Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance
Abstract
:1. Introduction
- Non-proliferative (NPDR)
- Proliferative (PDR) [9]
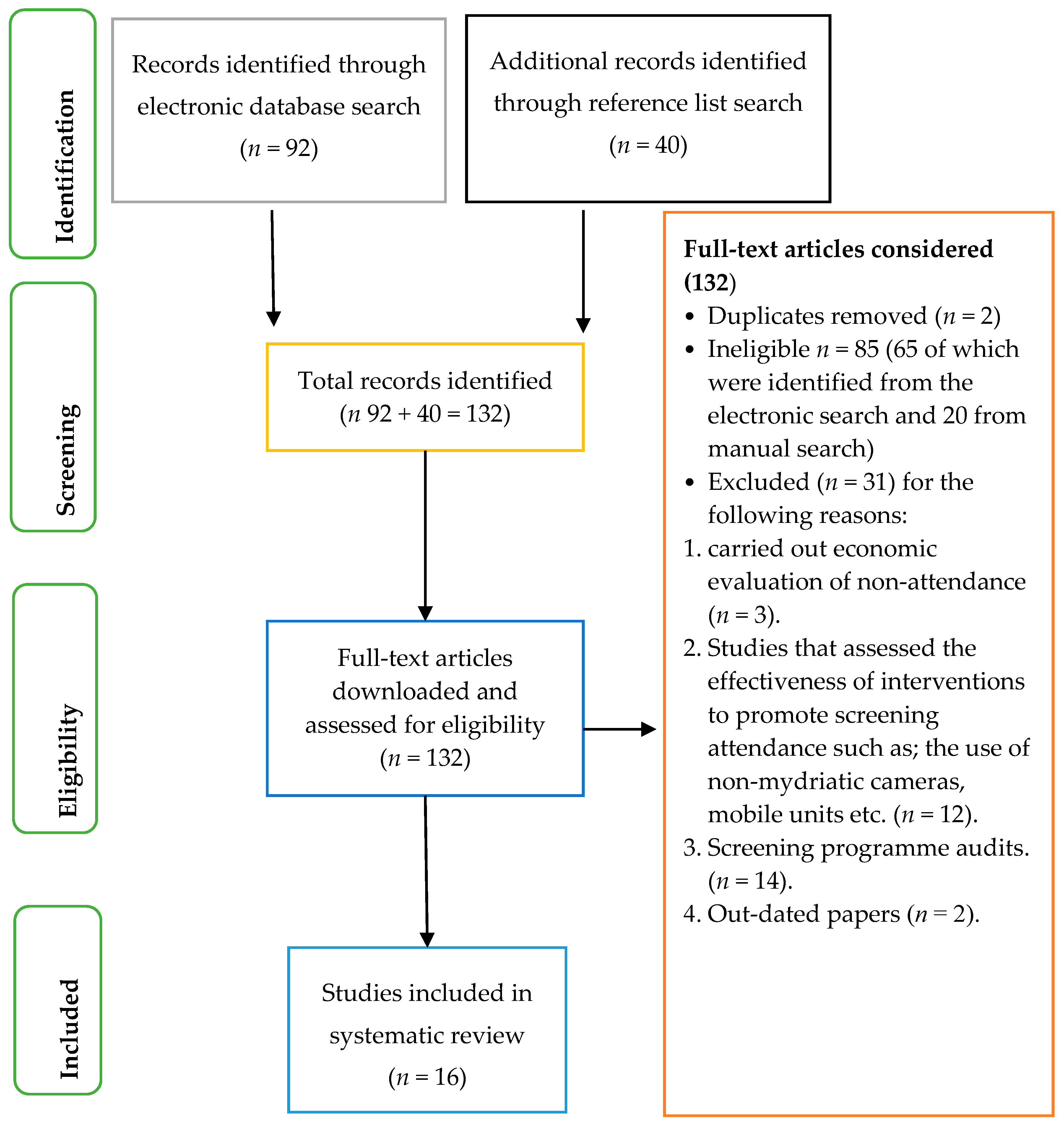
2. Methods
3. Results
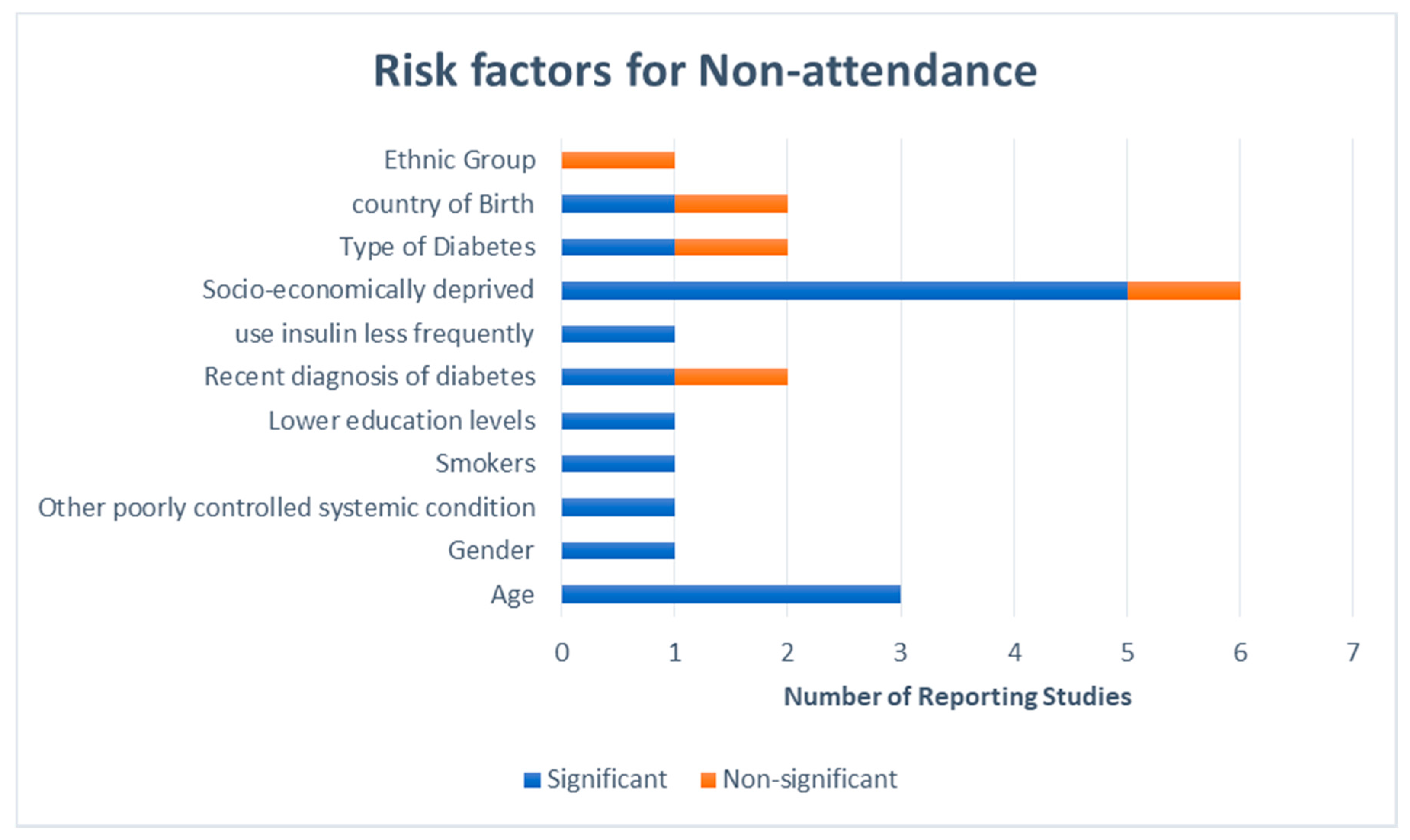
3.1. Demographics of Non-Attenders
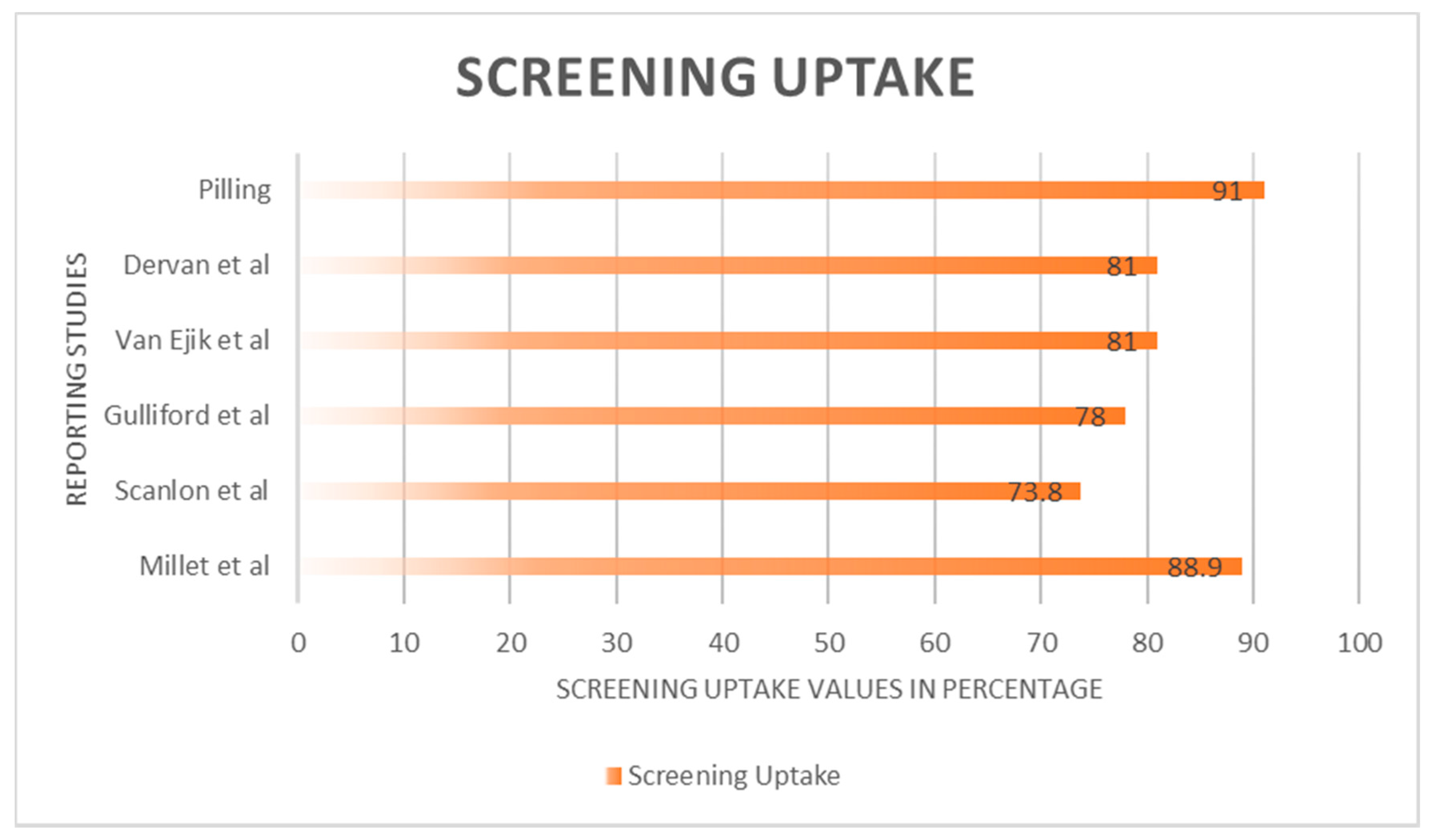
3.2. Screening Invitation, Uptake and Outcome
3.3. Facilitators and Barriers to Screening Compliance
3.4. Patients’ Perceptions and Experiences of Screening
3.5. Factors that Could Contribute to Better Screening Uptake
4. Discussion
Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Index Term | Synonyms | Related Terms |
|---|---|---|
| Patients non-attendance | (1a) Patients no show
(1b) Patients absenteeism (1c) do not attend (1d) non-compliance (1e) non-concordance | (1f) AWOL
(1g) non- appearance (1h) Barriers to access |
| Retinal Screening | (2a) Diabetic eye test
(2b) Diabetic screening (2c) Diabetic Retinopathy | (2d) retinal monitoring
(2e) Retinal photography (2f) Retinal checks (2g) Diabetic maculopathy |
| motives | (3a) Attitudes
(3b) Reason (3c) Rationale (3d) Experiences | (3e) Drive
(3f) Stimulus (3g) Basis |
| Date | Search Terms Used | No. of Papers Excluded | No. of Papers Included |
|---|---|---|---|
| Ebscohost | |||
| 20/3/17 | 1 [OR] 1a and 2 | 10 | 2 |
| 1 [OR] 1c and 2 | 30 | 7 | |
| 22/3/17 | |||
| 1c and 2a | 2 | 0 | |
| 1 and 2b | 3 | 2 | |
| 1 and 2e | 1 | 0 | |
| 1c and 2 and 3d | 2 | 1 | |
| Web of Science | |||
| 24/3/17 | 1 [OR] 1a and 2 | 1 | 2 |
| 3 [OR] 3d and 2 | 95 | 7 | |
| Science Direct | |||
| 27/3/17 | 3d and 2 | 2018 | 6 |
| 1c and 2 | 1908 | 4 | |
| 1 and 2 [OR] 2b | 124 | 0 | |
| 1a [OR] 1c and 2 | 39,708 | 2 | |
| 1a [OR] 1h and 2 | 2686 | 9 | |
| 1a–1h and 2 [OR] 2b | 8 | ||
| Cochrance Library | |||
| 03/4/17 | 3d and 2 | 24 | 0 |
| 3d and 2 | 1 | ||
| Nice Database | |||
| 06/4/17 | NICE and 2d | 215 | 35 |
| Scopus | |||
| 15/06/17 | 1 [OR] 1c and 2 | 5 | 1 |
| 3 [OR] 3d and 2 [OR] 2b and 1 | 7 | 5 | |
| Total: 92 | |||
References
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy for the meta-analysis for eye disease (meta-eye) study group. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenwick, E.; Rees, G.; Pesudovs, K.; Dirani, M.; Kawasaki, R.; Wong, T.Y.; Lamoureux, E. Social and emotional impact of diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2012, 40, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H.; Carter, S.C.; Foy, C.; Husband, R.F.A.; Abbas, J.; Bachmann, M.O. Diabetic retinopathy and socioeconomic deprivation in Gloucestershire. J. Med. Screen. 2008, 15, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanlon, P.H.; Aldington, S.J.; Stratton, I.M. Epidemiological issues in diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2013, 20, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C. Sex differences in micro-and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Ophthalmologist. The Royal College of Ophthalmologists Diabetic Retinopathy Guidelines; The Royal College of Ophthalmologist: London, UK, 2012; Available online: www.rcophth.ac.uk (accessed on 6 April 2017).
- Lowe, J. Screening for diabetic retinopathy item type article publisher nursing in general practice journal nursing in general practice screening for diabetic retinopathy. Nurs. Gen. Pract. 2017, 34–38. Available online: http://hdl.handle.net/10147/558980 (accessed on 27 September 2017).
- NHS, UK. Diabetic Eye Screening. NHS Choices. 2016. Available online: https://www.nhs.uk/conditions/diabetic-eye-screening/ (accessed on 6 November 2017).
- Peto, T.; Tadros, C. Screening for diabetic retinopathy and diabetic macular Edema in the United Kingdom. Curr. Diabetes Rep. 2012, 12, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Diabetic Eye Screening: Commission and Provide—GOV.UK. 2017. Available online: https://www.gov.uk/government/collections/diabetic-eye-screening-commission-and-provide (accessed on 6 November 2017).
- Wales, E.C. Eye Care Wales—Diabetic Eye Screening Wales (DESW). 2017. Available online: http://www.eyecare.wales.nhs.uk/drssw (accessed on 6 November 2017).
- NHS Scotland. Scottish Diabetic Retinopathy Screening Collaborative. 2017. Available online: http://www.ndrs.scot.nhs.uk/Links/index.htm/ (accessed on 6 November 2017).
- Public Health Agency. Diabetic Eye Screening Programme (also Known as Diabetic Retinopathy Screening) | HSC Public Health Agency. 2017. Available online: http://www.publichealth.hscni.net/directorate-public-health/service-development-and-screening/diabetic-eye-screening-programme-also-kn (accessed on 6 November 2017).
- Public Health England. NHS Diabetic Eye Screening Programme: Summary Statistics for England, 1 April 2015 to 31 March 2016; Public Health England: London, UK, 2016. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/605040/Diabetic_eye_screening_summary_infographic_2015_to_2016.pdf (accessed on 16 June 2017).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Screening for Diabetic Retinopathy in Europe 15 Years after the St. Vincent Declaration: The Liverpool Declaration 2005. 2005. Available online: http://reseau-ophdiat.aphp.fr/Document/Doc/confliverpool.pdf (accessed on 6 November 2017).
- Letts, L.; Wilkins, S.; Law, M.; Stewart, D.; Bosch, J.; Westmorland, M. Critical Review form—Qualitative Studies (Version 2.0); 2007. Available online: https://www.unisa.edu.au/Global/Health/Sansom/ Documents/iCAHE/CATs/McMasters_qualreview_version2 0.pdf (accessed on 13 November 2017).
- Lake, A.J.; Browne, J.; Rees, G.; Speight, J. What factors influence uptake of retinal screening among young adults with type 2 diabetes? A qualitative study informed by the theoretical domains framework. J. Diabetes Complicat. 2017, 31, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, A.; Al-Hassan, A.; Chauhan, D.; Al-Futais, M.; Khandekar, R. Knowledge, attitude, and perception of barriers for eye care among diabetic persons registered at employee health department of a tertiary eye hospital of central Saudi Arabia. Middle East Afr. J. Ophthalmol. 2016, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Strutton, R.; Du Chemin, A.; Stratton, I.M.; Forster, A.S. System-level and patient-level explanations for non-attendance at diabetic retinopathy screening in Sutton and Merton (London, UK): A qualitative analysis of a service evaluation: Table 1. BMJ Open 2016, 6, e010952. [Google Scholar] [CrossRef] [PubMed]
- Pilling, R.F. Screening for diabetic retinopathy in adults with learning disability: Current uptake and adjustments to facilitate equality of access. Br. J. Learn. Disabil. 2015, 43, 62–65. [Google Scholar] [CrossRef]
- Hipwell, A.E.; Sturt, J.; Lindenmeyer, A.; Stratton, I.; Gadsby, R.; O’Hare, P.; Scanlon, P.H. Attitudes, access and anguish: A qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open 2014, 4, e005498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenmeyer, A.; Sturt, J.A.; Hipwell, A.; Stratton, I.M.; Al-Athamneh, N.; Gadsby, R.; O’Hare, J.P.; Scanlon, P.H. Influence of primary care practices on patients’ uptake of diabetic retinopathy screening: A qualitative case study. Br. J. Gen. Pract. 2014, 64, e484–e492. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.S.; Forbes, A.; Dodhia, H.; Connor, C.; Du Chemin, A.; Sivaprasad, S.; Mann, S.; Gulliford, M.C. Non-attendance at diabetic eye screening and risk of sight-threatening diabetic retinopathy: A population-based cohort study. Diabetologia 2013, 56, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, K.N.D.; Blom, J.W.; Gussekloo, J.; Polak, B.C.P.; Groeneveld, Y. Diabetic retinopathy screening in patients with diabetes mellitus in primary care: Incentives and barriers to screening attendance. Diabetes Res. Clin. Pract. 2012, 96, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.; Bullen, G.; Chant, S.; Salman, R.; Vaidya, B.; Ling, R. Cost implications, deprivation and geodemographic segmentation analysis of non-attenders (DNA) in an established diabetic retinopathy screening programme. Diabetes Metab. Syndr. Clin. Res. Rev. 2012, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Gulliford, M.C.; Dodhia, H.; Chamley, M.; McCormick, K.; Mohamed, M.; Naithani, S.; Sivaprasad, S. Socio-economic and ethnic inequalities in diabetes retinal screening. Diabet. Med. 2010, 27, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Leese, G.P.; Boyle, P.; Feng, Z.; Emslie-smith, A.; Ellis, J.D. Screening uptake in a well-established diabetic retinopathy screening program: The role of geographical access and deprivation. Diabetes Care 2008, 31, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Dervan, E.; Lillis, D.; Flynn, L.; Staines, A.; O’Shea, D. Factors that influence the patient uptake of diabetic retinopathy screening. Ir. J. Med. Sci. 2008, 177, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Millett, C.; Dodhia, H. Diabetes retinopathy screening: Audit of equity in participation and selected outcomes in South East London. J. Med. Screen. 2006, 13, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Zoega, G.M.; Gunnarsdóttir, Þ.; Björnsdóttir, S.; Hreiðarsson, Á.B.; Viggósson, G.; Stefánsson, E. Screening compliance and visual outcome in diabetes. Acta Ophthalmol. 2005, 83, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Key, I.J.; Loyacano, N.M.; Horswell, R.L.; Desalvo, K.B. Perceived barriers to diabetic eye care. Arch. Ophthalmol. 2005, 123, 387. [Google Scholar] [CrossRef] [PubMed]
| Studies | Participants | N | Methods Used | Aim | Main Outcome |
|---|---|---|---|---|---|
| Lake et al. 2017 (UK) [21] | Young adults with T2D | 30 | Structured interviews | Explore factors associated with non-attendance | Some factors such as social influences, etc. are peculiar to younger adults with T2D. |
| Al-Alawi et al. 2016 (Saudi Arabia) [22] | Hospital staff with diabetes | 45 | close ended questionnaire | Evaluate knowledge, attitudes and barriers to screening | Most of the participants had excellent knowledge about diabetic eye complications, absence of gender specific-professionals was a cause for non-attendance. |
| Strutton et al. 2016 (UK)[23] | Screening DNAs (≥18 months) | 146 | Telephone Interview | Identify patient and system level reasons for non-attendance | Patients reasons included, having other commitments, anxiety etc. and system level reasons were mostly about miscommunication. |
| Piling et al. 2015 (UK) [24] | Diabetics with learning disability | 71 | Retrospective analysis | Find out if there is equality of access for patients with learning disability | National standards are not met for diabetic patients with learning disabilities |
| Hipwell et al. 2014 (UK) [25] | Staff and diabetic patients | 62 | Semi-structured interviews | Examine experiences with screening and how that affects uptake | Knowledge of DR was a major antecedent to screening, and psychological, pragmatic and social factors were antecedents to non-attendance. |
| Lindenmeyer et al. 2014 (UK) [26] | GPs | 9 GP practices | Semi-structured interviews | Identify factors that contribute to screening uptake | Some factors are modifiable such as improving communication, others were not such as diversity of ethnicity and languages. |
| Forster et al. 2013 (UK) [27] | Patients first screened in 2008 | 6556 | Retrospective analysis | Evaluate whether repeated non-attendance was linked to STDR | Repeated non-attendance increases risk of STDR. |
| Van Eijk et al. 2012 (The Netherlands) [28] | Diabetic patients ≥ 18 years | 2363 | Focus groups & Questionnaires | Examine barriers and incentives to screening | 81% attendance; non-attenders had lower education levels, shorter duration of diabetes and were less likely to use insulin or be checked by and internist. |
| Waqar et al. 2012 (UK) [29] | Screening DNAs (04/09–03/10) | 22,651 | Retrospective analysis | Evaluate relationships between socio-economic status and non-attendance | Increasing non-attendance with deprivation, lowest DNAs seen in successful professionals and highest DNAs seen in areas of social housing |
| Gulliford et al. 2010 (UK) [30] | Diabetic patients on a screening database | 59,495 | Retrospective analysis | Quantify socio-economic and ethnic in-equalities in screening | Only a weak association between non-attendance and deprivation, and smaller than previously reported inequality in screening. |
| Leese et al. 2008 (UK) [31] | Diabetic patients on a screening database | 15,150 | Retrospective analysis | Identify characteristics that determine attendance status | Socio-economic deprivation increases non-attendance |
| Dervan et al. 2008 (Ireland) [32] | Patients at diabetic centres | 209 | Questionnaires | Assess if patients are receiving regular screening and factors that influence uptake. | 81% of patients had screening done. Recommendation from a physician was a major factor in increasing uptake. |
| Scanlon et al. 2008 (UK) [4] | Diabetic patients on a screening database | 10,312 | Retrospective analysis | Investigate socio-economic differences in prevalence of diabetes, DR and screening uptake | Probability of being screened decreased with deprivation, prevalence of diabetes and developing STDR was associated with increasing deprivation |
| Millet et al. 2006 (UK) [33] | Diabetic patients on a screening database | 9750 | Retrospective analysis | Assess screening and screening outcome in South East London | 88.9% screening uptake, however, significant inequity in the delivery of the screening programme. |
| Zoega et al. 2005 (Iceland) [34] | Patients on the Icelandic blind registry | 22 | Retrospective analysis | Find relationship between screening compliance and visual outcome | Blind patients had worse pre-diagnosis screening compliance |
| Hartnett et al. 2005 (USA) [35] | Diabetic patients and physicians at an indigent clinic | 2145 records, 17 focus group participants | Focus group discussions and interviews | Address inadequate screening and explore perceived barriers. | Physicians and patients have different perceived barriers to screening, though they both agreed that accessing the care was also a barrier |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashim, R.M.; Newton, P.; Ojo, O. Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance. Int. J. Environ. Res. Public Health 2018, 15, 157. https://doi.org/10.3390/ijerph15010157
Kashim RM, Newton P, Ojo O. Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance. International Journal of Environmental Research and Public Health. 2018; 15(1):157. https://doi.org/10.3390/ijerph15010157
Chicago/Turabian StyleKashim, Rahima Muhammad, Paul Newton, and Omorogieva Ojo. 2018. "Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance" International Journal of Environmental Research and Public Health 15, no. 1: 157. https://doi.org/10.3390/ijerph15010157
APA StyleKashim, R. M., Newton, P., & Ojo, O. (2018). Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance. International Journal of Environmental Research and Public Health, 15(1), 157. https://doi.org/10.3390/ijerph15010157






