Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report
Abstract
:1. Background
2. Objectives
3. Methods
3.1. Setting and Sampling
3.2. Study Instrument
3.3. Sero-Testing and Genetic Testing
3.4. Statistical Analyses
4. Results
4.1. Genotyping
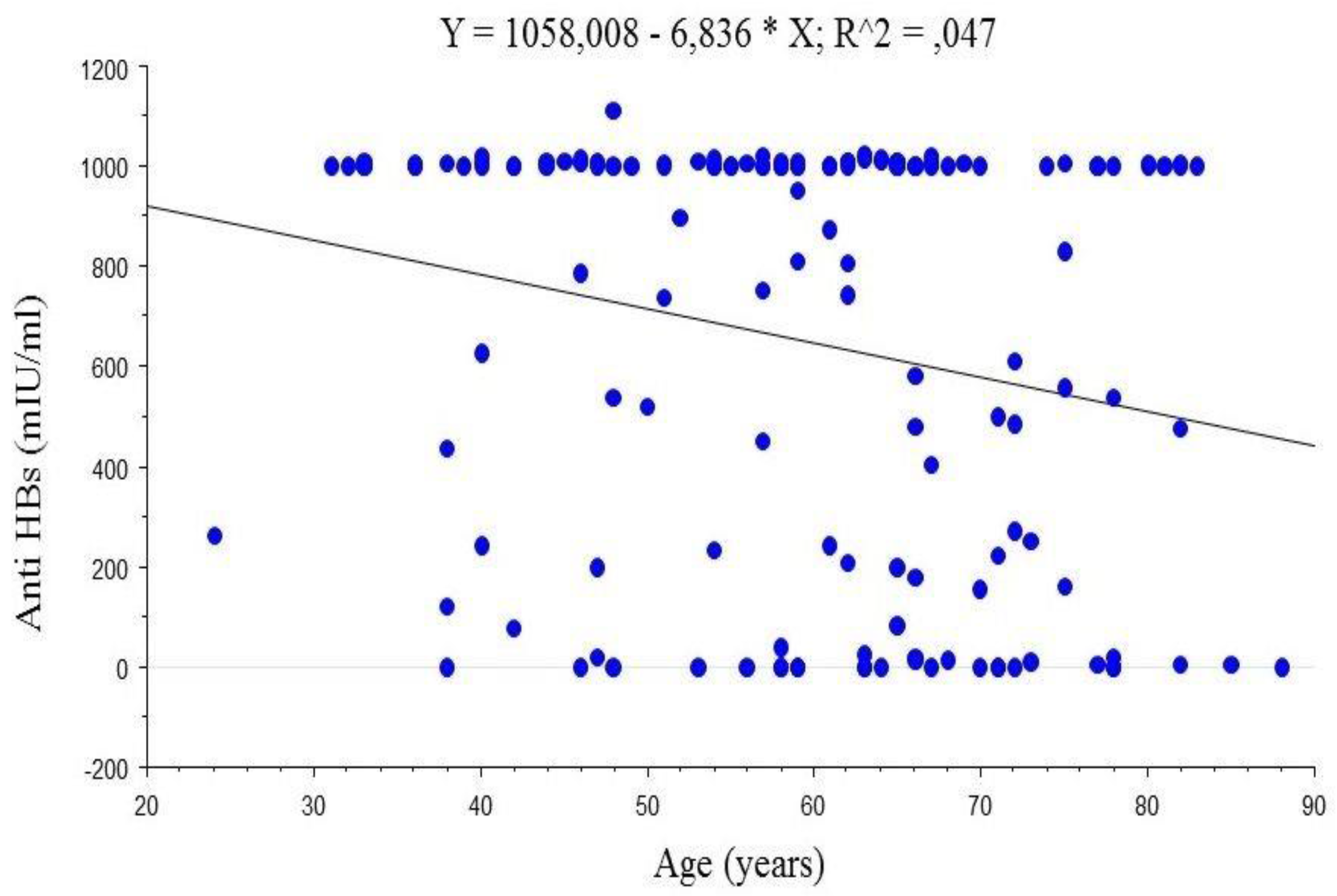
4.2. Anti-HBs Titres
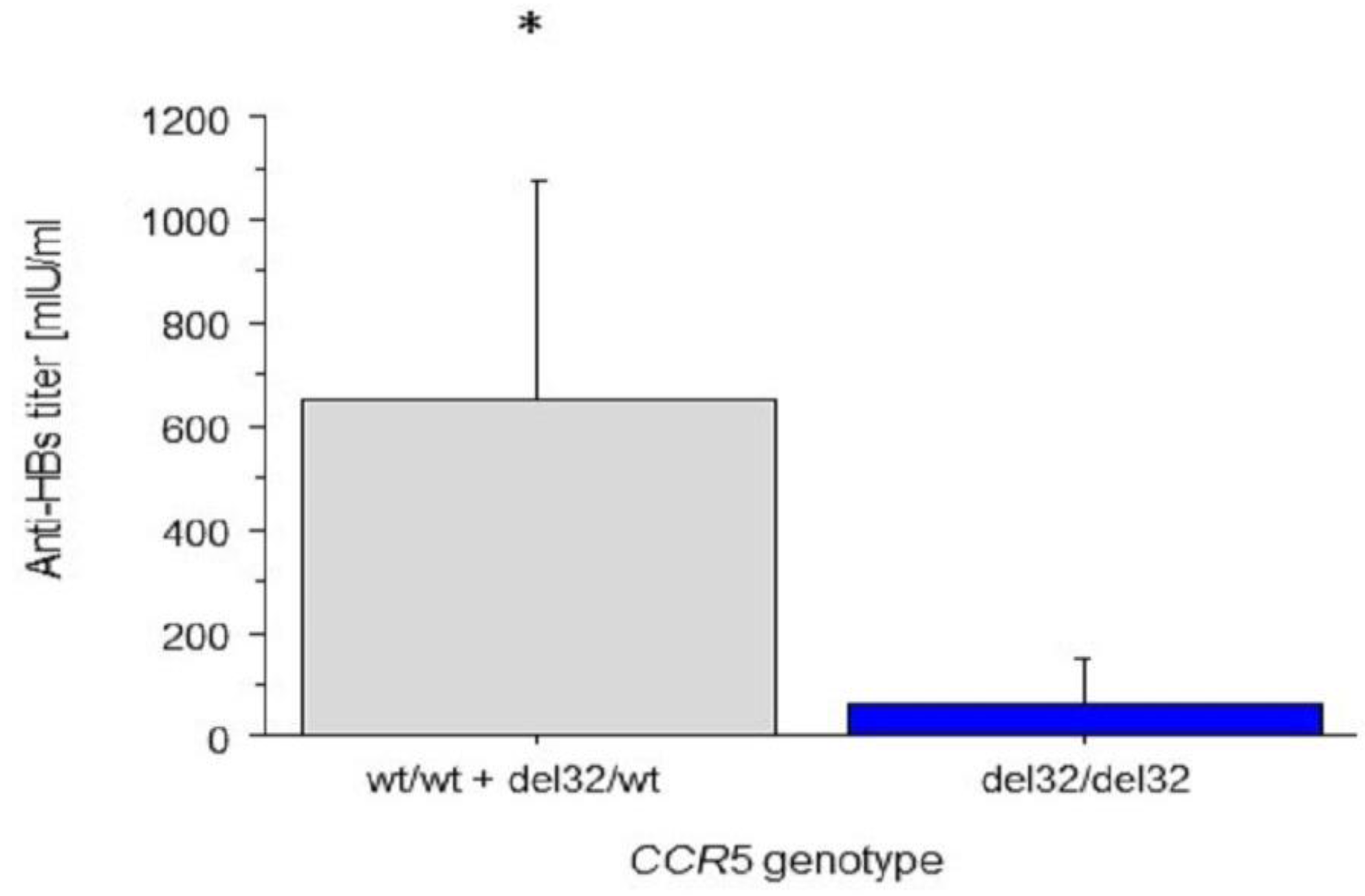
4.3. Associations between CCR2 and CCR5 Genotypes and Anti-HBs Titres
5. Discussion
5.1. Results Overview
5.2. Anti-HBs Titres
5.3. Frequencies of Δ32 CCR5 and 190G > A CCR2 Allele
5.4. The Association between Anti-HBs Titres and CCR5 and CCR2 Genotypes
6. Limitations
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Ślusarczyk, J. Vaccines and vaccination preventing viral hepatitis—Successes and misery. Zakażenia 2008, 6, 3–6. [Google Scholar]
- Van Damme, P.; Van Herck, K. A review of the long-term protection after hepatitis A and B vaccination. Travel. Med. Infect. Dis. 2007, 5, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.W.; Simard, E.P.; Finelli, L.; Fiore, A.E.; Bell, B.P. Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol. Rev. 2006, 28, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, M.; Van Damme, P.; Chlibek, R.; Smetana, J.; von Sonnenburg, F. Hepatitis A/B vaccination of adults over 40 years old: Comparison of three vaccine regimens and effect of influencing factors. Vaccine 2006, 24, 5509–5515. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M.; Fish, E.N. Chemokines: Attractive mediators of the immune response. Semin. Immunol. 2003, 15, 5–14. [Google Scholar] [CrossRef]
- Wu, L.; LaRosa, G.; Kassam, N.; Gordon, C.J.; Heath, H.; Ruffing, N.; Chen, H.; Humblias, J.; Samson, M.; Parmentier, M.; et al. Interaction of chemokine receptor CCR5 with its ligands: Multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 1997, 186, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Warr, G.; Loy, J.; Bravo, R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997, 186, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Carrington, M.; Winkler, C.; Huttley, G.A.; Smith, M.W.; Allikmets, R.; Goedert, J.J.; Buchbinder, S.P.; Vittinghoff, E.; Gomperts, E.; et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 1996, 273, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Adler, G.; Valjevac, A.; Skonieczna-Żydecka, K.; Mackic-Djurovic, M.; Parczewski, M.; Urbańska, A.; Salkic, N.N. Frequency of CCR5 Δ32 allele in healthy Bosniak population. Bosn. J. Basic Med. Sci. 2014, 14, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinski, P.P.; Lecybył, R.; Ignacak, M.; Juszczyk, J.; Trzeciak, W.H. Distribution of 32 alelle of the CCR5 gene in the population of Poland. J. Hum. Genet. 2000, 45, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Guergnon, J.; Combadière, C. Role of chemokines polymorphisms in diseases. Immunol. Lett. 2012, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Rebbani, K.; Ezzikouri, S.; Marchio, A.; Ababou, M.; Kitab, B.; Dejean, A.; Kandil, M. Common polymorphic effectors of immunity against hepatitis B and C modulate susceptibility to infection and spontaneous clearance in a Moroccan population. Infect. Genet. Evol. 2014, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Kim, D.Y.; Chang, H.Y.; Hong, S.P.; Shin, J.S.; Kim, Y.S.; Kim, H.; Kim, J.K.; Paik, Y.H.; Lee, K.S.; et al. Association of genetic variations in CCR5 and its ligand, RANTES with clearance of hepatitis B virus in Korea. J. Med. Virol. 2006, 78, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.L.; Astemborski, J.; Bashirova, A.; Mosbruger, T.; Greer, S.; Witt, M.D.; Goedert, J.J.; Hilgartner, M.; Majeske, A.; O’Brien, S.J.; et al. Genetic protection against hepatitis B virus conferred by CCR5 Delta32: Evidence that CCR5 contributes to viral persistence. J. Virol. 2007, 81, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.U.; Lima, G.D.; de Oliveira, M.L.; Heráclio Sde, A.; Silva, H.D.; Crovella, S.; Maia Mde, M.; Souza, P.R. CCR2 and CCR5 genes polymorphisms in women with cervical lesions from Pernambuco, northeast region of Brazil: A case-control study. Mem. Inst. Oswaldo Cruz (Rio de Janeiro) 2016, 111, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, J.Y.; Cho, S.W.; Choi, J.Y.; Lee, J.A.; Kim, M.H.; Lee, J.E.; Hahm, K.B.; Kim, J.H. RANTES, MCP-1, CCR2, CCR5, CXCR1 and CXCR4 gene polymorphisms are not associated with the outcome of hepatitis B virus infection: Results from a large scale single ethnic population. J. Korean Med. Sci. 2007, 22, 529–535. [Google Scholar] [CrossRef]
- Prasad, P.; Tiwari, A.K.; Kumar, K.M.; Ammini, A.C.; Gupta, A.; Gupta, R. Association of TGF beta1, TNF alpha, CCR2 and CCR5 gene polymorphisms in type-2 diabetes and renal insufficiency among Asian Indians. BMC Med. Genet. 2007, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; Vermeire, S.; Thoelen, I.; Keyaerts, E.; Struyf, F.; Vlietinck, R.; Rutgeerts, P.; Van Ranst, M. Analysis of the CC chemokine receptor 5 (CCR5) delta-32 polymorphism in inflammatory bowel disease. Hum. Genet. 2001, 108, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Thorisson, G.A.; Smith, A.V.; Krishnan, L.; Stein, L.D. The international HapMap project web site. Genome Res. 2005, 15, 1592–1593. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Van Der Meeren, O.; Crasta, P.; Cheuvart, B.; De Ridder, M. Characterization of an age-response relationship to GSK’s recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum. Vaccines Immunother. 2015, 11, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Sena, A.C.; Moorman, A.C.; Moore, Z.S.; Sharapov, U.M.; Drobenuic, J.; Hu, D.J.; Wood, H.W.; Xing, J.; Spradling, P.R. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012, 30, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Ganczak, M.; Bohatyrewicz, A.; Szych, Z.; Białecki, P. Markers of hepatitis B, C and HIV in trauma orthopedics patients and staff from a Polish teaching hospital. Chir. Narz. Ruchu Ortop. Pol. 2008, 73, 83–88. [Google Scholar]
- Parczewski, M.; Leszczyszyn-Pynka, M.; Kaczmarczyk, M.; Adler, G.; Binczak-Kuleta, A.; Loniewska, B. Sequence variants of chemokine receptor genes and susceptibility to HIV-1 infection. J. Appl. Genet. 2009, 50, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Moudi, B.; Mahmoudzadeh-Sagheb, H.; Hashemi, M. The correlation between interferon Lambda 3 gene polymorphisms and susceptibility to hepatitis B virus infection. Hepat. Mon. 2016, 16. [Google Scholar] [CrossRef]
- Nansen, A.; Christensen, J.P.; Andreasen, S.O.; Bartholdy, C.; Christensen, J.E.; Thomsen, A.R. The role of CC chemokine receptor 5 in antiviral immunity. Blood 2002, 99, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Ng-Cashin, J.; Kuhns, J.J.; Burkett, S.E.; Powderly, J.D.; Craven, R.R.; van Deventer, H.W.; Kirby, S.L.; Serody, J.S. Host absence of CCR5 potentiates dendritic cell vaccination. J. Immunol. 2003, 170, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W.; Dean, M.; Carrington, M.; Winkler, C.; Huttley, G.A.; Lomb, D.A.; Goedert, J.J.; O’Brien, T.R.; Jacobson, L.P.; Kaslow, R.; et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science 1997, 277, 959–996. [Google Scholar] [CrossRef] [PubMed]
- Algood, H.M.; Flynn, J.L. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J. Immunol. 2004, 173, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Kurihara, T.; Ryseck, R.P.; Yang, Y.; Ryan, C.; Loy, J.; Warr, G.; Bravo, R. Impaired macrophage function and enhanced T-cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 co-receptor. J. Immunol. 1998, 160, 4018–4025. [Google Scholar] [PubMed]
- Chang, H.Y.; Ahn, S.H.; Kim, D.Y.; Shin, J.S.; Kim, Y.S.; Hong, S.P.; Chung, H.J.; Kim, S.O.; Yoo, W.D.; Han, K.H. Association between CCR5 promoter polymorphisms and hepatitis B virus infection. Korean J. Hepatol. 2005, 11, 116–124. [Google Scholar] [CrossRef]
| Variable | All (n = 149) | Women (n = 85) | Men (n = 64) | p ** |
|---|---|---|---|---|
| Age (years) | 59.4 ± 13.6 | 61.0 ± 13.8 | 57.2 ± 13.0 | 0.08 |
| Body mass (kg) | 76.7 | 68.0 | 84.5 | <0.0001 |
| Height (cm) | 167.4 ± 8.5 | 162.5 ± 6.4 | 173.9 ± 6.3 | <0.0001 |
| BMI (kg/m2) | 26.7 | 26.1 | 27.9 | 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganczak, M.; Skonieczna-Żydecka, K.; Drozd-Dąbrowska, M.; Adler, G. Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report. Int. J. Environ. Res. Public Health 2017, 14, 166. https://doi.org/10.3390/ijerph14020166
Ganczak M, Skonieczna-Żydecka K, Drozd-Dąbrowska M, Adler G. Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report. International Journal of Environmental Research and Public Health. 2017; 14(2):166. https://doi.org/10.3390/ijerph14020166
Chicago/Turabian StyleGanczak, Maria, Karolina Skonieczna-Żydecka, Marzena Drozd-Dąbrowska, and Grażyna Adler. 2017. "Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report" International Journal of Environmental Research and Public Health 14, no. 2: 166. https://doi.org/10.3390/ijerph14020166
APA StyleGanczak, M., Skonieczna-Żydecka, K., Drozd-Dąbrowska, M., & Adler, G. (2017). Possible Impact of 190G > A CCR2 and Δ32 CCR5 Mutations on Decrease of the HBV Vaccine Immunogenicity—A Preliminary Report. International Journal of Environmental Research and Public Health, 14(2), 166. https://doi.org/10.3390/ijerph14020166





