Usability of a Medication Event Reminder Monitor System (MERM) by Providers and Patients to Improve Adherence in the Management of Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aims of the Study
- The identification of unrecognized defects in the design and function of the MERM prior to scaled deployment [16].
- The very specific identification of patient or provider usability issues, permitting:
- Targeted instruction, graphics, or FAQs to prevent or resolve such issues.
- Targeted training to prevent or resolve such issues.
- The assessment of user performance, satisfaction, and acceptability.
2.2. Study Design
2.3. Setting
2.4. Patient and Provider Selection
2.5. Variable and Measurement/Procedures
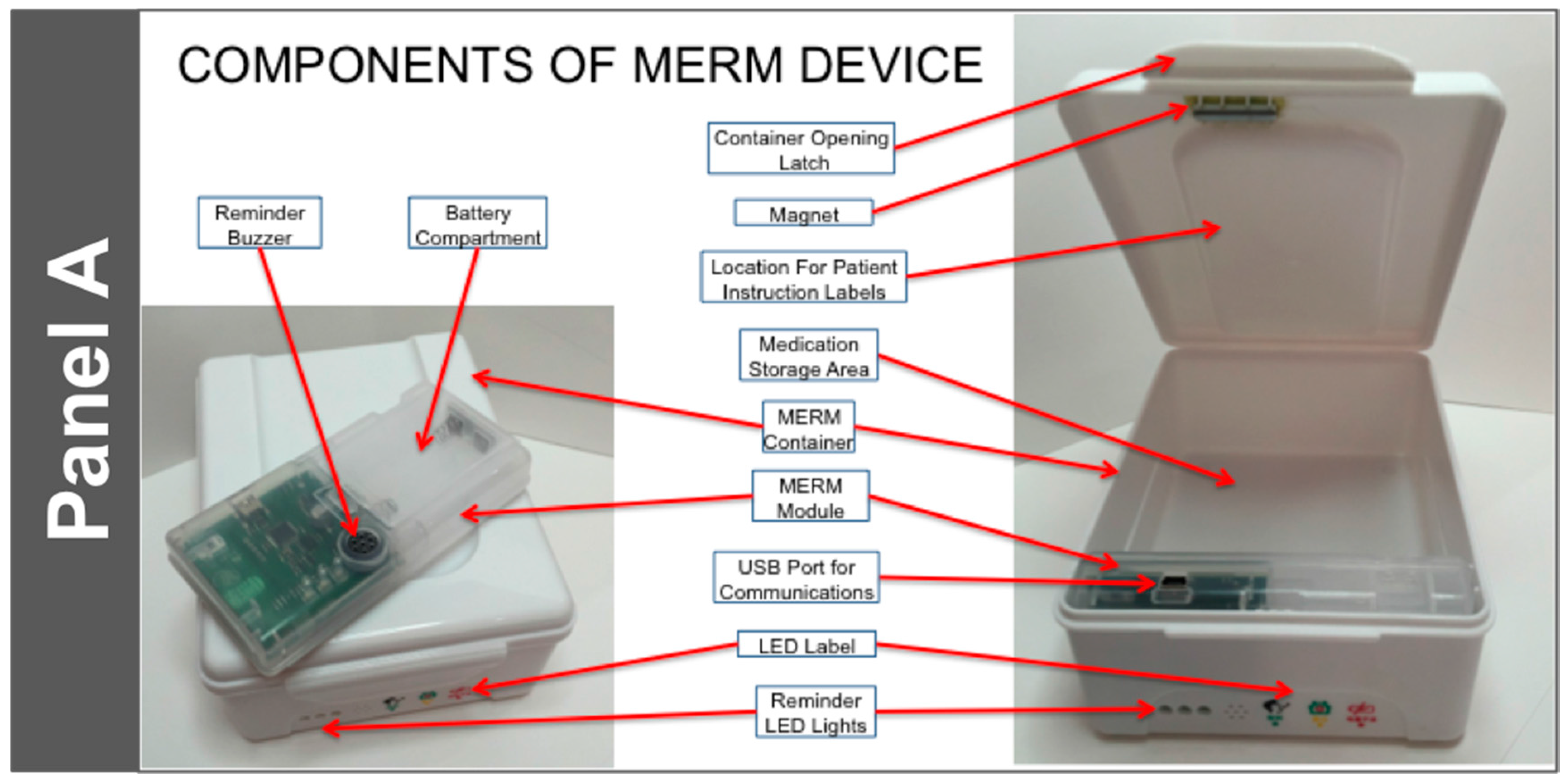
- It is suitable for use with blister packaged TB medications by TB patients. It can hold one month of DS-TB medication and is highly portable. The electronic module is removable and can, if desired, be transported discretely to the clinic.
- It is highly durable. The container is made of food grade plastic that is resistant to damage from impact. In addition, the MERM has been designed to be resistant to vibration (from transportation or otherwise).
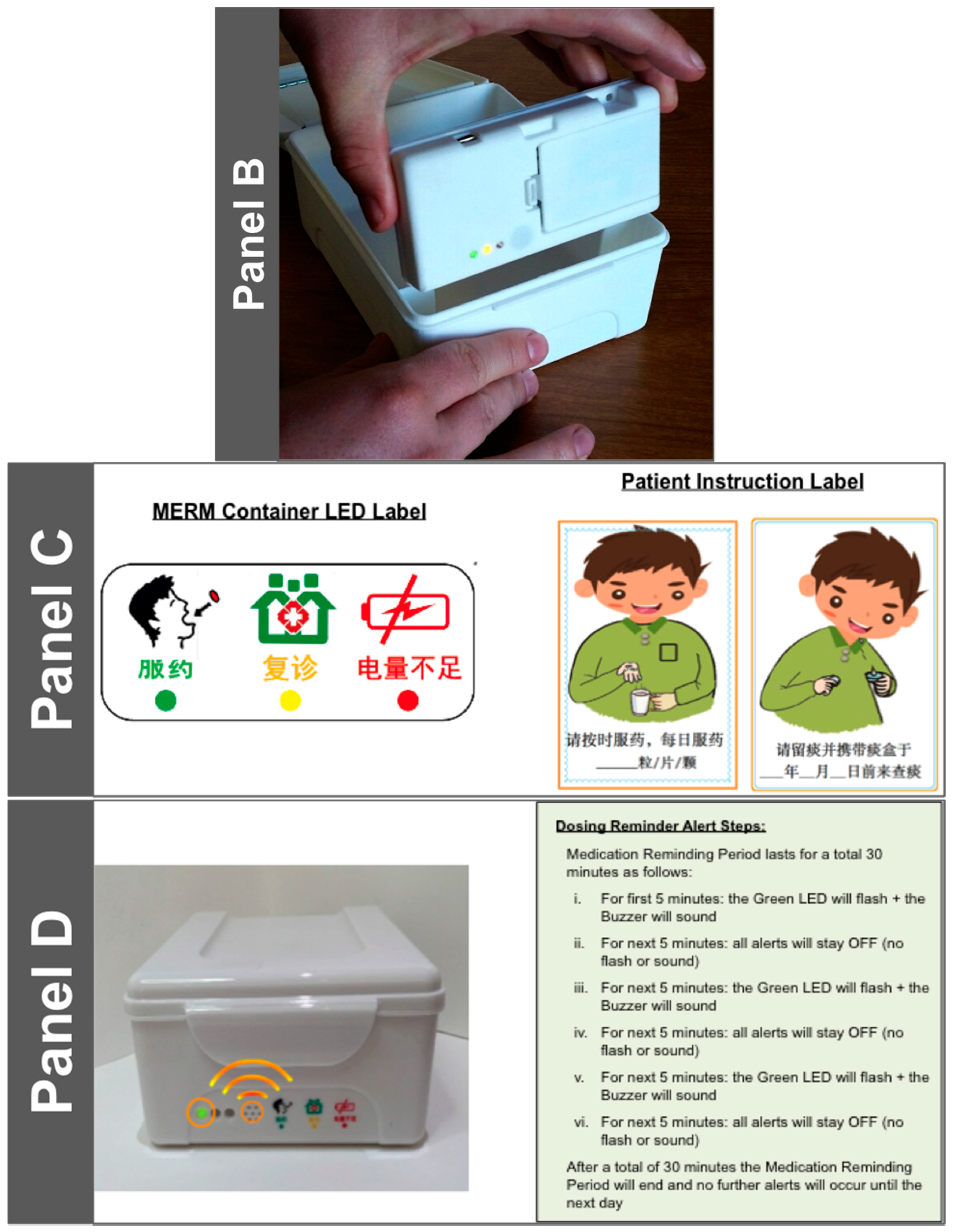
- Data acquisition is simple: It uses a magnetic sensor switch that is triggered upon the opening of the drug container box.
- It has programmable alert mechanisms consisting of:
- Three LED Lights: Green—Dose Alert; Yellow—Refill; Red—Low Battery
- An audible tone to indicate that a dose is due.
- At a specific time each day, it produces a ‘heartbeat’ to indicate to the data center that the MERM was functioning that day. The ‘heartbeat’ is downloaded along with the patient dosing data when the patient returns to the clinic for follow up.
- Data transfer is straightforward. A USB port is available to connect to the provider’s data system.
- The power source is simple, and does not require a connection to an electrical outlet or recharging. It utilizes two (2) AA disposable alkaline batteries, and the battery life is greater than 180 days.
- The MERM is affordable: its cost is approximately 9 USD, plus cost of the disposable batteries ($0.50 for the two required). It is highly re-usable, resulting in a lower per-patient cost.
- At treatment initiation, the MERM is registered to the patient and daily dosing refill alerts are set per the medication regimen and patient preference.
- The MERM is filled with one month of medication and given to the patient.
- Visual and audible dosing reminders indicate to patients that medication should be taken.
- Patient opens the MERM and takes medication, which action is captured and stored by the MERM as a proxy for the medication-taking event [20].
- Patient brings MERM with them to their next provider visit and/or for refill.
- Care providers connect the MERM via a USB cable to download dosing history and ‘heartbeat’ information, in the process populating the patient-specific adherence dashboard.
- Care providers print out the patient’s dosing history and use that information to evaluate adherence “patterns”, investigate and understand patient’s specific challenges with medication adherence and counsel the patient about how to recognize and overcome those challenges.
- The dosing history also drives an algorithm that identifies the patient as high, medium, or low risk, based on the patient’s level and pattern of non-adherence.
- Refill medications are inserted into the MERM.
- Daily dosing and refill alerts are adjusted (if required).
- Patient is sent home again with his/her MERM and medications.
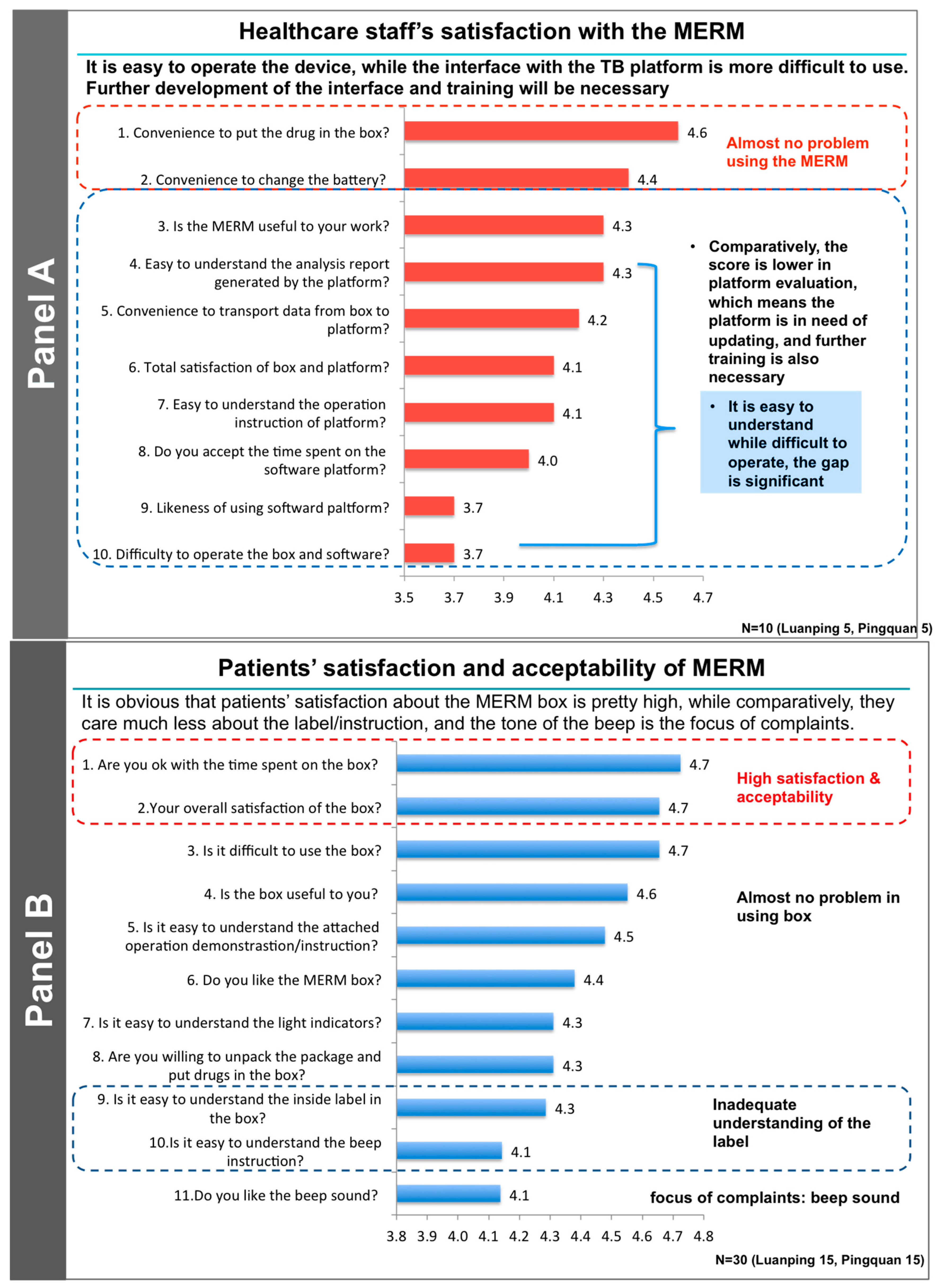
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horsburgh, C.R., Jr.; Barry, C.E., 3rd; Lange, C. Treatment of Tuberculosis. N. Engl. J. Med. 2015, 373, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, H.M.; Burman, W.J.; Chaisson, R.E.; Daley, C.L.; Etkind, S.C.; Friedman, L.N.; Fujiwara, P.; Grzemska, M.; Hopewell, P.C.; Iseman, M.D.; et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 603–662. [Google Scholar] [PubMed]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Adane, A.A.; Alene, K.A.; Koye, D.N.; Zeleke, B.M. Non-adherence to anti-tuberculosis treatment and determinant factors among patients with tuberculosis in northwest Ethiopia. PLoS ONE 2013, 8, e78791. [Google Scholar]
- Bayer, R.; Wilkinson, D. Directly observed therapy for tuberculosis: History of an idea. Lancet 1995, 345, 1545–1548. [Google Scholar] [CrossRef]
- Fox, W. Self-administration of medicaments. A review of published work and a study of the problems. Bull. Int. Union Tuberc. 1962, 32, 307–331. [Google Scholar] [PubMed]
- Weis, S.E.; Slocum, P.C.; Blais, F.X.; King, B.; Nunn, M.; Matney, G.B.; Gomez, E.; Foresman, B.H. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N. Engl. J. Med. 1994, 330, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Volmink, J.; Garner, P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst. Rev. 2007, CD003343. [Google Scholar] [CrossRef]
- McLaren, Z.M.; Milliken, A.A.; Meyer, A.J.; Sharp, A.R. Does directly observed therapy improve tuberculosis treatment? More evidence is needed to guide tuberculosis policy. BMC Infect. Dis. 2016, 16, 537. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert-Pijnen, J.E.W.C.; Nijland, N.; van Limburg, M.; Ossebaard, H.C.; Kelders, S.M.; Eysenbach, G.; Seydel, E.R. A Holistic Framework to Improve the Uptake and Impact of eHealth Technologies. J. Med. Internet Res. 2011, 13, e111. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.W. A comparison of usability methods for testing interactive health technologies: Methodological aspects and empirical evidence. Int. J. Med. Inform. 2009, 78, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J. SUS: A ’Quick and Dirty’ Usability Scale. In Usability Evaluation in Industry; Jordan, P.W.T.B., Weerdmeester, B.A., McClelland, I.A., Eds.; Taylor & Francis Ltd.: London, UK, 1996; pp. 189–194. [Google Scholar]
- Haberer, J.E.; Sabin, L.; Amico, K.R.; Orrell, C.; Galarraga, O.; Tsai, A.C.; Vreeman, R.C.; Wilson, I.; Sam-Agudu, N.A.; Blaschke, T.F.; et al. Improving antiretroviral therapy adherence in resource-limited settings at scale: A discussion of interventions and recommendations. J. Int. AIDS Soc. 2017, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Demonceau, J.; Ruppar, T.; Kristanto, P.; Hughes, D.A.; Fargher, E.; Kardas, P.; De Geest, S.; Dobbels, F.; Lewek, P.; Urquhart, J.; et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: A systematic literature review and meta-analysis. Drugs 2013, 73, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lewis, J.J.; Zhang, H.; Lu, W.; Zhang, S.; Zheng, G.; Bai, L.; Li, J.; Li, X.; Chen, H.; et al. Effectiveness of Electronic Reminders to Improve Medication Adherence in Tuberculosis Patients: A Cluster-Randomised Trial. PLoS Med. 2015, 12, e1001876. [Google Scholar] [CrossRef] [PubMed]
- Denhaerynck, K.; Schäfer-Keller, P.; Young, J.; Steiger, J.; Bock, A.; De Geest, S. Examining assumptions regarding valid electronic monitoring of medication therapy: Development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med. Res. Methodol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Wood, C.E.; Johnston, M.; Abraham, C.; Francis, J.J.; Hardeman, W. Behaviour change techniques: The development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol. Assess. 2015, 19, 1–188. [Google Scholar] [PubMed]
- Bleser, D.E.L.; Geest, S.D.E.; Vincke, B.; Ruppar, T.; Vanhaecke, J.; Dobbels, F. How to test electronic adherence monitoring devices for use in daily life: A conceptual framework. CIN Comput. Inf. Nurs. 2011, 29, 489–495. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, L.; De Geest, S.; Vandenbroeck, S.; Vanhaecke, J.; Dobbels, F. How accurate are electronic monitoring devices? A laboratory study testing two devices to measure medication adherence. Sensors 2010, 10, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, B.; Tousset, E.; Rode, R.; Bertz, R.; Mayer, S.; Urquhart, J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J. Clin. Pharmacol. 2005, 45, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Haberer, J.E.; Musinguzi, N.; Tsai, A.C.; Boum, Y., 2nd; Bwana, B.M.; Muzoora, C.; Hunt, P.W.; Martin, J.N.; Bangsberg, D.R. Real-time electronic adherence monitoring plus follow-up improves adherence compared with standard electronic adherence monitoring. AIDS 2017, 31, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.A.; Lewin, S.A.; Smith, H.J.; Engel, M.E.; Fretheim, A.; Volmink, J. Patient adherence to tuberculosis treatment: A systematic review of qualitative research. PLoS Med. 2007, 4, e238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellappa, V.; Lefèvre, P.; Battaglioli, T.; Narayanan, D.; Van der Stuyft, P. Coping with tuberculosis and directly observed treatment: A qualitative study among patients from South India. BMC Health Serv. Res. 2016, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Treatment of Drug-Susceptible Tuberculosis and Patient Care; 2017 Update; WHO: Geneva, Switzerland, 2017; p. 25.
- Menzies, N.A.; Gomez, G.B.; Bozzani, F.; Chatterjee, S.; Foster, N.; Baena, I.G.; Laurence, Y.V.; Qiang, S.; Siroka, A.; Sweeney, S.; et al. Cost-effectiveness and resource implications of aggressive action on tuberculosis in China, India, and South Africa: A combined analysis of nine models. Lancet Glob. Health 2016, 4, e816–e826. [Google Scholar] [CrossRef]
| County of Study in China | Dates of Study | Focus Group Participants | In-Depth Interviews | ||
|---|---|---|---|---|---|
| Medical Staff | Patients | Medical Staff | Patients | ||
| Luanping | 17 March 2016 | 0 | 8 | 5 | 7 |
| Pingquan | 18 and 19 March 2016 | 5 | 7 | 0 | 8 |
| Age Range (Years) | <30 | 30–39 | 40–49 | 50–60 | >60 |
|---|---|---|---|---|---|
| Number of Participants | 5 | 3 | 7 | 8 | 7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Blaschke, T.; Thomas, B.; De Geest, S.; Jiang, S.; Gao, Y.; Li, X.; Buono, E.W.; Buchanan, S.; Zhang, Z.; et al. Usability of a Medication Event Reminder Monitor System (MERM) by Providers and Patients to Improve Adherence in the Management of Tuberculosis. Int. J. Environ. Res. Public Health 2017, 14, 1115. https://doi.org/10.3390/ijerph14101115
Liu X, Blaschke T, Thomas B, De Geest S, Jiang S, Gao Y, Li X, Buono EW, Buchanan S, Zhang Z, et al. Usability of a Medication Event Reminder Monitor System (MERM) by Providers and Patients to Improve Adherence in the Management of Tuberculosis. International Journal of Environmental Research and Public Health. 2017; 14(10):1115. https://doi.org/10.3390/ijerph14101115
Chicago/Turabian StyleLiu, Xiaoqiu, Terrence Blaschke, Bruce Thomas, Sabina De Geest, Shiwen Jiang, Yongxin Gao, Xinxu Li, Elizabeth Whalley Buono, Stacy Buchanan, Zhiying Zhang, and et al. 2017. "Usability of a Medication Event Reminder Monitor System (MERM) by Providers and Patients to Improve Adherence in the Management of Tuberculosis" International Journal of Environmental Research and Public Health 14, no. 10: 1115. https://doi.org/10.3390/ijerph14101115




