Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
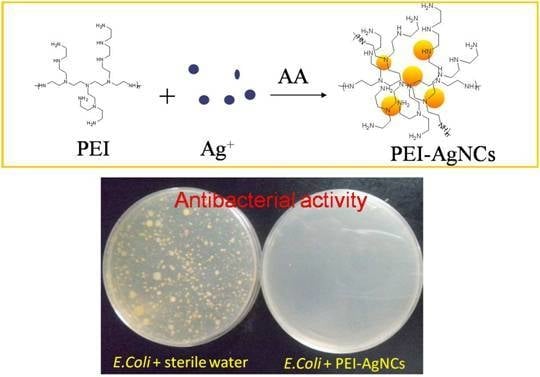
2.2. Syntheses of PEI-AgNCs and AgNPs
2.3. Antibacterial Tests
3. Results
Preparation of PEI-AgNCs and AgNPs and Their Antibacterial Properties.
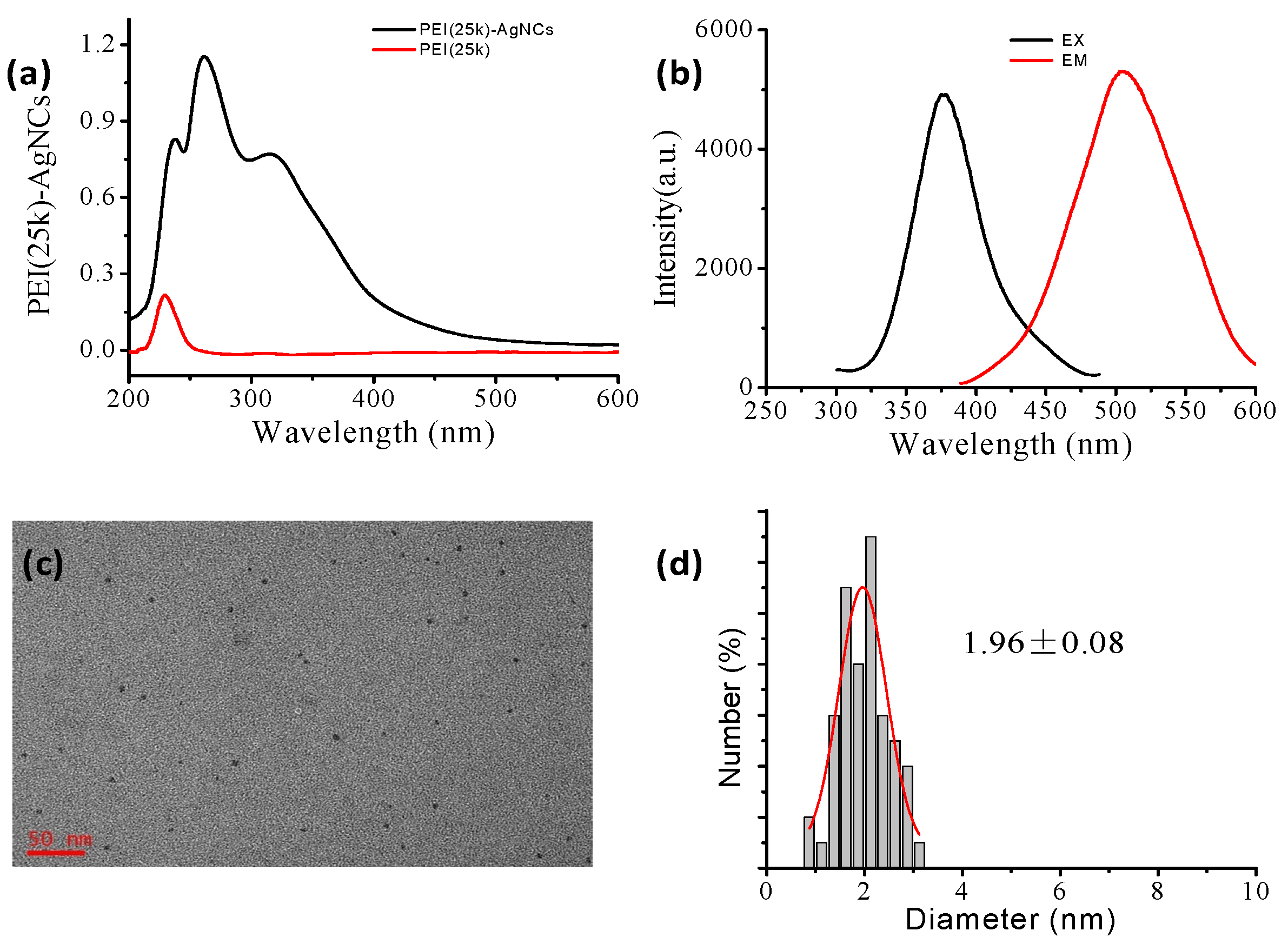
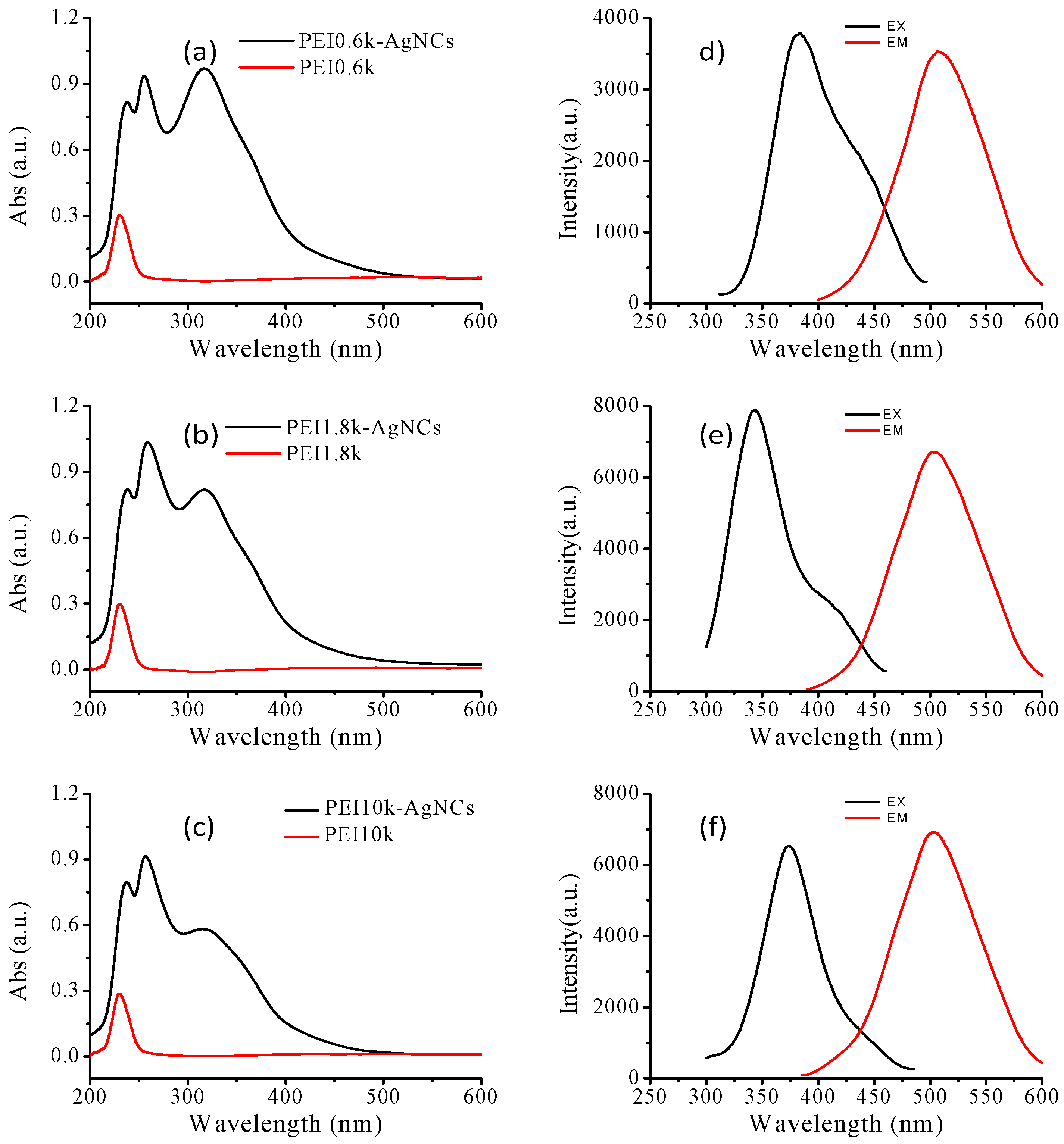
Syntheses and Characterization of PEI- AgNCs and AgNPs
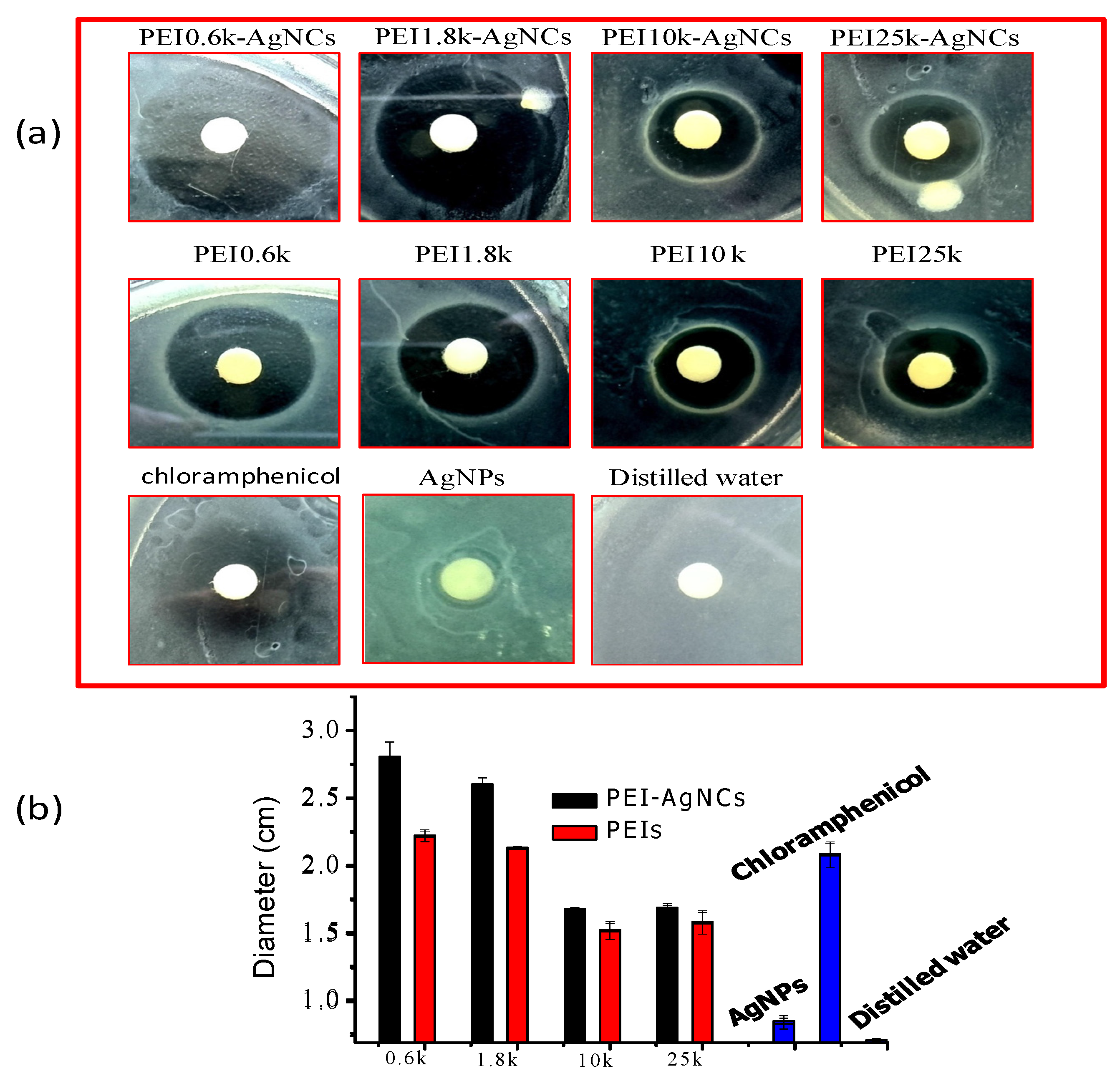
The Assessment of PEI-AgNCs Antibacterial Activity
4. Discussion
4.1. The Formation of PEI-AgNCs
4.2. Antibacterial Properties of PEI-AgNCs
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNC | Silver nanocluster |
| AgNPs | Silver nanoparticles |
| HRTEM | High resolution transmission electron microscopy |
| LB | Luria Bertani |
| MICs | Minimum inhibitory concentrations |
| PEI | Polyethyleneimine |
| PEI-AgNCs | Polyethyleneimine capped silver nanoclusters |
| PVP | Polyvinylpyrrolidone |
| EX/EM | Excitation/emission wavelength |
References
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J.; Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S. Antimicrobials: Reactive resistance. Nat. Rev. Microbiol. 2010, 8, 248–248. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Wiener, J. Antibacterial agents in textile industry. In Antimicrobial Agents; Bobbarala, V., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 387–406. [Google Scholar]
- Daschner, F.D.; Ruden, H.; Simon, R.; Clotten, J. Microbiological contamination of drinking water in a commercial household water filter system. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fafliora, E.; Bampalis, V.G.; Lazarou, N.; Mantzouranis, G.; Anastassiou, E.D.; Spiliopoulou, I.; Christofidou, M. Bacterial contamination of medical devices in a Greek emergency department: Impact of physicians’ cleaning habits. Am. J. Infect. Control 2014, 42, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.; Orlewska, K.; Kępa, M.; Idzik, D.; Dziedzic, A.; Mularz, T.; Krawczyk, M.; Miklasińska, M.; Wąsik, T. Biofilm formation and antimicrobial susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int. J. Environ. Res. Public Health 2014, 11, 4619–4633. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: nano-antimicrobial materials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 2014, 246012–246012. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interfac. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Le, A.-T. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of size-dependent antimicrobial and cytotoxic properties of silver nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Díez, I.; Ras, R.H. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, Y.-C.; Li, H.-W.; Chang, H.-T. Fluorescent silver nanoclusters stabilized by DNA scaffolds. Chem. Commun. 2014, 50, 9800–9815. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.J.; Lee, C.-H.; Hsieh, J.-T.; Wang, H.-H.; Li, J.K.; Shen, J.-L.; Chan, W.-H.; Yeh, H.-I.; Chang, W.H. Synthesis of fluorescent metallic nanoclusters toward biomedical application: Recent progress and present challenges. J. Med. Biol. Eng. 2009, 29, 276–283. [Google Scholar]
- Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A. Polyethylenimine in medicinal chemistry. Curr. Med. Chem. 2008, 15, 2826–2839. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Chen, T.; Riffon, R.; Wang, R.; Wang, Z. Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Haldar, J.; An, D.; de Cienfuegos, L.Á.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Pilo, R.; Weiss, E.I. Antibacterial activity of dental cements containing quaternary ammonium polyethylenimine nanoparticles. J. Nanomater. 2012, 2012, 58. [Google Scholar] [CrossRef]
- Shvero, D.K.; Davidi, M.P.; Weiss, E.I.; Srerer, N.; Beyth, N. Antibacterial effect of polyethyleneimine nanoparticles incorporated in provisional cements against Streptococcus mutans. J. Biomed. Mater. Res. 2010, 94, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Jose Ruben, M.; Jose Luis, E.; Alejandra, C.; Katherine, H.; Juan, B.K.; Jose Tapia, R.; Miguel Jose, Y. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar]
- Lee, H.J.; Lee, S.G.; Oh, E.J.; Chung, H.Y.; Han, S.I.; Kim, E.J.; Seo, S.Y.; Ghim, H.D.; Yeum, J.H.; Choi, J.H. Antimicrobial polyethyleneimine-silver nanoparticles in a stable colloidal dispersion. Colloids Surf. B 2011, 88, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Li, N.B.; Luo, H.Q. Polyethyleneimine-templated Ag nanoclusters: A new fluorescent and colorimetric platform for sensitive and selective sensing halide ions and high disturbance-tolerant recognitions of iodide and bromide in coexistence with chloride under condition of high ionic strength. Anal. Chem. 2012, 84, 10373–10379. [Google Scholar] [PubMed]
- Yuan, Z.; Cai, N.; Du, Y.; He, Y.; Yeung, E.S. Sensitive and selective detection of copper ions with highly stable polyethyleneimine-protected silver nanoclusters. Anal. Chem. 2013, 86, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Li, N.B.; Luo, H.Q. Highly sensitive fluorescent and colorimetric pH sensor based on polyethylenimine-capped silver nanoclusters. Langmuir 2013, 29, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.Y.; Li, H.F.; Gao, Z.F.; Qu, F.; Li, N.B.; Luo, H.Q. Utilizing polyethyleneimine-capped silver nanoclusters as a new fluorescence probe for Sudan I–IV sensing in ethanol based on fluorescence resonance energy transfer. Sens. Actuators B 2014, 193, 730–736. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. Polyethyleneimine-capped silver nanoclusters as a fluorescence probe for sensitive detection of hydrogen peroxide and glucose. Anal. Chim. Acta 2012, 749, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mavani, K.; Shah, M. Synthesis of silver nanoparticles by using sodium borohydride as a reducing agent. Int. J. Eng. Res. Technol. 2013, 2, 3. [Google Scholar]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobicall; APPROVED Standard—Ninth Edition. Available online: http://120.52.72.40/antimicrobianos.com.ar/c3pr90ntcsf0/ATB/wp-content/uploads/2012/11/03-CLSI-M07-A9-2012.pdf (accessed on 14 March 2016).
- Seow, W.Y.; Liang, K.; Kurisawa, M.; Hauser, C.A. Oxidation as a facile strategy to reduce the surface charge and toxicity of polyethyleneimine gene carriers. Biomacromolecules 2013, 14, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Gibney, K.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Peng, Q.; Chen, F.; Zhong, Z.; Zhuo, R. Low molecular weight polyethylenimine conjugated gold nanoparticles as efficient gene vectors. Bioconjugate Chem. 2010, 21, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Erol, M.; Attygalle, A.; Du, H.; Sukhishvili, S. Synthesis of positively charged silver nanoparticles via photoreduction of Agno3 in branched polyethyleneimine/hepes solutions. Langmuir 2007, 23, 9836–9843. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ali, A. Synthesis of nanostructured cadmium and zinc sulfides in aqueous solutions of hyperbranched polyethyleneimine. J. Cryst. Growth 2008, 310, 5252–5258. [Google Scholar] [CrossRef]
- Mock, J.; Barbic, M.; Smith, D.; Schultz, D.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Wang, Q.; Yang, T.; Cao, J.; Lin, Q.; Yuan, Z.; Li, L. Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents. Int. J. Environ. Res. Public Health 2016, 13, 334. https://doi.org/10.3390/ijerph13030334
Xu D, Wang Q, Yang T, Cao J, Lin Q, Yuan Z, Li L. Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents. International Journal of Environmental Research and Public Health. 2016; 13(3):334. https://doi.org/10.3390/ijerph13030334
Chicago/Turabian StyleXu, Dong, Qingyun Wang, Tao Yang, Jianzhong Cao, Qinlu Lin, Zhiqin Yuan, and Le Li. 2016. "Polyethyleneimine Capped Silver Nanoclusters as Efficient Antibacterial Agents" International Journal of Environmental Research and Public Health 13, no. 3: 334. https://doi.org/10.3390/ijerph13030334