Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Sources and Culture Media
2.2. Microorganisms
2.3. Enrichment and Selection of PAH-Degrading Bacteria
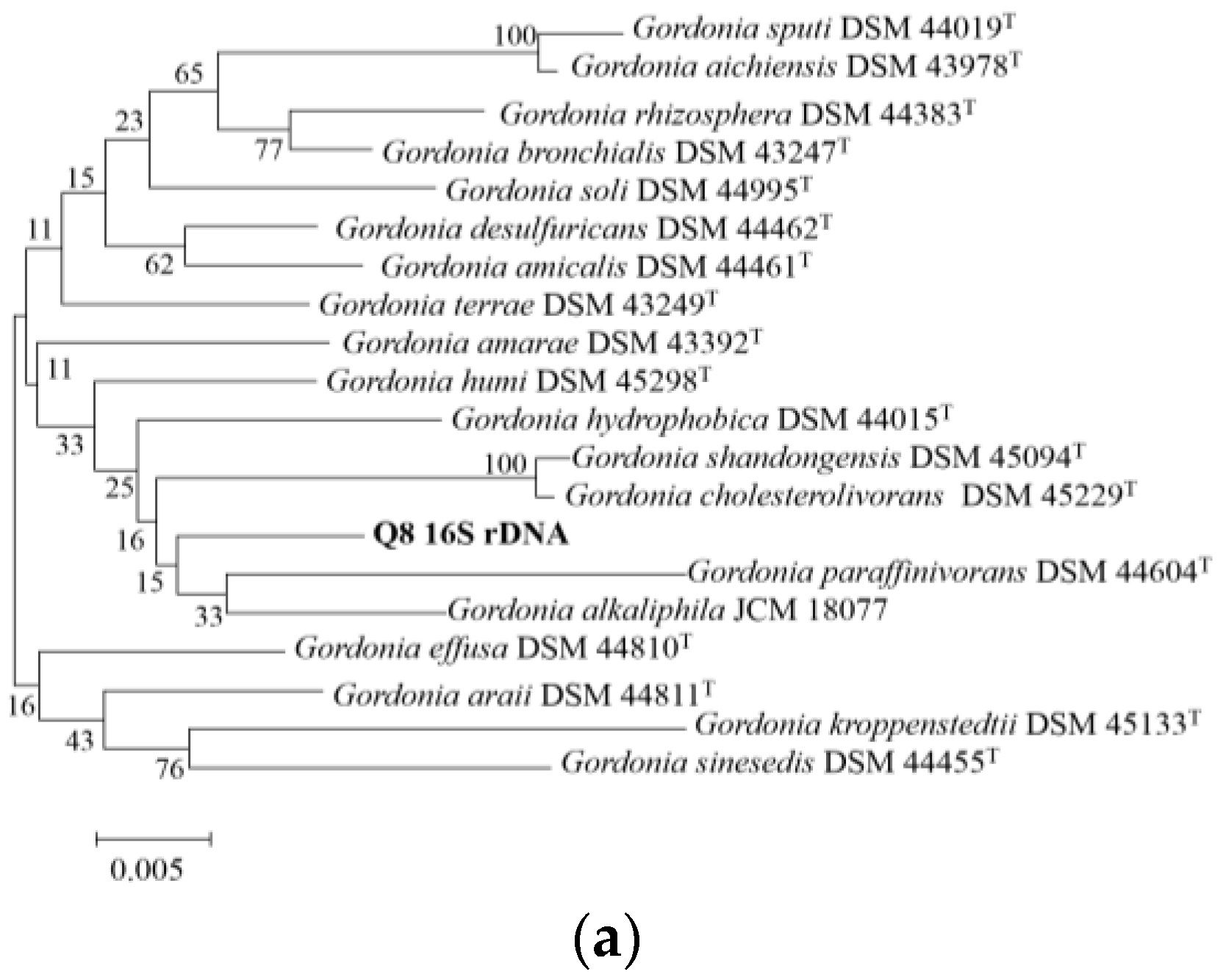
2.4. Analysis of 16S rDNA Sequence
2.5. Determination of GC Content and DNA–DNA Hybridization
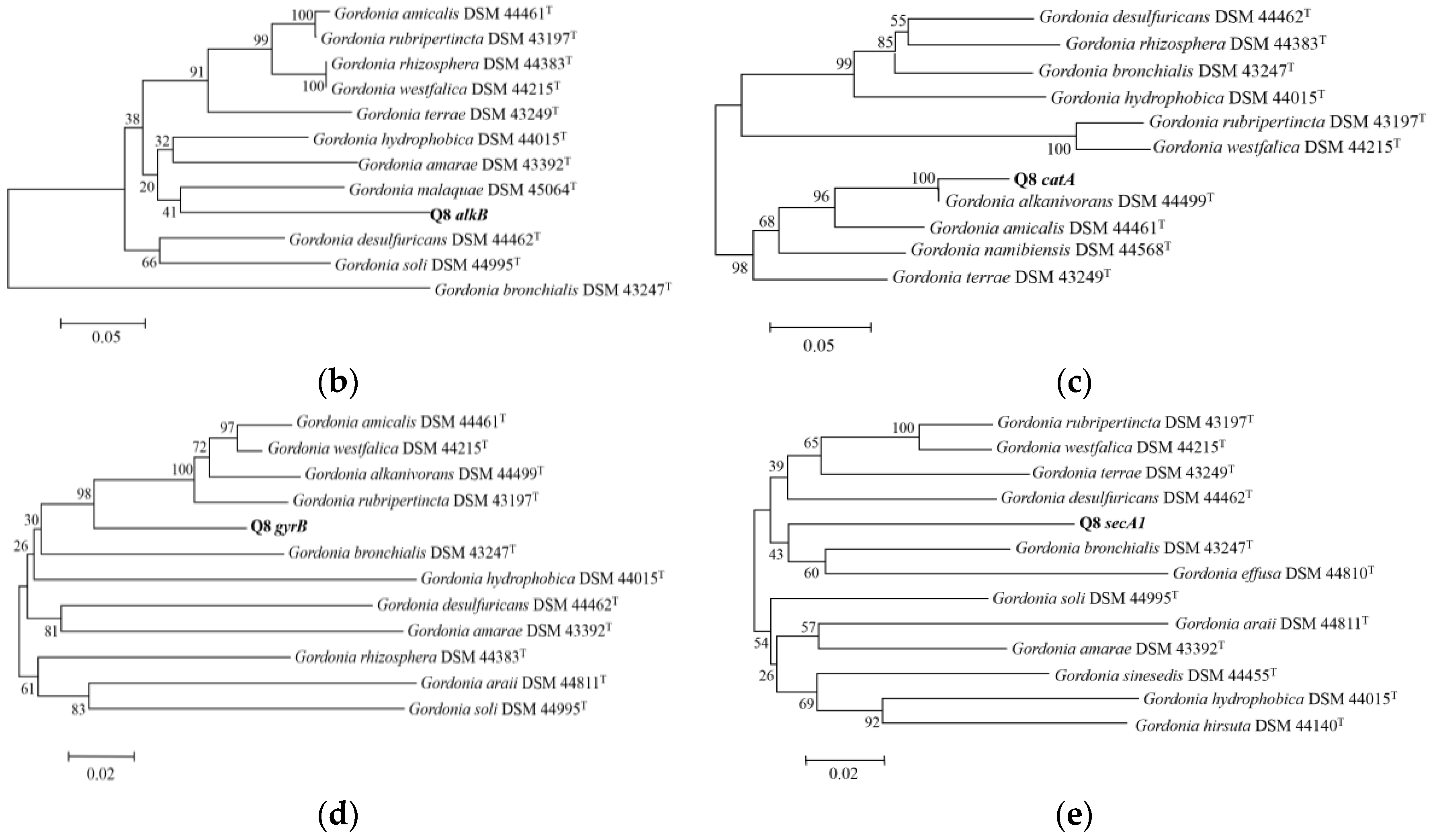
2.6. Amplification and Sequencing of Housekeeping Genes alkB, catA, gyrB and secA1
2.7. NCBI Accession Number
2.8. Biodegradation of PAHs by Strain
2.9. Measurement of PAH Degradation in Petroleum
3. Results
3.1. Description and Identification of Strain Q8
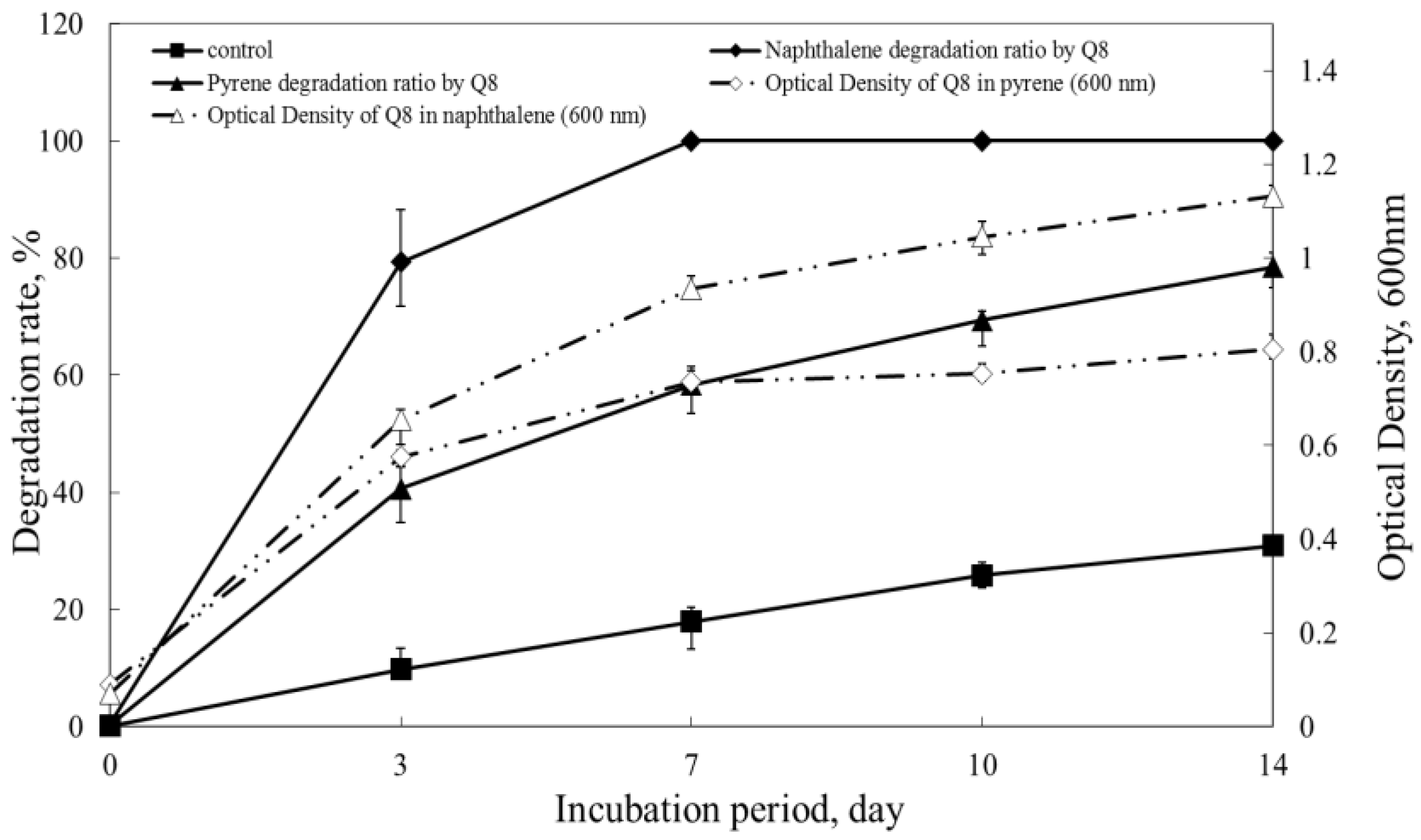
3.2. Growth with a Single PAH as the Sole Source of Carbon and Energy
3.3. Biodegradation of PAHs
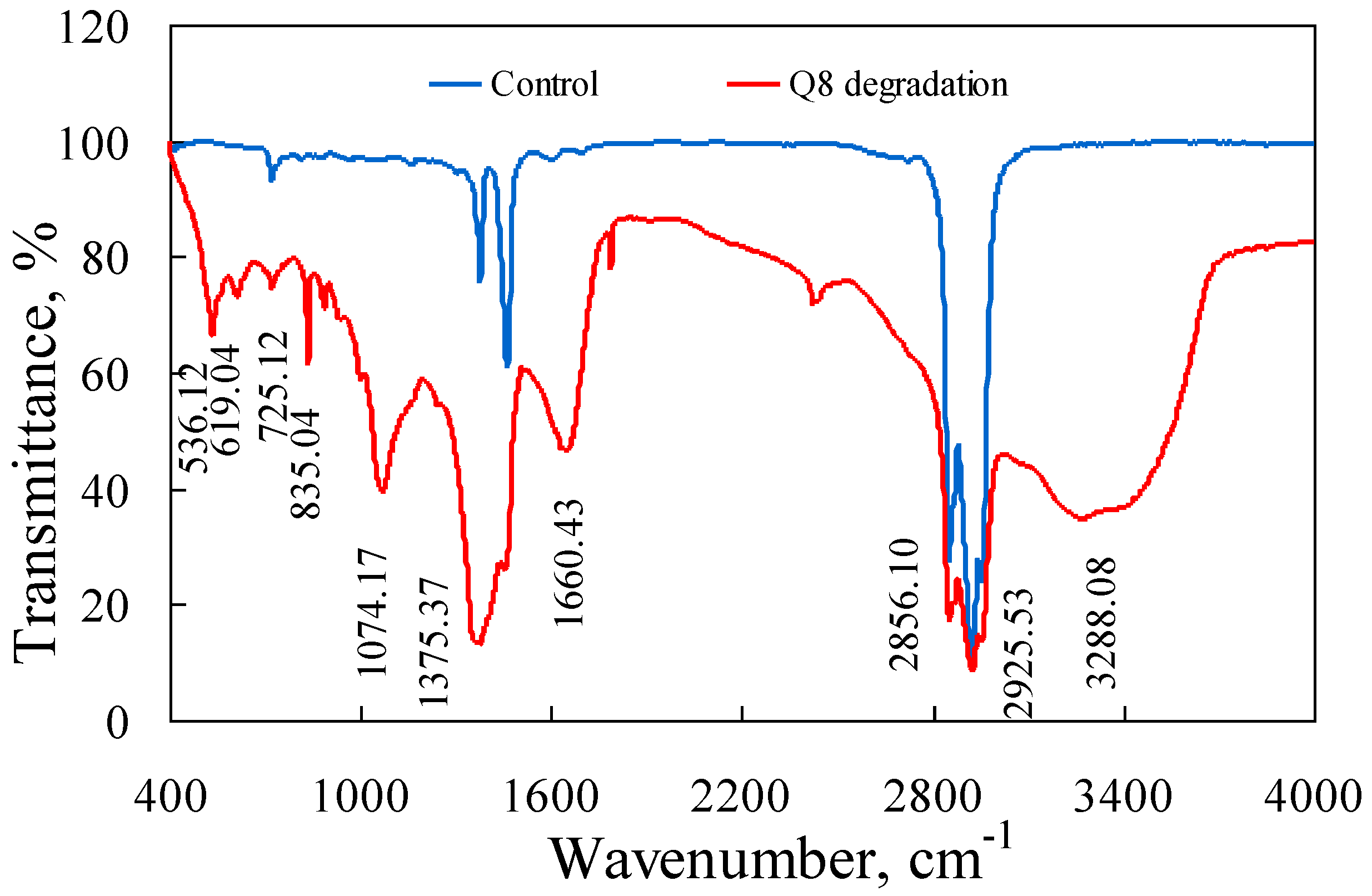
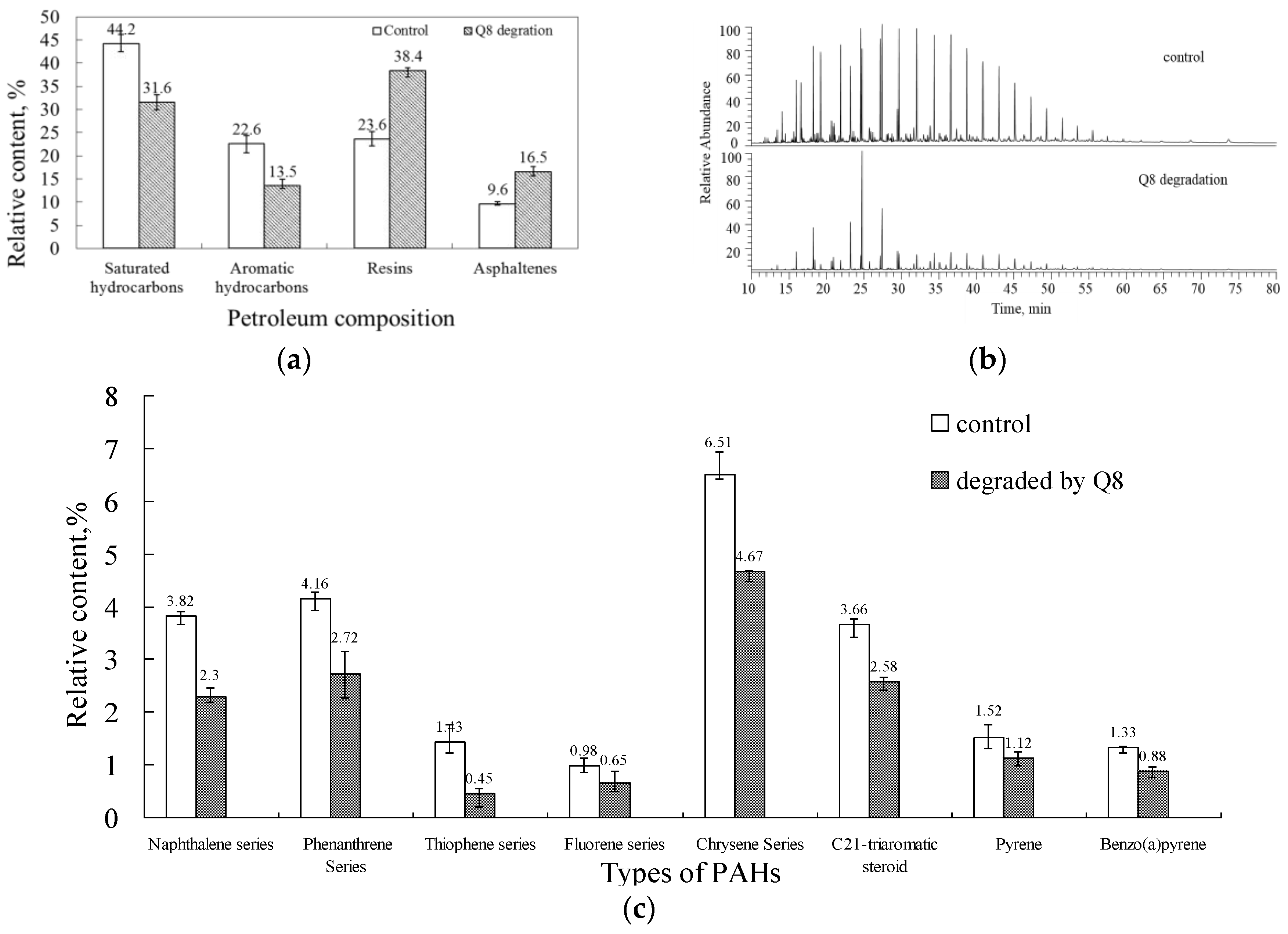
3.4. PAH Degradation in Crude Oil
4. Discussion
4.1. Advances in Bioremediation of PAHs
4.2. Screening Efficient and Adaptable Bacteria with Capacities to Degrade PAHs
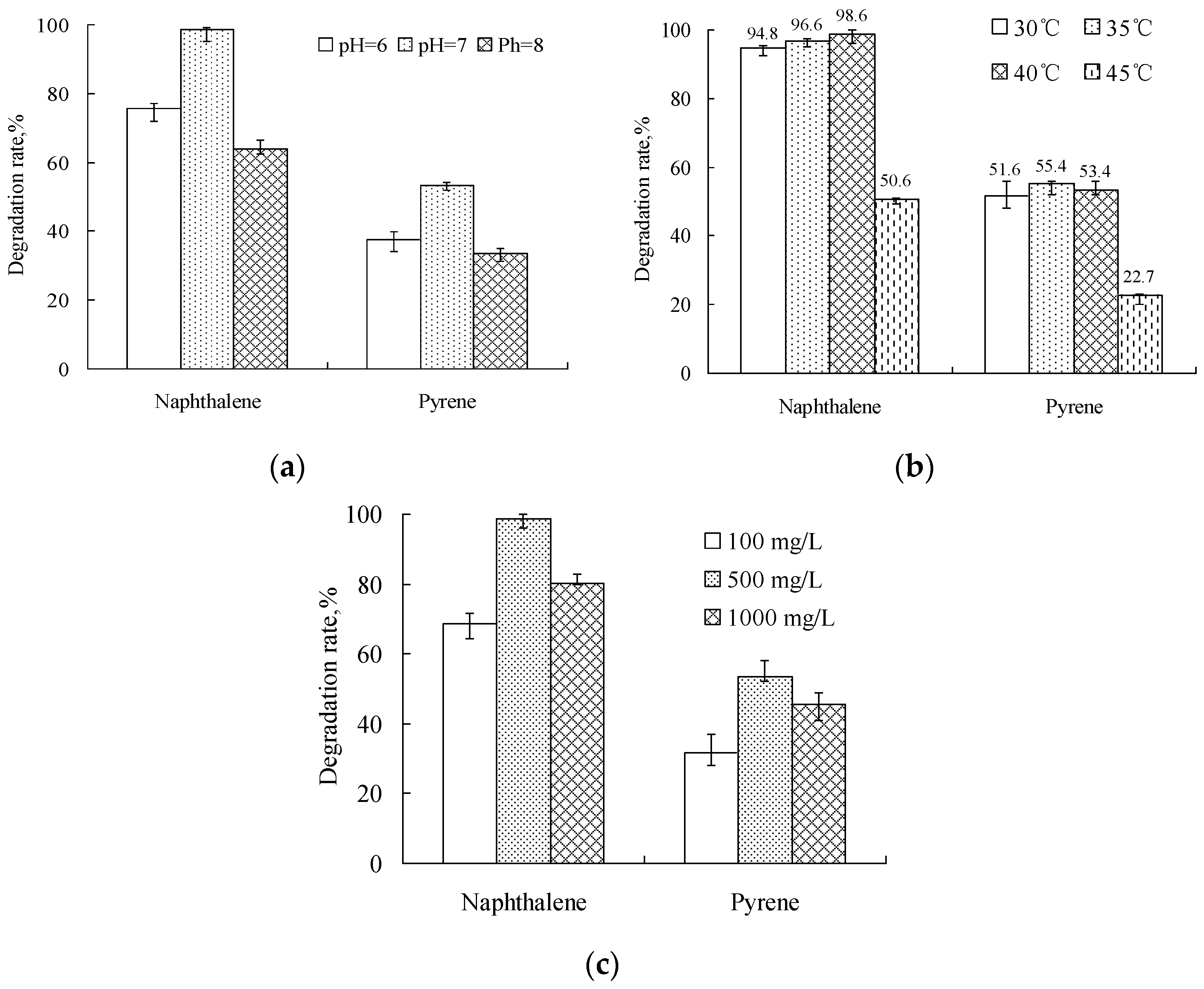
4.3. Effect of Environmental Conditions on the Degradation of PAHs
4.4. PAH Degradation in Oily Sludge and Sewage
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gu, Z.P.; Feng, J.L.; Han, W.L.; Li, L.; Wu, M.H.; Fu, J.M.; Sheng, G.Y. Diurnal variations of polycyclic aromatic hydrocarbons associated with PM 2.5 in Shanghai, China. J. Environ. Sci. 2010, 22, 389–396. [Google Scholar]
- Dong, D.M.; Liu, X.X.; Hua, X.Y.; Guo, Z.Y.; Li, L.F.; Zhang, L.W.; Xie, Y.J. Sedimentary record of polycyclic aromatic hydrocarbons in Songhua River, China. Environ. Earth Sci. 2016, 75, 508–515. [Google Scholar] [CrossRef]
- Schneider, I.L.; Teixeira, E.C.; Agudelo-Castañeda, D.M.; Silva e Silva, G.; Balzaretti, N.; Braga, M.F.; Oliveira, L.F. FTIR analysis and evaluation of carcinogenic and mutagenic risks of nitro-polycyclic aromatic hydrocarbons in PM1.0. Sci. Total Environ. 2016, 541, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Alrumman, S.A.; Hesham, A.L.; Alamri, S.A. Isolation, fingerprinting and genetic identification of indigenous PAHs degrading bacteria from oil-polluted soils. J. Environ. Biol. 2016, 37, 75–81. [Google Scholar] [PubMed]
- Ding, J.; Cong, J.; Zhou, J.; Gao, S.X. Polycyclic aromatic hydrocarbon biodegradation and extracellular enzyme secretion in agitated and stationary cultures of Phanerochaete chrysosporium. J. Environ. Sci. 2008, 20, 88–93. [Google Scholar] [CrossRef]
- Zhao, H.P.; Wang, L.; Ren, J.R.; Li, Z.; Li, M.; Gao, H.W. Isolation and characterization of phenanthrene-degrading strains Sphingomonas sp. ZP1 and Tistrella sp. ZP5. J. Hazard. Mater. 2008, 152, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Muckian, L.M.; Grant, R.J.; Clipson, N.J.W.; Doyle, E.M. Bacterial community dynamics during bioremediation of phenanthrene and fluoranthene amended soil. Int. Biodeterior. Biodegrad. 2009, 63, 52–56. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, X.; Waigi, M.G.; Liu, J.; Sun, K.; Gao, Y. Biodegradation of Mixed PAHs by PAH-Degrading Endophytic Bacteria. Int. J. Environ. Res. Public. Health 2016, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Lorgeoux, C.; Faure, P.; Billet, D.; Cébron, A. Isolation and substrate screening of polycyclic aromatic hydrocarbon degrading bacteria from soil with long history of contamination. Int. Biodeterior. Biodegrad. 2016, 107, 1–9. [Google Scholar] [CrossRef]
- Maki, T.; Masahito, S.; Ariani, H.; Shigeaki, H. The potential of Cycloclasticus and Altererythrobacter strains for use in bioremediation of petroleum-aromatic-contaminated tropical marine environments. J. Biosci. Bioeng. 2010, 110, 48–52. [Google Scholar]
- Shen, F.T.; Ho, M.J.; Huang, H.R.; Arun, A.B.; Rekha, P.D.; Young, C.C. Molecular detection and phylogenetic characterization of Gordonia species in heavily oil-contaminated soils. Res. Microbiol. 2008, 159, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Ling, J.Y.; Sun, H.B.; Luo, J.; Fan, Y.Y.; Cui, Z.J. Isolation and characterization of a newly isolated polycyclic aromatic hydrocarbons-degrading Janibacter anophelis strain JY11. J. Hazard. Mater. 2009, 172, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.W.; Luan, T.G.; Zou, F.; Tam, N.F.Y. Different bacterial groups for biodegradation of three- and four-ring PAHs isolated from a Hong Kong mangrove sediment. J. Hazard. Mater. 2008, 152, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Tachibana, S.; Itoh, K. Biodegradation of chrysene, an aromatic hydrocarbon by Polyporus sp. S133 in liquid medium. J. Hazard. Mater. 2009, 164, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.P.; Wu, Q.S.; Wang, L.; Zhao, X.T.; Gao, H.W. Degradation of phenanthrene by bacterial strain isolated from soil in oil refinery fields in Shanghai China. J. Hazard. Mater. 2009, 164, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Wang, N.; Li, H.J.; Cai, C. Microbial degradation mechanisms of soil high molecular weight PAHs and affecting factors: A review. J. Ecol. 2011, 30, 2621–2627. [Google Scholar]
- Cajthaml, T.; Erbanová, P.; Kollmann, A.; Novotný, C.; Sasek, V.; Mougin, C. Degradation of PAHs by Ligninolytic Enzymes of Irpex lacteus. Folia Microbiol. 2008, 53, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Pinyakong, O.; Habe, H.; Omori, T. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). J. Gen. Appl. Microbiol. 2003, 49, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Bai, X.; Sheng, H.; Jiao, L.; Zhou, H.; Shao, Z. Distribution of PAHs and the PAH-degrading bacteria in the deep-sea sediments of the high-latitude Arctic Ocean. Biogeosciences 2015, 12, 2163–2177. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Li, J.; Gao, Y.Z. Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant Soil 2014, 374, 251–262. [Google Scholar] [CrossRef]
- Anjali, J.; Fulekar, M.H. Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J. Hazard. Mater. 2011, 187, 333–340. [Google Scholar]
- Zhao, L.X.; Ma, T.; Gao, M.L.; Gao, P.K.; Cao, M.N.; Zhu, X.D.; Li, G.Q. Characterization of microbial diversity and community in waterflooding oil reservoirs in China. World J. Microbiol. Biotechnol. 2012, 28, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Tamaoka, J.; Komagata, K. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol. Lett. 1984, 25, 125–128. [Google Scholar] [CrossRef]
- Adnan, S.; Li, N.; Miura, H.; Hashimoto, Y.; Yamamoto, H.; Ezaki, T. Covalently immobilized DNA plate for luminometric DNA-DNA hybridization to identify viridans streptococci in under 2 h. FEMS Microbiol. Lett. 1993, 106, 139–142. [Google Scholar] [CrossRef]
- Shen, F.T.; Young, L.S.; Hsieh, M.F.; Lin, S.Y.; Young, C.C. Molecular detection and phylogenetic analysis of the alkane 1-monooxygenase gene from Gordonia spp. Syst. Appl. Microbiol. 2010, 33, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Z.; Cui, Z.S.; Dong, C.M.; Lai, Q.L.; Chen, L. Analysis of a PAH-degrading bacterial population in subsurface sediments on the Mid-Atlantic Ridge. Part I Oceanogr. Res. Pap. 2010, 157, 724–730. [Google Scholar] [CrossRef]
- Young, M.K.; Chi, K.A.; Seung, H.W.; Gyoo, Y.J.; Jong, M.P. Synergic degradation of phenanthrene by consortia of newly isolated bacterial strains. J. Biotechnol. 2009, 144, 293–298. [Google Scholar]
- Darmawan, R.; Nakata, H.; Ohta, H.; Niidome, T.; Takikawa, K.; Morimura, S. Isolation and Evaluation of PAH Degrading Bacteria. J. Biosci. Bioeng. 2015, 6, 2978–2983. [Google Scholar]
- Guo, C.L.; Zhou, H.W.; Wong, Y.S.; Tam, N.F.Y. Biodegradation ability and dioxgenase genes of PAH-degrading Sphingomonas and Mycobacterium strains isolated from mangrove sediments. Int. Biodeterior. Biodegrad. 2010, 64, 419–426. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gai, L.X.; Hou, Z.W.; Yang, C.Y.; Ma, C.Q.; Wang, Z.G.; Sun, B.P.; He, X.F.; Tang, H.Z.; Xu, P. Characterization and biotechnological potential of petroleum-degrading bacteria isolated from oil-contaminated soils. Bioresour. Technol. 2010, 101, 8452–8456. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.K.; Li, G.Q.; Dai, X.C.; Dai, L.B.; Wang, H.B.; Zhao, L.X.; Ma, T. Nutrients and oxygen alter reservoir biochemical characters and enhance oil recovery during biostimulation. World J. Microbiol. Biotechnol. 2013, 55, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindström, K. Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 2015, 36, 1–7. [Google Scholar] [CrossRef]
- Kummer, C.; Schumann, P.; Stackebrandt, E. Gordonia alkanivorans sp. nov., isolated from tar-contaminated soil. Int. J. Syst. Bacteriol. 1999, 49, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.F.; Sun, X.S.; Zhou, P.J.; Liu, R.L.; Liang, F.L.; Ma, Y.H. Gordonia paraffinivorans sp. nov., a hydrocarbon-degrading actinomycete isolated from an oil-producing well. Int. J. Syst. Evol. Microbiol. 2003, 53, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, B.; Pakshirajan, K.; Venkata Dasu, V. Biodegradation of pyrene by Mycobacterium frederiksbergense in a two-phase partitioning bioreactor system. Bioresour. Technol. 2008, 99, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Korlević, M.; Zucko, J.; Dragić, M.N.; Blažina, M.; Pustijanac, E.; Zeljko, T.V.; Gacesa, R.; Baranasic, D.; Starcevic, A.; Diminic, J.; et al. Bacterial diversity of polluted surface sediments in the northern Adriatic Sea. Syst. Appl. Microbiol. 2015, 38, 189–197. [Google Scholar]
- Toledo, F.L.; Calvo, C.; Rodelas, B.; González-López, J. Selection and identification of bacteria isolated from waste crude oil with polycyclic aromatic hydrocarbons removal capacities. Syst. Appl. Microbiol. 2006, 29, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.S.; Bari, S.B.; Surana, S.J. Isolation of stigmasterol and γ-sitosterol from petroleum ether extract of woody stem of Abelmoschus manihot. Asian J. Biol. Life Sci. 2009, 55, 1767–1773. [Google Scholar] [CrossRef]
- Aske, N.; Kallevik, H.; Sjöblom, J. Determinaton of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuel 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Saranya, K.; Palanisami, T.; Mallavarapu, M.; Yong, B.L.; Ravi, N. Polyaromatic hydrocarbon (PAH) degradation potential of a new acid tolerant, diazotrophic P-solubilizing and heavy metal resistant bacterium Cupriavidus sp. MTS-7 isolated from long-term mixed contaminated soil. Chemosphere 2016, 162, 31–39. [Google Scholar]
- Debajyoti, G.; Shreya, G.; Tapan, K.D.; Youngho, A. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1–27. [Google Scholar]
- Saranya, K.; Palanisami, T.; Mallavarapu, M.; Ravi, N. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by novel bacterial consortia tolerant to diverse physical settings-assessments in liquid- and slurry-phase systems. Int. Biodeterior. Biodegrad. 2016, 108, 149–157. [Google Scholar]
- Sharma, A.; Singh, S.B.; Sharma, R.; Chaudhary, P.; Pandey, A.K.; Ansari, R.; Vasudevan, V.; Arora, A.; Singh, S.; Saha, S.; et al. Enhanced biodegradation of PAHs by microbial consortium with different amendment and their fate in in-situ condition. J. Environ. Manag. 2016, 181, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kang, S.H.; Mulchandani, A.; Chen, W. Bioremediation: Environmental clean-up through pathway engineering. Curr. Opin. Biotechnol. 2008, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Wang, Y.S.; Hu, D.F. Biosorption and biodegradation of polycyclic aromatic hydrocarbons in aqueous solutions by a consortium of white-rot fungi. J. Hazard. Mater. 2010, 179, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, L.X. PAH-degrading microbial consortium and its pyrene-degrading plasmids from mangrove sediment samples in Huian, China. Mar. Pollut. Bull. 2008, 57, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Fathepure, B.Z. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front. Microbiol. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Zheng, Y.; Wu, Y.H.; Huo, Y.Y.; Cheng, H.; Wang, C.S.; Xu, X.W. Complete genome sequence of a benzo[a]pyrene-degrading bacterium Altererythrobacter epoxidivorans CGMCC 1.7731T. Mar. Genom. 2016, 25, 39–41. [Google Scholar]
- Tao, X.Q.; Lu, G.N.; Dang, Z.; Yang, C.; Yi, X.Y. A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem. 2007, 42, 401–408. [Google Scholar] [CrossRef]
- Jacques, R.J.S.; Okeke, B.C.; Bento, F.M.; Teixeira, A.S.; Peralba, M.C.; Camargo, F.A. Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour. Technol. 2008, 99, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.F.; Teng, Y.; Luo, Y.M.; Christie, P.; Ma, W.T.; Liu, F.; Wu, Y.G.; Luo, Y.; Li, Z.G. Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Trichoderma reesei FS10-C and Effect of Bioaugmentation on an Aged PAH-Contaminated Soil. Bioremediat. J. 2015, 19, 9–17. [Google Scholar] [CrossRef]
- Meehan, C.; Banat, I.M.; McMullan, G.; Nigam, P.; Smyth, F.; Marchant, R. Decolorization of Remazol Black-B using a thermotolerant yeast, Kluyveromyces marxianus IMB3. Environ. Int. 2000, 26, 75–79. [Google Scholar] [CrossRef]
| Strain | Naphthalene | Phenanthrene | Anthracene | Pyrene | ||||
|---|---|---|---|---|---|---|---|---|
| 72 h | 168 h | 72 h | 168 h | 72 h | 168 h | 72 h | 168 h | |
| Control | 7.9 ± 0.2 | 18.6 ± 0.4 | 5.8 ± 0.2 | 6.6 ± 0.1 | 2.3 ± 0.1 | 3.9 ± 0.2 | 0.6 ± 0.3 | 0.8 ± 0.5 |
| Q8 removal ratio (%) | 75.6 ± 2.8 | 100 ± 0 | 45.9 ± 4.8 | 95.4 ± 4.6 | 40.4 ± 0.2 | 73.8 ± 2.5 | 37.7 ± 0.5 | 53.4 ± 0.1 |
| 18077T removal ratio (%) | 38.5 ± 4.6 | 70.5 ± 2.1 | 31.5 ± 0.8 | 65.3 ± 2.2 | 28.6 ± 4.2 | 45.3 ± 1.1 | 18.6 ± 3.0 | 38.7 ± 0.6 |
| 44604T removal ratio (%) | 48.5 ± 3.7 | 89.8 ± 0.6 | 42.7 ± 0.8 | 66.7 ± 1.7 | 33.6 ± 2.4 | 42.3 ± 0.2 | 28.6 ± 4.5 | 34.5 ± 2.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.-B.; Wang, C.-Y.; Lv, C.-Y.; Lun, Z.-M.; Zheng, C.-G. Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. Int. J. Environ. Res. Public Health 2017, 14, 215. https://doi.org/10.3390/ijerph14020215
Qi Y-B, Wang C-Y, Lv C-Y, Lun Z-M, Zheng C-G. Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. International Journal of Environmental Research and Public Health. 2017; 14(2):215. https://doi.org/10.3390/ijerph14020215
Chicago/Turabian StyleQi, Yi-Bin, Chen-Yu Wang, Cheng-Yuan Lv, Zeng-Min Lun, and Cheng-Gang Zheng. 2017. "Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water" International Journal of Environmental Research and Public Health 14, no. 2: 215. https://doi.org/10.3390/ijerph14020215





