Changes in Cosmetics Use during Pregnancy and Risk Perception by Women
Abstract
:1. Introduction
2. Subjects and Method
2.1. Subjects
2.2. Method
2.3. Statistical Analysis
3. Results
3.1. Women’s Characteristics
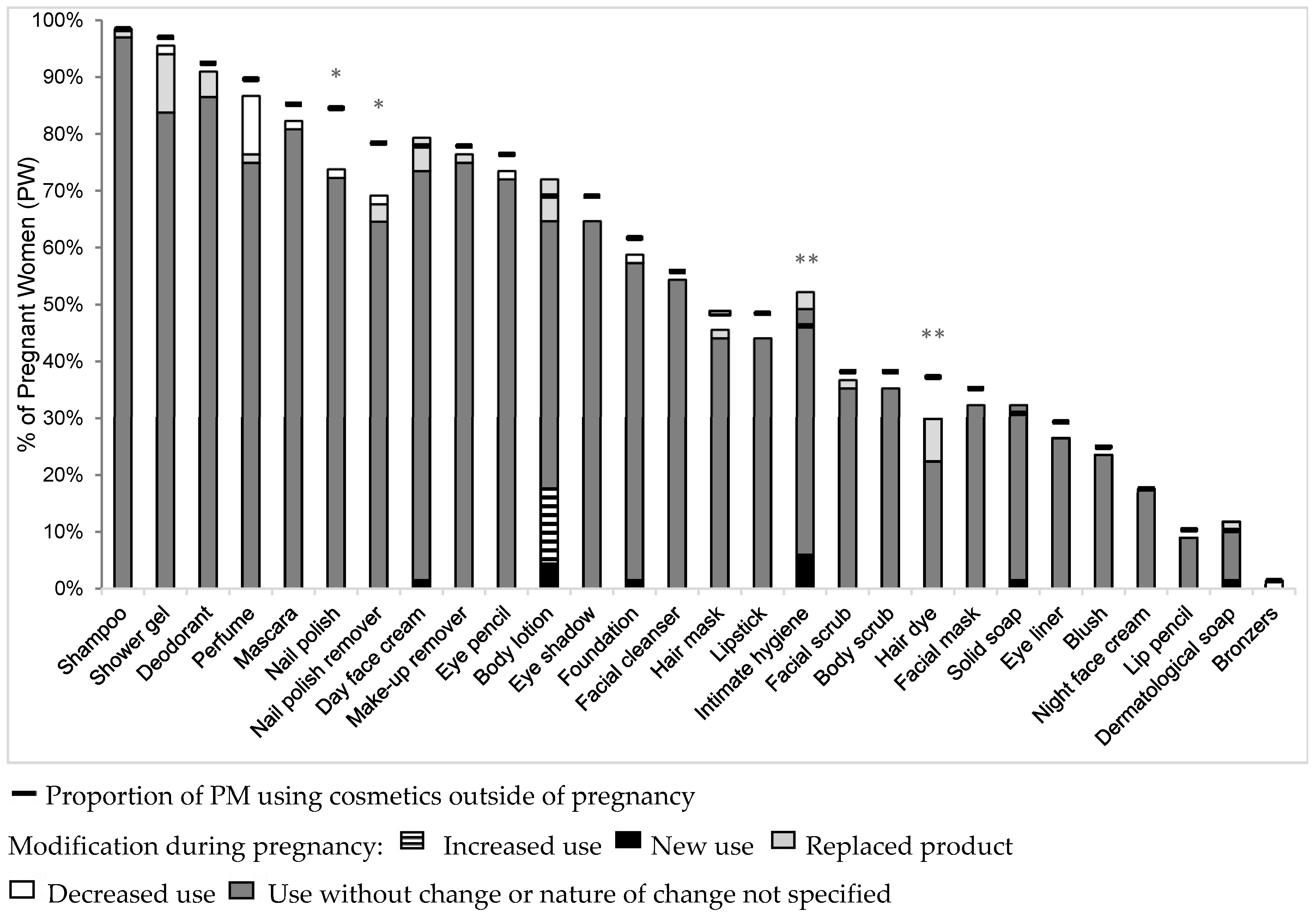
3.2. Proportion of Women Using Cosmetics Outside of Pregnancy
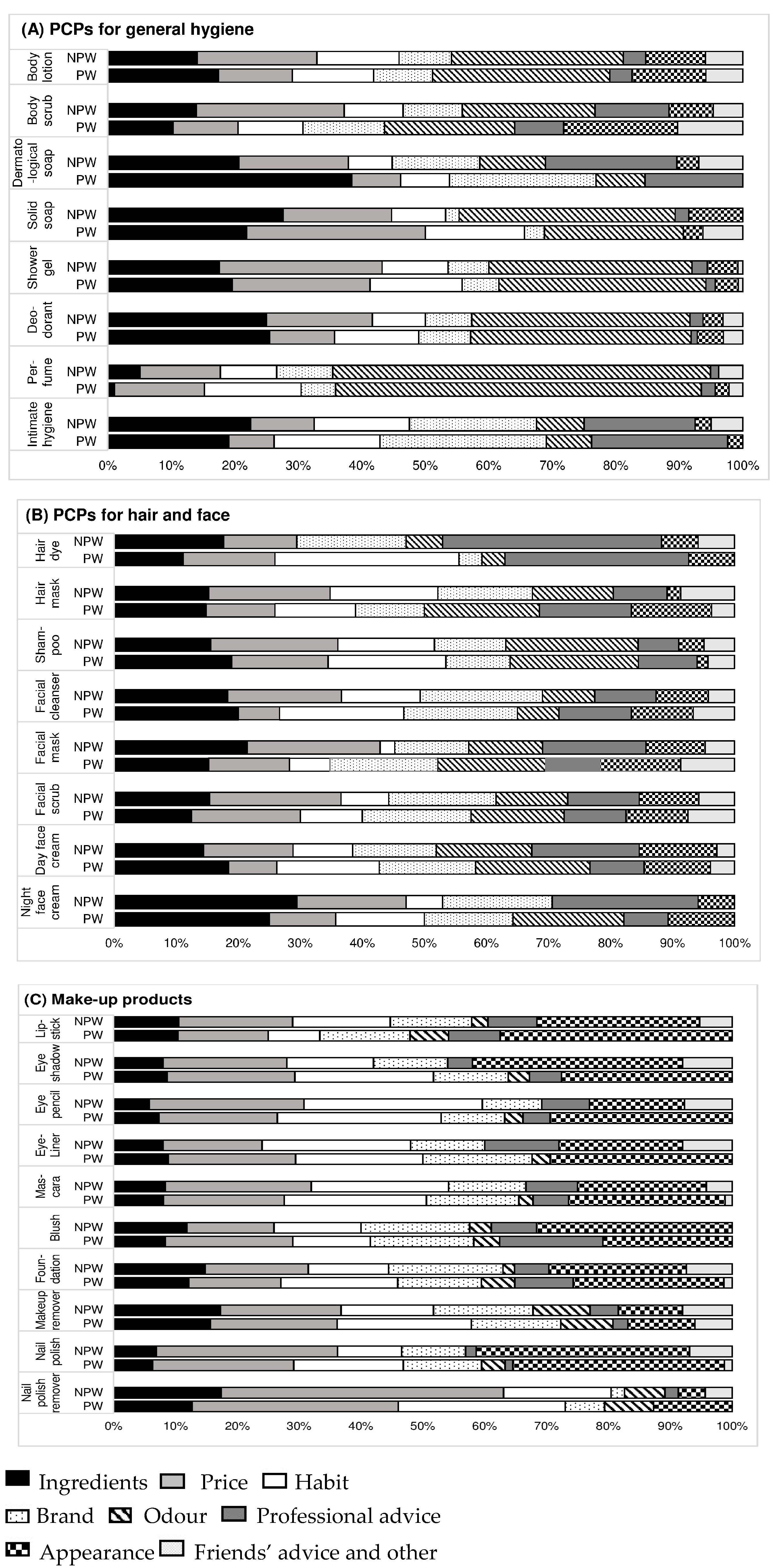
3.3. Criteria of Choice of Cosmetics Outside of Pregnancy
3.4. Proportion of Women Intending to Change (NPW) and Having Changed (PW) Cosmetics Use during Pregnancy
3.5. Types of Changes Made during Pregnancy
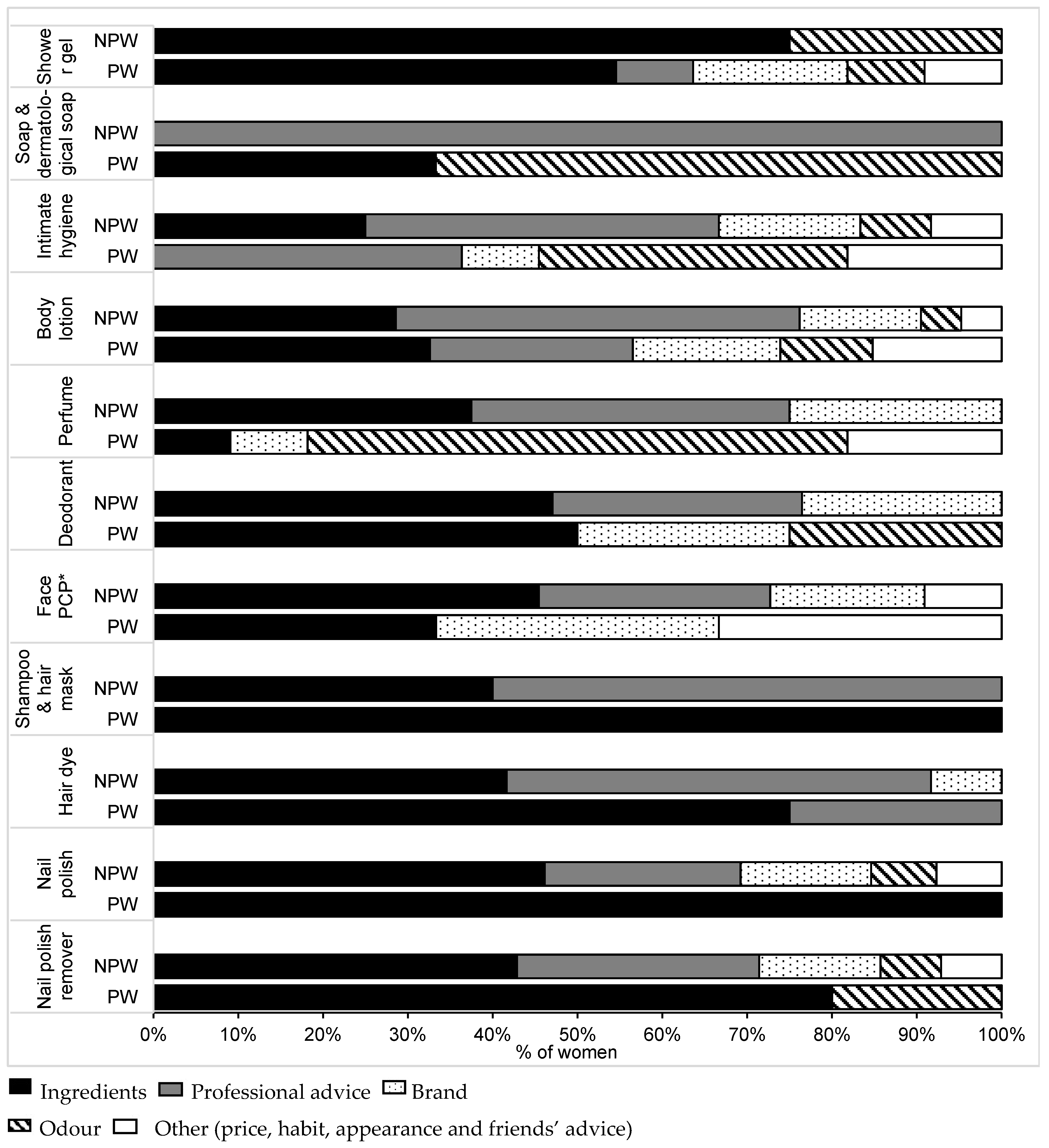
3.6. Criteria of Choice of Cosmetics during Pregancy
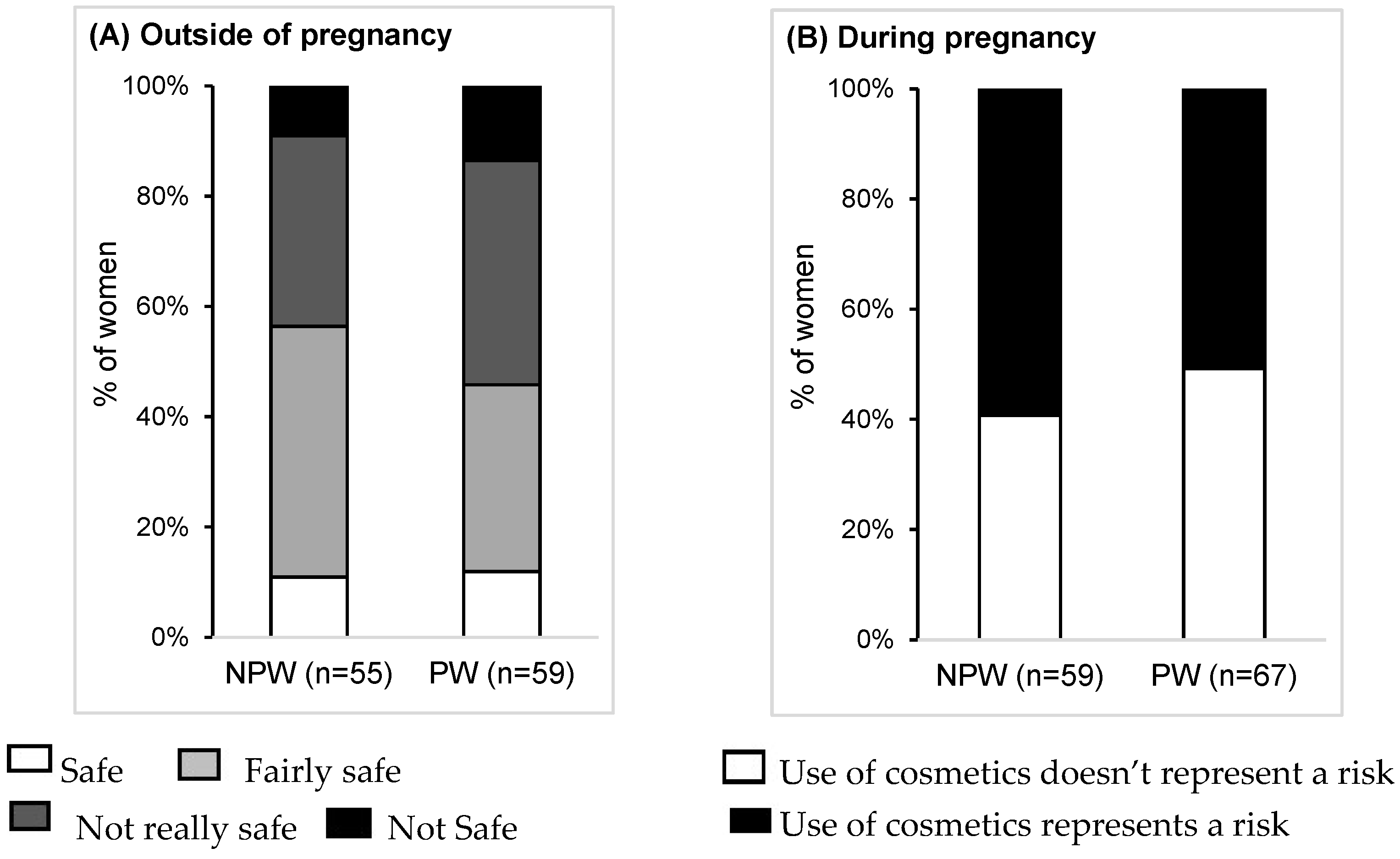
3.7. Risk Perception and Advice from Health Professionals
4. Discussion
4.1. Use of Cosmetics Outside of Pregnancy
4.2. Use of Cosmetics during Pregnancy
4.3. Perception of Risk Related to Cosmetics and Their Ingredients
4.4. Impact of Pregnancy on the Risk Perception of Cosmetics
4.5. Need of Advice from Health Professionals about the Safe Use of Cosmetics
4.6. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EU | European Union |
| NPW | non-pregnant women |
| PCP | personal care products |
| PW | pregnant women |
| TPHP | Triphenyl phosphate |
References
- European Union. Regulation (EC) no 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu (accessed on 7 December 2015).
- Juhász, M.L.; Marmur, E.S. A review of selected chemical additives in cosmetic products. Dermatol. Ther. 2014, 27, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Chevillotte, G.; Ficheux, A.S.; Morisset, T.; Roudot, A.C. Exposure method development for risk assessment to cosmetic products using a standard composition. Food Chem. Toxicol. 2014, 68, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Mortamais, M.; Chevrier, C.; Petit, C.; Calafat, A.M.; Ye, X.; Silva, M.J.; Brambilla, C.; Pin, I.; Charles, M.; et al. Exposure to Phthalates and Phenols during Pregnancy and Offspring Size at Birth. Environ. Health Perspect. 2012, 120, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, A.; Kang, H.S.; Park, J.H.; Kwon, J.E.; Moon, G.I.; Hwang, M.S.; Hwang, I.G. The association between urinary benzophenone concentrations and personal care product use in Korean adults. Arch. Environ. Contam. Toxicol. 2015. (in process). [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.M.; Kongshoj, B.; Skakkebaek, N.E.; Wulf, H.C. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Just, A.C.; Williams, P.L.; Smith, K.W.; Calafat, A.M.; Hauser, R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Ljung Björklund, K.; Palm, B.; Wennberg, M.; Kaj, L.; Lindh, C.H.; Jönsson, B.A.; Berglund, M. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ. Int. 2014, 73, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Paulussen, M.; Geens, T.; Bruckers, L.; Baeyens, W.; David, F.; Dumont, E.; Loots, I.; Morrens, B.; de Bellevaux, B.N.; et al. Biomarkers of human exposure to personal care products: Results from the Flemish Environment and Health Study (FLEHS 2007–2011). Sci. Total Environ. 2013, 463, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Martina, C.A.; Weiss, B.; Swan, S.H. Lifestyle behaviors associated with exposures to endocrine disruptors. Neurotoxicology 2012, 33, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Palmieri, R.T.; Matuszewski, J.M.; Herring, A.H.; Baird, D.D.; Hartmann, K.E.; Hoppin, J.A. Consumer product exposures associated with urinary phthalate levels in pregnant women. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, E.; Hagopian, A.; Hoffman, K.; Butt, C.M.; Lorenzo, A.; Congleton, J.; Webster, T.F.; Stapleton, H.M. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 2015, 86, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lemini, C.; Jaimez, R.; Avila, M.E.; Franco, Y.; Larrea, F.; Lemus, A.E. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol. Ind. Health 2003, 19, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Wilson, V.S.; Furr, J.; Lambright, C.R.; Rider, C.V.; Blystone, C.R.; Hotchkiss, A.K.; Gray, L.E. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008, 105, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Axelstad, M.; Boberg, J.; Vinggaard, A.M.; Christiansen, S.; Hass, U. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem. Toxicol. 2013, 59, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, K.; Choi, K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat. Toxicol. 2012, 114, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, R.H.; Sonneveld, E.; Jansen, J.H.; Seinen, W.; van der Burg, B. Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci. 2005, 83, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharmacol. 2005, 203, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Sathyanarayana, S.; Barrett, E.S.; Janssen, S.; Liu, F.; Nguyen, R.H.N.; Redmon, J.B.; TIDES Study Team. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 2015, 30, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Vendittelli, F.; Sauvant-Rochat, M.P. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015, 83, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Stapleton, H.M. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 2010, 118, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Kannan, K.; Sapra, K.J.; Maisog, J.; Sundaram, R. Urinary concentrations of benzophenone-type ultraviolet radiation filters and couples’ fecundity. Am. J. Epidemiol. 2014, 180, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Chen, Z.; Buck Louis, G.M.; Sundaram, R.; Hediger, M.L.; Sun, L.; Kannan, K. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012, 46, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Female exposure to phenols and phthalates and time to pregnancy: The Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil. Steril. 2015, 103, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.H.; Dudzina, T.; Delmaar, C.J.E.; Bakker, M.I.; Russel, F.G.M.; von Goetz, N.; Scheepers, P.T.; Roeleveld, N. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem. Toxicol. 2013, 55, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Manová, E.; von Goetz, N.; Keller, C.; Siegrist, M.; Hungerbühler, K. Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int. J. Environ. Res. Public Health 2013, 10, 2778–2798. [Google Scholar] [CrossRef] [PubMed]
- Just, A.C.; Whyatt, R.M.; Perzanowski, M.S.; Calafat, A.M.; Perera, F.P.; Goldstein, I.F.; Chen, Q.; Rundle, A.G.; Miller, R.L. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ. Health Perspect. 2012, 120, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Fisher, M.; Neisa, A.; MacKinnon, L.; Kuchta, S.; MacPherson, S.; Probert, A.; Arbuckle, T.E. Personal Care Product Use in Pregnancy and the Postpartum Period: Implications for Exposure Assessment. Int. J. Environ. Res. Public Health 2016, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Sathyanarayana, S.; Janssen, S.; Redmon, J.B.; Nguyen, R.H.N.; Kobrosly, R.; Swan, S.H.; TIDES Study Team. Environmental health attitudes and behaviors: Findings from a large pregnancy cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Bennett, D.H.; Ritz, B.; Cassady, D.L.; Lee, K.; Hertz-Picciotto, I. Usage pattern of personal care products in California households. Food Chem. Toxicol. 2010, 48, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Sellier, S.; Houivet, E.; Marpeau, L.; Fournet, P.; Thobois, B.; Bénichou, J.; Joly, P. Incidence and risk factors for striae gravidarum. J. Am. Acad. Dermatol. 2015, 73, 699–700. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). Report on Carcinogens, Thirteenth Edition. Formaldehyde, 2014. Available online: http://ntp.niehs.nih.gov/ntp/roc/content/profiles/formaldehyde.pdf (accessed on 7 December 2015).
- National Toxicology Program (NTP). Report on Carcinogens, Thirteenth Edition. 1,4-Dioxan, 2014. Available online: http://ntp.niehs.nih.gov/ntp/roc/content/profiles/dioxane.pdf (accessed on 7 December 2015).
- Møller, P.; Wallin, H. Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene. Mutat. Res. 2000, 462, 13–30. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 884/2007 of 26 July 2007 on Emergency Measures Suspending the Use of E 128 Red 2G as Food Colour. Available online: http://eur-lex.europa.eu (accessed on 8 December 2015).
- Fernandez, M.F.; Olmos, B.; Granada, A.; López-Espinosa, M.J.; Molina-Molina, J.M.; Fernandez, J.M.; Cruz, M.; Olea-Serrano, F.; Olea, N. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: A nested case-control study. Environ. Health. Perspect. 2007, 115, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cabut, S. Automédication et Critères de Choix des Cosmétiques et des Produits D’hygiène Corporelle chez les Femmes Enceintes. Ph.D. Thesis, The University of Auvergne, Clermont-Ferrand, France, 5 October 2015. [Google Scholar]
- Ashley, J.M.; Hodgson, A.; Sharma, S.; Nisker, J. Pregnant women’s navigation of information on everyday household chemicals: Phthalates as a case study. BMC Pregnancy Childbirth 2015, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- République Française. Loi no 2009-879 du 21 Juillet 2009 Portant Réforme de L’hôpital et Relative Aux Patients, à la Santé et Aux Territoires, Article 38. Available online: http://legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000020879475&categorieLien=id (accessed on 19 February 2016).
- République Française. Code de la Santé Publique, Article R4235–2. Available online: http://www.legifrance.gouv.fr (accessed on 8 December 2015).
- République Française, Institut National de Prévention et D’éducation Pour la Santé (INPES). Guide de la Pollution de l’air Intérieur. Available online: http://www.inpes.sante.fr/30000/images/0904_air_interieur/Guide.pdf (accessed on 19 February 2016).
- Danish Ministry of the Environment. Expecting a Baby? Advice about Chemicals and Pregnancy. Available online: http://eng.mst.dk/media/mst/69080/Expecting%20a%20baby.pdf (accessed on 19 February 2016).
- Delmaar, C.; Bokkers, B.; ter Burg, W.; Schuur, G. Validation of an aggregate exposure model for substances in consumer products: A case study of diethyl phthalate in personal care products. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Korgavkar, K.; Wang, F. Stretch marks during pregnancy: A review of topical prevention. Br. J. Dermatol. 2015, 172, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Kermarrec, M. Enquête Nationale Périnatale 2010. Les Naissances en 2010 et Leur Evolution Depuis 2003. Institut National de la Santé et de la Recherche Médicale (INSERM): Paris, 2011. Available online: http://www.sante.gouv.fr/IMG/pdf/Les_naissances_en_2010_et leur_evolution_depuis_2003.pdf (accessed on 19 February 2016). [Google Scholar]
- Association des Utilisateurs de Dossiers Informatisés en Pédiatrie, Obstétrique et Gynécologie (Audipog). The Online Perinatal Database. Available online: http://www.audipog.net/interro-choix.php (accessed on 19 February 2016).
- Tourangeau, R.; Yan, T. Sensitive questions in surveys. Psychol. Bull. 2007, 133, 859–883. [Google Scholar] [CrossRef] [PubMed]


| Personal Information | Total N = 128 (%) (m ± SD) a | NPW Group N = 60 (%) (m ± SD) a | PW Group N = 68 (%) (m ± SD) a | p-Value |
|---|---|---|---|---|
| Age | n = 127 | n = 60 | n = 67 | - |
| in years | 30.5 ± 5.8 | 30.7 ± 7.4 | 30.2 ± 3.9 | 0.67 |
| Town of residence | n = 124 | n = 57 | n = 67 | - |
| ≤5000 inhabitants | 82 (66.1) | 30 (52.6) | 52 (77.6) | <0.01 |
| >5000 inhabitants | 42 (33.9) | 27 (47.4) | 15 (22.4) | - |
| Educational level | n = 127 | n = 59 | n = 68 | - |
| Junior school | 8 (6.3) | 5 (8.5) | 3 (4.4) | 0.70 |
| High school | 24 (18.9) | 11 (18.6) | 13 (19.1) | - |
| University level | 95 (74.8) | 43 (72.9) | 52 (76.5) | - |
| Socioprofessional category b | n = 128 | n = 60 | n = 68 | - |
| Executive and intellectual professions | 28 (21.9) | 13 (21.7) | 15 (22.1) | 1.00 |
| Intermediate professions c | 46 (35.9) | 21 (35.0) | 25 (36.8) | - |
| Employees | 40 (31.3) | 19 (31.7) | 21 (30.9) | - |
| Others d | 4 (3.1) | 2 (3.3) | 2 (2.9) | - |
| No occupation | 10 (7.8) | 5 (8.3) | 5 (7.4) | - |
| Previous pregnancy | n = 127 | n = 60 | n = 67 | - |
| ≥1 | 65 (51.2) | 32 (53.3) | 33 (49.3) | 0.78 |
| Chronic treatment e | n = 128 | n = 60 | n = 68 | - |
| Outside of pregnancy | 19 (14.8) | 13 (21.7) | 6 (8.8) | 0.05 |
| Personal Care Product | Total n = 128 (%) | NPW Group n = 60 (%) | PW Group n = 68 (%) | p-Value |
|---|---|---|---|---|
| General hygiene | ||||
| Shower gel | 120 (93.7) | 54 (90.0) | 66 (97.1) | 0.15 |
| Solid soap | 45 (32.5) | 24 (40.0) | 21 (30.9) | 0.37 |
| Dermatological soap a | 22 (17.5) | 15 (25.9) | 7 (10.3) | 0.03 |
| Intimate hygiene product a | 58 (46.0) | 27 (45.8) | 31 (46.3) | 1 |
| Body scrub | 51 (39.8) | 25 (41.7) | 26 (38.2) | 0.83 |
| Body lotion | 92 (71.9) | 46 (76.7) | 47 (69.1) | 0.45 |
| Deodorant b | 117 (92.1) | 55 (91.7) | 62 (92.5) | 1 |
| Perfume b | 116 (91.3) | 56 (93.3) | 61 (91.0) | 0.75 |
| Bronzers | 1 (0.8) | 0 | 1 (1.5) | |
| Face hygiene | ||||
| Facial cleanser a | 77 (60.6) | 39 (66.1) | 38 (55.9) | 0.32 |
| Day face cream | 105 (82.0) | 52 (86.7) | 53 (77.9) | 0.25 |
| Night face cream | 24 (18.7) | 12 (20.0) | 12 (17.7) | 0.91 |
| Facial scrub | 56 (43.7) | 30 (50.0) | 26 (38.2) | 0.25 |
| Facial mask | 45 (35.2) | 21 (35.0) | 24 (35.3) | 1 |
| Hair hygiene | ||||
| Shampoo | 126 (98.4) | 59 (98.3) | 67 (98.5) | 1 |
| Hair mask | 55 (43.0) | 22 (36.7) | 33 (48.5) | 0.24 |
| Hair dye a | 43 (34.1) | 18 (30.5) | 25 (37.3) | 0.55 |
| Make-Up Product | Total n = 128 (%) | NPW Group n = 60 (%) | PW Group n = 68 (%) | p-Value |
|---|---|---|---|---|
| Make-up foundation | 74 (57.8) | 32 (53.3) | 42 (61.8) | 0.43 |
| Blush | 36 (28.1) | 19 (31.7) | 17 (25.0) | 0.52 |
| Mascara | 110 (85.9) | 52 (86.7) | 58 (85.3) | 1 |
| Eye-liner | 43 (33.6) | 23 (38.3) | 20 (29.4) | 0.35 |
| Eye pencil | 93 (72.7) | 41 (68.3) | 52 (76.5) | 0.41 |
| Eye shadow | 88 (68.7) | 41 (68.3) | 47 (69.1) | 1 |
| Lipstick | 58 (45.3) | 25 (41.7) | 33 (48.5) | 0.55 |
| Lip pencil a | 12 (9.4) | 6 (10.2) | 7 (10.5) | 1 |
| Make-up remover | 104 (81.3) | 51 (85.0) | 53 (77.9) | 0.38 |
| Nail polish | 96 (75.0) | 41 (68.3) | 55 (80.9) | 0.15 |
| Nail polish remover b | 92 (73.6) | 41 (68.3) | 51 (78.5) | 0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marie, C.; Cabut, S.; Vendittelli, F.; Sauvant-Rochat, M.-P. Changes in Cosmetics Use during Pregnancy and Risk Perception by Women. Int. J. Environ. Res. Public Health 2016, 13, 383. https://doi.org/10.3390/ijerph13040383
Marie C, Cabut S, Vendittelli F, Sauvant-Rochat M-P. Changes in Cosmetics Use during Pregnancy and Risk Perception by Women. International Journal of Environmental Research and Public Health. 2016; 13(4):383. https://doi.org/10.3390/ijerph13040383
Chicago/Turabian StyleMarie, Cécile, Sophie Cabut, Françoise Vendittelli, and Marie-Pierre Sauvant-Rochat. 2016. "Changes in Cosmetics Use during Pregnancy and Risk Perception by Women" International Journal of Environmental Research and Public Health 13, no. 4: 383. https://doi.org/10.3390/ijerph13040383






