Epidemiological Features and Forecast Model Analysis for the Morbidity of Influenza in Ningbo, China, 2006–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Definition and Study Population
2.2. Specimen and Data Collection
2.3. Laboratory Testing
2.4. Ethics Statement
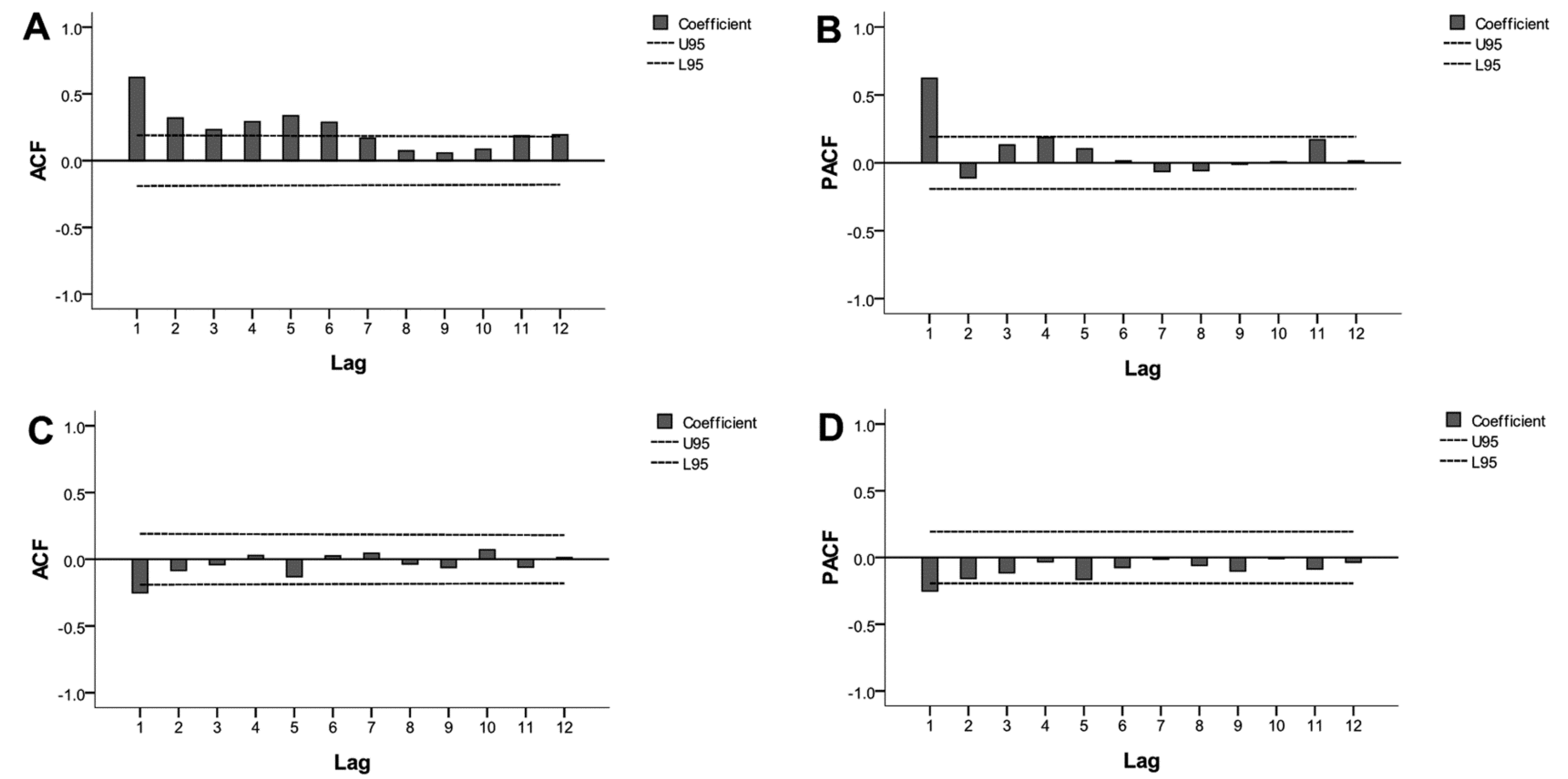
2.5. ARIMA Model
2.6. Statistical Analysis
3. Results
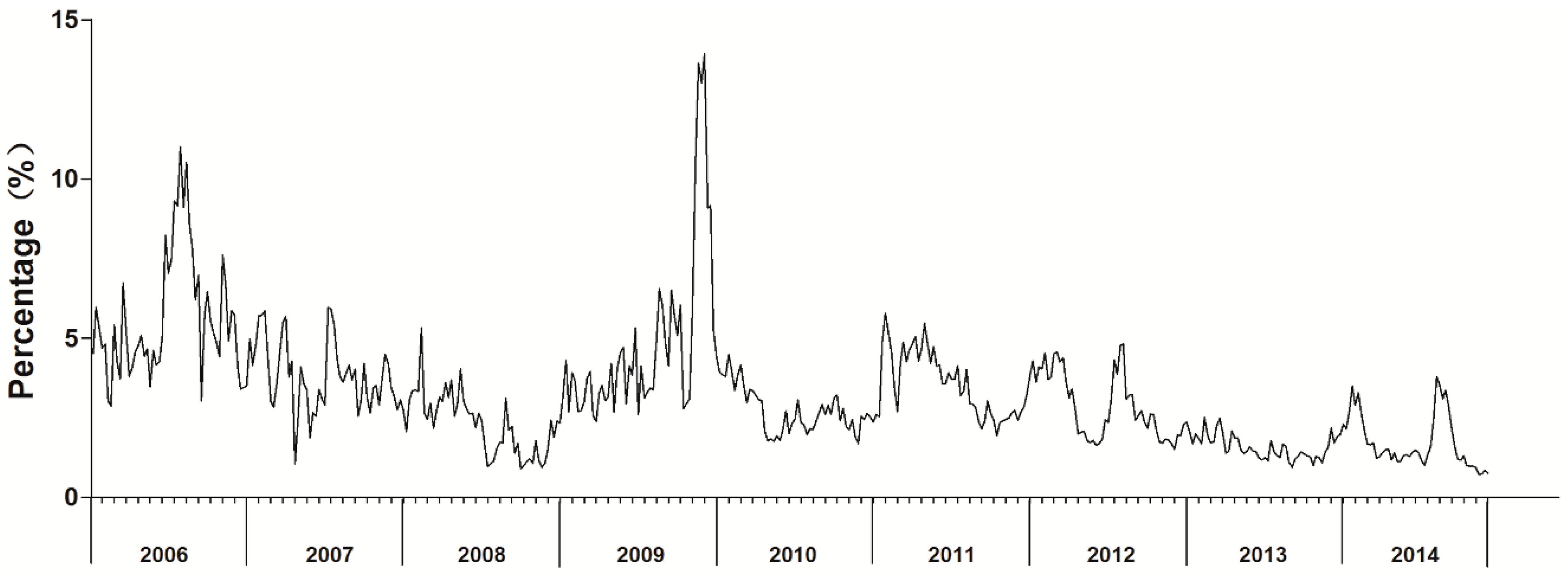
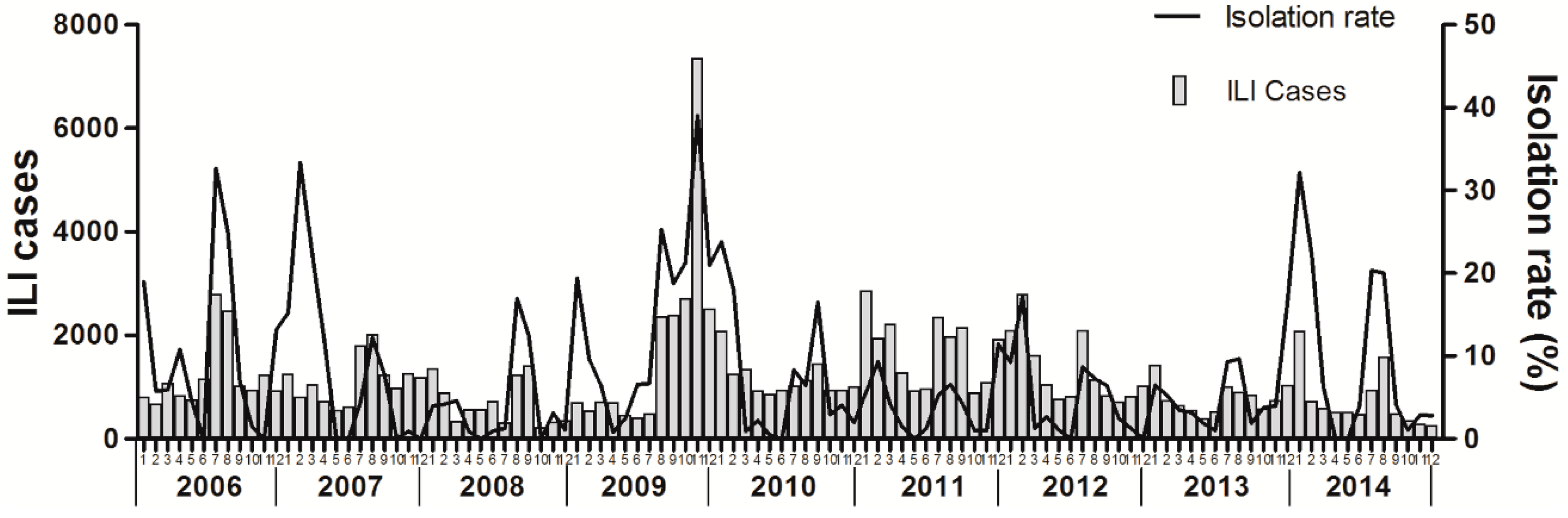
3.1. Influenza Surveillance from 2006 to 2014
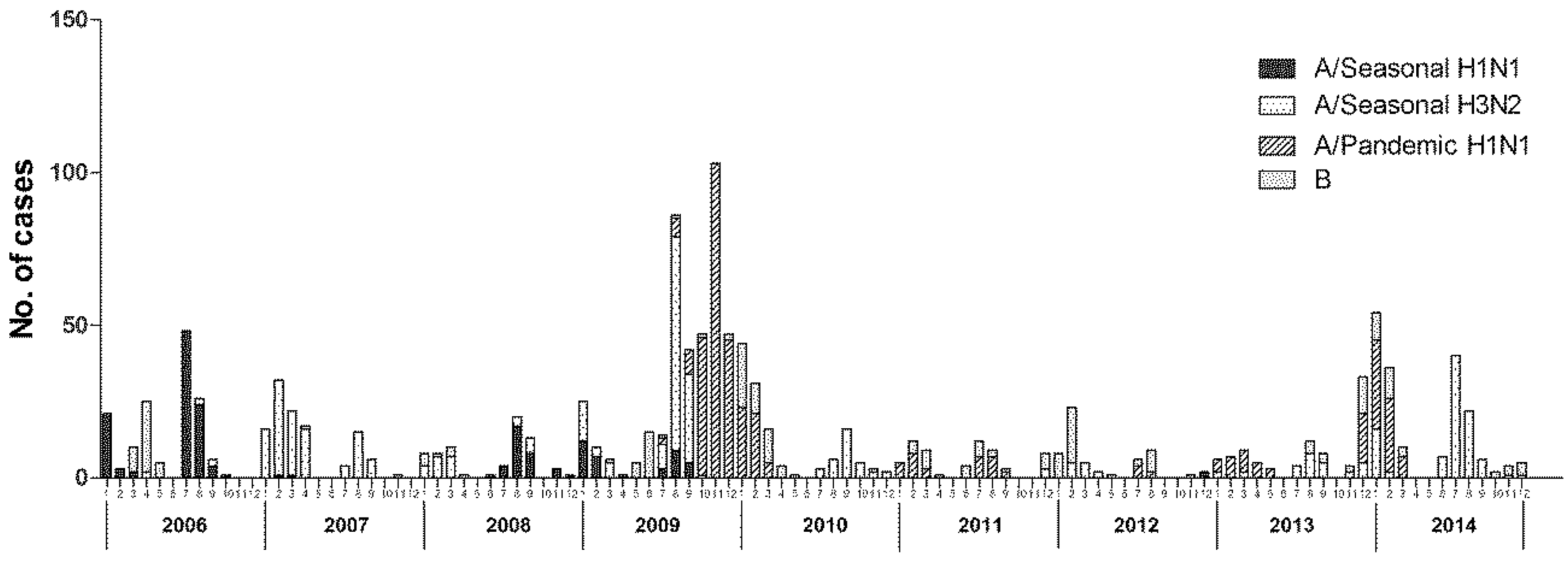
3.2. Viral Etiology of Patients with Influenza-Like Illness
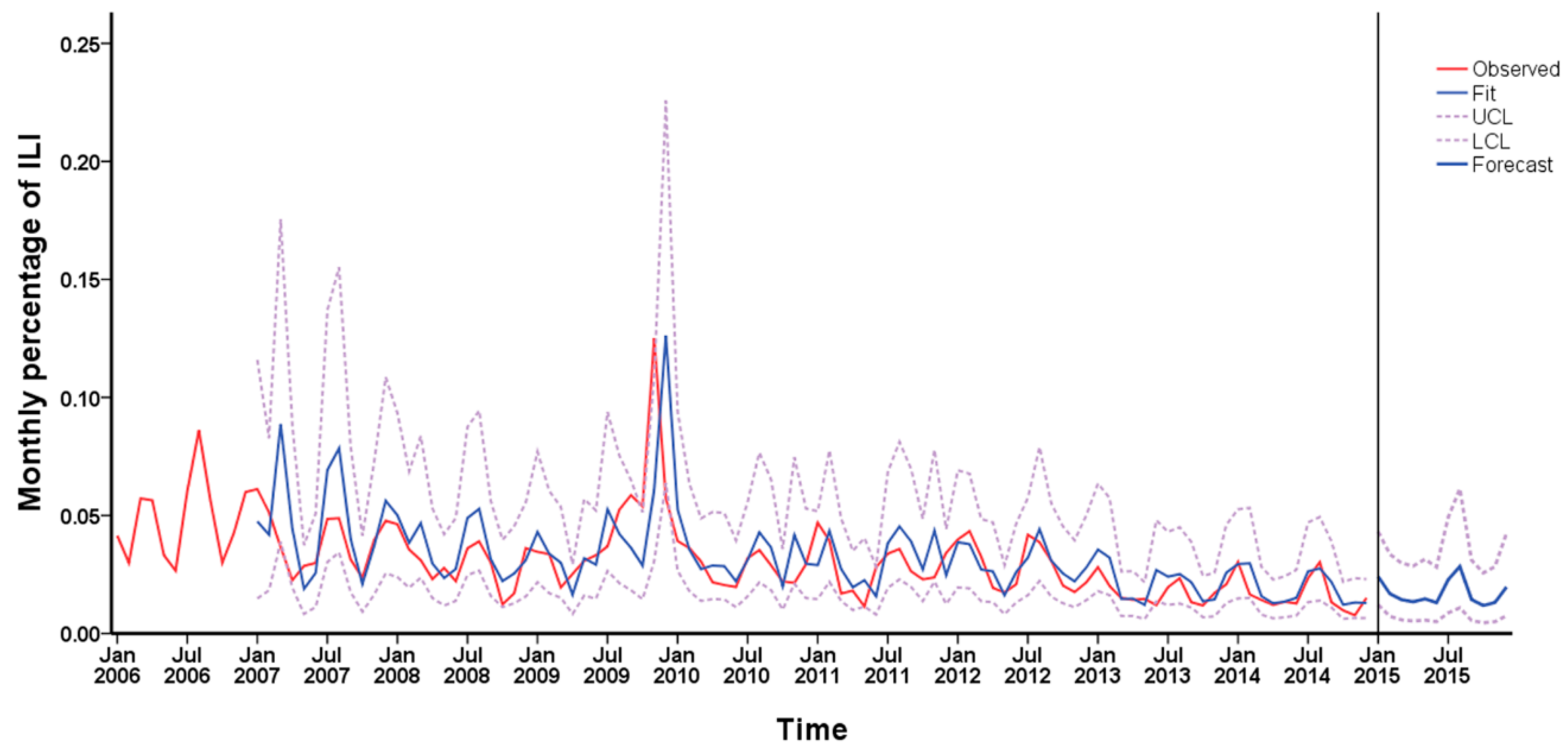
3.3. Time Series Analysis of Monitoring Data
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russell, C.A.; Jones, T.C.; Barr, I.G.; Cox, N.J.; Garten, R.J.; Gregory, V.; Gust, I.D.; Hampson, A.W.; Hay, A.J.; Hurt, A.C.; et al. The global circulation of seasonal influenza A(H3N2) viruses. Science 2008, 320, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhong, H.; He, J.; Rutherford, S.; Yang, F. Using Google Trends for influenza surveillance in South China. PLoS ONE 2013, 8, e55205. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Torres, V.A.; Gómez, J.; Ocaña, V.; Aguilar, P.; Saldarriaga, T.; Chavez, E.; Perez, J.; Zamalloa, H.; Forshey, B.; Paz, I.; et al. Influenza-like illness sentinel surveillance in Peru. PLoS ONE 2009, 4, e6118. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.E.; Hjertqvist, M.; Lundkvist, A.; Hornfeldt, B. Predicting high risk for human hantavirus infections, Sweden. Emerg. Infect. Dis. 2009, 15, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Hattaf, K.; Yousfi, N. Mathematical Model of the Influenza A (H1N1) Infection. Adv. Stud. Biol. 2009, 8, 383–390. [Google Scholar]
- Zhang, L.P.; Zheng, Y.L.; Wang, W.; Zhang, X.L.; Zheng, Y.J. An optimized Nash nonlinear grey Bernoulli model based on particle swarm optimization and its application in prediction for the incidence of Hepatitis B in Xinjiang, China. Comput. Biol. Med. 2014, 49, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wang, P.X.; Zhang, S.Y.; Zhu, D.H. Drought forecasting based on the remote sensing data using ARIMA models. Math. Comput. Model. 2010, 51, 1398–1403. [Google Scholar] [CrossRef]
- Yurekli, K.; Kurunc, A.; Ozturk, F. Application of linear stochastic models to monthly flow data of Kelkit Stream. Ecol. Model. 2005, 183, 67–75. [Google Scholar] [CrossRef]
- Hu, W.; Tong, S.; Mengersen, K.; Connell, D. Weather variability and the incidence of cryptosporidiosis: Comparison of time series Poisson regression and SARIMA models. Ann. Epidemiol. 2007, 17, 679–688. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Infection with Pandemic (H1N1) 2009 Virus: Updated Interim WHO Guidance on Global Surveillance; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Ho, S.L.; Xie, M.; Goh, T.N. A comparative study of neural network and Box-Jenkins ARIMA modeling in time series prediction. Comput. Ind. Eng. 2002, 42, 371–375. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, L.; Wang, Y.; Lu, Z.; Tian, L.; Tan, L.; Shi, Y.; Nie, S.; Liu, L. A hybrid model for predicting the prevalence of schistosomiasis in humans of Qianjiang City, China. PLoS ONE 2014, 9, e104875. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Duan, W.; Lv, M.; Shi, W.; Peng, X.; Wang, X.; Lu, Y.; Liang, H.; Seale, H.; Pang, X.; et al. Review of an influenza surveillance system, Beijing, People’s Republic of China. Emerg. Infect. Dis. 2009, 15, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Scuffham, P.A. Estimating influenza-related hospital admissions in older people from GP consultation data. Vaccine 2004, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.C.; Su, M.C.; Chen, C.H.; Huang, S.H.; Chen, W.L.; Tien, N.; Lin, C.W. Epidemiology of pandemic influenza A/H1N1 virus during 2009–2010 in Taiwan. Virus Res. 2013, 177, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, L.Q.; Zhang, Y.; Qian, Q.; Li, Y.P.; Xu, C.L.; Zhang, Y.; Yan, L.; Yang, H.; Bai, Y.C.; et al. Spatiotemporal characteristics of seasonal influenza in mainland China. Chin. J. Epidemiol. 2009, 30, 1097–1101. (In Chinese) [Google Scholar]
- Niang, M.N.; Dosseh, A.; Ndiaye, K.; Sagna, M.; Gregory, V.; Goudiaby, D.; Hay, A.; Diop, O.M. Sentinel surveillance for influenza in Senegal, 1996–2009. J. Infect. Dis. 2012, 206, S129–S135. [Google Scholar] [CrossRef] [PubMed]
- Kamigaki, T.; Chaw, L.; Tan, A.G.; Tamaki, R.; Alday, P.P.; Javier, J.B.; Olveda, R.M.; Oshitani, H.; Tallo, V.L. Seasonality of Influenza and Respiratory Syncytial Viruses and the Effect of Climate Factors in Subtropical-Tropical Asia Using Influenza-Like Illness Surveillance Data, 2010–2012. PLoS ONE 2016, 11, e0167712. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.J.; Xu, G.Z.; Dong, H.J.; Liu, J.Y.; Chen, K.J.; Fang, T. Analysis on influenza viruses circulating and dominant strain switchover in Ningbo during 2005–2006. Chin. J. Health Lab. Technol. 2007, 17, 428–430. (In Chinese) [Google Scholar]
- Bonney, J.H.; Kronmann, K.C.; Lindan, C.P.; Asante, I.A.; Parbie, P.; Aboagye, J.; Amankwah, J.; Odoom, J.K.; Adjabeng, M.; Nzussouo, N.T.; et al. Virological surveillance of influenza-like illness among children in Ghana, 2008–2010. J. Infect. Dis. 2012, 206, S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guo, J.; Cui, S.J.; Lv, Y.N.; Gao, X.Y.; Li, W.H.; Yan, H.Q.; Qu, M.; Shi, W.X.; Lu, G.L.; et al. Etiological characteristics of influenza A (H1N1) 2009 virus in Beijing. Chin. J. Epidemiol. 2010, 31, 494–496. (In Chinese) [Google Scholar]
- Badar, N.; Bashir Aamir, U.; Mehmood, M.R.; Nisar, N.; Alam, M.M.; Kazi, B.M.; Zaidi, S.S. Influenza virus surveillance in Pakistan during 2008–2011. PLoS ONE 2013, 8, e79959. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Ortega, O.; Kuan, G.; Reingold, A.; Saborio, S.; Balmaseda, A.; Harris, E. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005–2007. Emerg. Infect. Dis. 2009, 15, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.R.; Heffernan, R.T.; Paladini, M.; Konty, K.; Weiss, D.; Mostashari, F. Monitoring the impact of influenza by age: Emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007, 4, e247. [Google Scholar] [CrossRef] [PubMed]
- Burmaa, A.; Kamigaki, T.; Darmaa, B.; Nymadawa, P.; Oshitani, H. Epidemiology and impact of influenza in Mongolia, 2007–2012. Influenza Other Respir. Viruses 2014, 8, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, A.; Dasgupta, S.; Chao, E.; Lemmings, J.; Goodin, K.; Harris, M.; Martin, K.; Feist, M.; Wu, W.; Boulton, R.; et al. Estimating influenza incidence and rates of influenza-like illness in the outpatient setting. Influenza Other Respir. Viruses 2013, 7, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Portuguese Laboratory Network for the Diagnosis of Influenza Infection. Contribution of the Portuguese Laboratory Network for the Diagnosis of Influenza A(H1N1)pdm09 infection during the 2009/10 and 2010/11 influenza seasons. Euro Surveill. 2012, 17, 18–27. [Google Scholar]
- Prachayangprecha, S.; Makkoch, J.; Suwannakarn, K.; Vichaiwattana, P.; Korkong, S.; Theamboonlers, A.; Poovorawan, Y. Epidemiology of seasonal influenza in Bangkok between 2009 and 2012. J. Infect. Dev. Ctries. 2013, 7, 734–740. [Google Scholar] [CrossRef] [PubMed]
| Year | 0~ | 5~ | 15~ | 25~ | ≥60 | Total |
|---|---|---|---|---|---|---|
| No. of ILI | No. of ILI | No. of ILI | No. of ILI | No. of ILI | ||
| 2006 | 7835 (52.76%) a | 3430 (23.10%) | 957 (6.44%) | 2388 (16.08%) | 240 (1.62%) | 14,850 |
| 2007 | 7181 (52.72%) | 3559 (26.13%) | 828 (6.08%) | 1771 (13.00%) | 282 (2.07%) | 13,621 |
| 2008 | 4744 (53.57%) | 1871 (21.13%) | 854 (9.64%) | 1175 (13.27%) | 212 (2.39%) | 8856 |
| 2009 | 9081 (40.39%) | 7005 (31.16%) | 2915 (12.97%) | 3133 (13.94%) | 347 (1.54%) | 22,481 |
| 2010 | 6957 (49.51%) | 2776 (19.75%) | 1711 (12.18%) | 2112 (15.03%) | 497 (3.54%) | 14,053 |
| 2011 | 9991 (48.28%) | 4321 (20.88%) | 2960 (14.30%) | 3041 (14.70%) | 380 (1.84%) | 20,693 |
| 2012 | 8027 (47.69%) | 2499 (14.85%) | 2870 (17.05%) | 3078 (18.29%) | 359 (2.13%) | 16,833 |
| 2013 | 3708 (40.52%) | 1102 (12.04%) | 1890 (20.66%) | 2194 (23.98%) | 256 (2.80%) | 9150 |
| 2014 | 2989 (33.25%) | 940 (10.46%) | 1911 (21.26%) | 2713 (30.18%) | 437 (4.86%) | 8990 |
| Total | 60,513 (46.72%) | 27,503 (21.23%) | 16,896 (13.04%) | 21,605 (16.68%) | 3010 (2.32%) | 129,528 |
| Year | No. of Samples | No. of Positive | Positive Rate (%) | Influenza A Virus | Influenza B Virus | ||
|---|---|---|---|---|---|---|---|
| H1N1 | H3N2 | pdm H1N1 | |||||
| 2006 | 1290 | 145 | 11.24 | 103 (71.03%) | 6 (4.14%) | 0 (0%) | 36 (24.83%) |
| 2007 | 1223 | 113 | 9.24 | 2 (1.77%) | 109 (96.46%) | 0 (0%) | 2 (1.77%) |
| 2008 | 1230 | 56 | 4.56 | 31 (55.36%) | 19 (33.93%) | 0 (0%) | 6 (10.71%) |
| 2009 | 2359 | 401 | 17.00 | 37 (9.23%) | 129 (32.17%) | 209 (52.12%) | 26 (6.48%) |
| 2010 | 1821 | 128 | 7.03 | 0 (0%) | 31 (24.22%) | 49 (38.28%) | 48 (37.50%) |
| 2011 | 864 | 26 | 3.01 | 0 (0%) | 3 (11.54%) | 11 (42.31%) | 12 (46.15%) |
| 2012 | 866 | 24 | 2.77 | 0 (0%) | 6 (25.00%) | 0 (0%) | 18 (75.00%) |
| 2013 | 1658 | 69 | 4.16 | 0 (0%) | 18 (26.09%) | 33 (47.83%) | 18 (26.09%) |
| 2014 | 1983 | 186 | 9.38 | 0 (0%) | 95 (51.08%) | 60 (32.26%) | 31 (16.67%) |
| Total | 13,294 | 1148 | 8.64 | 173 (15.07%) | 416 (36.24%) | 362 (31.53%) | 197 (17.16%) |
| Parameter | ARIMA (1, 1, 0) (1, 1, 0)12 | ARIMA (1, 1, 1) (1, 1, 0)12 | ARIMA (0, 1, 0) (1, 1, 0)12 | ARIMA (0, 1, 1) (1, 1, 0)12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | t | P | SE | t | P | SE | t | P | SE | t | P | |
| Constant | 0.002 | −0.191 | 0.849 | 0.000 | −0.491 | 0.625 | 0.002 | −0.155 | 0.877 | 0.002 | −0.224 | 0.823 |
| AR1 | 0.103 | −1.386 | 0.169 | 0.104 | 5.984 | 0.000 | - | - | - | - | - | - |
| MA1 | - | - | - | 1.835 | 0.544 | 0.588 | - | - | - | 0.098 | 3.347 | 0.001 |
| SAR1 | 0.084 | −6.590 | 0.000 | 0.086 | −6.363 | 0.000 | 0.084 | −6.520 | 0.000 | 0.084 | −6.623 | 0.000 |
| Statistic | RMSE | MAE | MAPE | BIC |
|---|---|---|---|---|
| ARIMA (1, 1, 0) (1, 1, 0)12 | 0.016 | 0.009 | 31.663 | −8.181 |
| ARIMA (1, 1, 1) (1, 1, 0)12 | 0.014 | 0.009 | 28.785 | −8.311 |
| ARIMA (0, 1, 0) (1, 1, 0)12 | 0.016 | 0.009 | 31.701 | −8.197 |
| ARIMA (0, 1, 1) (1, 1, 0)12 | 0.015 | 0.009 | 31.984 | −8.230 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, Y.; Feng, W.; Liu, K.; Zhang, S.; Hu, F.; Jiao, S.; Lao, X.; Ni, H.; Xu, G. Epidemiological Features and Forecast Model Analysis for the Morbidity of Influenza in Ningbo, China, 2006–2014. Int. J. Environ. Res. Public Health 2017, 14, 559. https://doi.org/10.3390/ijerph14060559
Wang C, Li Y, Feng W, Liu K, Zhang S, Hu F, Jiao S, Lao X, Ni H, Xu G. Epidemiological Features and Forecast Model Analysis for the Morbidity of Influenza in Ningbo, China, 2006–2014. International Journal of Environmental Research and Public Health. 2017; 14(6):559. https://doi.org/10.3390/ijerph14060559
Chicago/Turabian StyleWang, Chunli, Yongdong Li, Wei Feng, Kui Liu, Shu Zhang, Fengjiao Hu, Suli Jiao, Xuying Lao, Hongxia Ni, and Guozhang Xu. 2017. "Epidemiological Features and Forecast Model Analysis for the Morbidity of Influenza in Ningbo, China, 2006–2014" International Journal of Environmental Research and Public Health 14, no. 6: 559. https://doi.org/10.3390/ijerph14060559
APA StyleWang, C., Li, Y., Feng, W., Liu, K., Zhang, S., Hu, F., Jiao, S., Lao, X., Ni, H., & Xu, G. (2017). Epidemiological Features and Forecast Model Analysis for the Morbidity of Influenza in Ningbo, China, 2006–2014. International Journal of Environmental Research and Public Health, 14(6), 559. https://doi.org/10.3390/ijerph14060559






