.7 | 75.4 | 36.2 | 0.53 | |
| No head injury | 0.8 | 86.9 | 38.1 | 0.63 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Rau, C.-S.; Kuo, S.C.H.; Chien, P.-C.; Hsieh, H.-Y.; Hsieh, C.-H. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. Int. J. Environ. Res. Public Health 2018, 15, 2346. https://doi.org/10.3390/ijerph15112346
Wu S-C, Rau C-S, Kuo SCH, Chien P-C, Hsieh H-Y, Hsieh C-H. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. International Journal of Environmental Research and Public Health. 2018; 15(11):2346. https://doi.org/10.3390/ijerph15112346
Chicago/Turabian StyleWu, Shao-Chun, Cheng-Shyuan Rau, Spencer C. H. Kuo, Peng-Chen Chien, Hsiao-Yun Hsieh, and Ching-Hua Hsieh. 2018. "The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data" International Journal of Environmental Research and Public Health 15, no. 11: 2346. https://doi.org/10.3390/ijerph15112346
APA StyleWu, S.-C., Rau, C.-S., Kuo, S. C. H., Chien, P.-C., Hsieh, H.-Y., & Hsieh, C.-H. (2018). The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. International Journal of Environmental Research and Public Health, 15(11), 2346. https://doi.org/10.3390/ijerph15112346







