Fungi from a Groundwater-Fed Drinking Water Supply System in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Water Treatment System
2.2. Water Supplies
2.3. Sampling Sites
2.4. Samples Collection
2.5. Physical-Chemical Analysis of Water
2.6. Mycological Analysis
2.6.1. Fungal Quantification
2.6.2. Isolation
2.6.3. Identification
3. Results and Discussion
3.1. Physical-Chemical Analysis
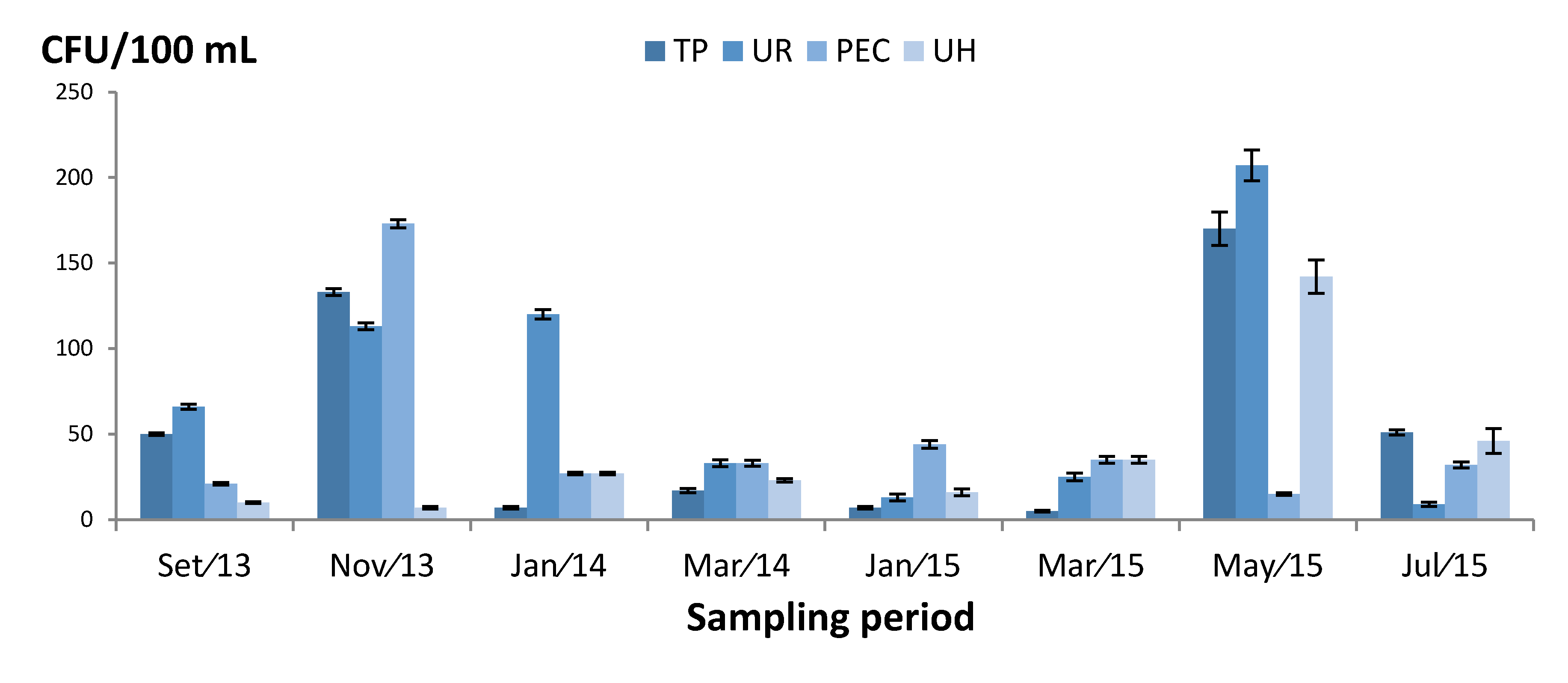
3.2. Quantification of Fungi
3.3. Fungal Identification
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagy, L.A.; Olson, B.H. Occurrence and significance of bacteria, fungi and yeasts associated with distribution pipe surfaces. In Proceedings of the American Water Works Association, Water Quality Technology Conference, Houston, TX, USA, December 1985; American Water Works Association: Denver, CO, USA, 1985; pp. 213–238. [Google Scholar]
- Franková, E.; Horecka, M. Filamentous soil fungi and unidentified bacteria in drinking water from wells and water mains near Bratislava. Microbiol. Res. 1995, 150, 311–313. [Google Scholar] [CrossRef]
- Kinsey, G.C.; Paterson, R.R.; Kelley, J. Methods for the determination of filamentous fungi in treated and untreated waters. J. Appl. Microbiol. Symp. Suppl. 1999, 85, 214S–224S. [Google Scholar] [CrossRef] [PubMed]
- Göttlich, E.; van der Lubbe, W.; Lange, B.; Fiedler, S.; Melchert, I.; Reifenrath, M.; Flemming, H.C.; de Hoog, S. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health 2002, 205, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.; Kinsey, G.; Paterson, R.; Brayford, D. Identification and Control of Fungi in Distribution Systems; AWWA Research Foundation and American Water Works Association: Denver, CO, USA, 2003. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Fungal contamination of drinking water. In Water Encyclopedia; Lehr, J., Keeley, J., Lehr, J., Kingery, T.B., III, Eds.; John Wiley & Sons: New York, NY, USA, 2005; pp. 1–7. [Google Scholar]
- Gonçalves, A.B.; Paterson, R.R.M.; Lima, N. Survey and significance of filamentous fungi from tap water. Int. J. Hyg. Environ. Health 2006, 209, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hageskal, G.; Knutsen, A.K.; Gaustad, P.; de Hoog, G.S.; Skaar, I. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol. 2006, 72, 7586–7593. [Google Scholar] [CrossRef] [PubMed]
- Hageskal, G.; Gaustad, P.; Heier, B.T.; Skaar, I. Occurrence of moulds in drinking water. J. Appl. Microbiol. 2007, 102, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Hageskal, G.; Lima, N.; Skaar, I. The study of fungi in drinking water. Mycol. Res. 2009, 113, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, V.J.; Basílio, M.C.; Fernandes, D.; Domingues, M.; Paiva, J.M.; Benoliel, M.J.; Crespo, M.T.; San Romão, M.V. Occurrence of filamentous fungi and yeasts in three different drinking water sources. Water Res. 2009, 43, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Fernandes, D.; Carvalho, G.; Benoliel, M.J.; San Romão, M.V.; Barreto Crespo, M.T. Assessment of the presence and dynamics of fungi in drinking water sources using cultural and molecular methods. Water Res. 2010, 44, 4850–4859. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, V.M.; Oliveira, H.M.; Santos, C.; Paterson, R.; Gusmão, N.; Lima, N. Filamentous fungi in drinking water, particularly in relation to biofilm formation. Int. J. Environ. Res. Public. Health 2011, 8, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, B.R.; Barreto Crespo, M.T.; San Romão, M.V.; Benoliel, M.J.; Samson, R.A.; Pereira, V.J. New insights concerning the occurrence of fungi in water sources and their potential pathogenicity. Water Res. 2013, 47, 6338–6347. [Google Scholar] [CrossRef] [PubMed]
- Skaar, I.; Hageskal, G. Fungi in drinking water. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 597–606. [Google Scholar]
- Kinsey, G.; Paterson, R.; Kelley, J. Filamentous fungi in water systems. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N., Eds.; Academic Press: London, UK, 2003; pp. 77–98. [Google Scholar]
- Grabinska-Loniewska, A.; Konillowicz-Kowalska, T.; Wardzynska, G.; Boryn, K. Occurrence of fungi in water distribution system. Pol. J. Environ. Stud. 2007, 16, 539–547. [Google Scholar]
- Hussain, T.; Ishtiaq, C.M.; Hussain, A.; Mahmood, T.; Sultana, K.; Ashraf, M. Incidence of fungi in water springs of Samahni Valley, District Bhiimber, Azad Kashmir, Parkistan. Int. J. Biol. 2010, 2, 94–101. [Google Scholar]
- Paterson, R.R.M.; Hageskal, G.; Skaar, I.; Lima, N. Occurrence, problems, analysis and removal of filamentous fungi in drinking water. Fungicides: Chemistry, Environmental Impact and Health Effects, DeCosta, P., Bezerra, P., Eds.; Nova Biomedical Books: New York, NY, USA, 2009; 379–399. [Google Scholar]
- Del Águila de la Puente, C.; Rodríguez, S.F.; Henriques-Gil, N. Encephalitozoon. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 267–291. [Google Scholar]
- Matos, O.; Lobo, M.L. Enterocytozoon. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 293–321. [Google Scholar]
- Sammon, N.B.; Harrower, K.M.; Fabbro, L.D.; Reed, R.H. Incidence and distribution of microfungi in a treated municipal water supply system in sub-tropical Australia. Int. J. Environ. Res. Public Health 2010, 7, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Health Risk from Microbial Growth and Biofilms in Drinking Water Distribution Systems. Available online: http://www.epa.gov/sites/production/files/2015-09/documents/2007_05_18_disinfection_tcr_whitepaper_tcr_biofilms.pdf (accessed on 9 February 2016).
- Besner, M.-C.; Prévost, M.; Regli, S. Assessing the public health risk of microbial intrusion events in distribution systems: Conceptual model, available data, and challenges. Water Res. 2011, 45, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.J.; Kuchar, R.T.; Rex, J.H.; Francesconi, A.; Kasai, M.; Muller, F.M.; Walsh, T.J. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: A new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Dis. 2001, 33, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Gaustad, P.; Meis, J.F.G.M.; Voss, A.; Verweij, P.E.; Abrahamsen, T.G. Recovery of filamentous fungi from water in a paediatric bone marrow transplantation unit. J. Hosp. Infect. 2001, 47, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.D.; Richardson, R. Aspergillus and aspergillosis. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 151–164. [Google Scholar]
- World Health Organization, Guidelines for Drinking-Water Quality, 4th ed.; WHO Library Cataloguing in Publication Data: Geneva, Switzerland, 2011.
- DEFRA, Review of Fungi in Drinking Water and the Implications for Human Health. Available online: http://dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-255.pdf (accessed on 9 February 2016).
- Paterson, R.R.M.; Lima, N. Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Food Microbiology Series ; CRC Press: Boca Rotan, FL, USA, 2015; pp. 1–618. [Google Scholar]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- APHA-American Public Health Association; American Water Works Association (AWWA); Water Environmental Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association; American Water Works Association; Water Environmental Federation: Washington, DC, USA, 2012. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Volume II, Blackie Academic and Professional: London, UK, 1997. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 3rd ed.; Burgess Publishing Co.: Minneapolis, MN, USA, 1972; pp. 1–273. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Introduction to Food and Airborne Fungi, 7th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2004. [Google Scholar]
- Portaria n° 2914 de 12/2011. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html (accessed on 11 February 2016).
- Barcelona, M.J. TOC determinations in ground water. Grand Water 1984, 22, 18–24. [Google Scholar] [CrossRef]
- Samah, A.M.; Shimaa, R.H.; Raed, R.A.-W. Relative diversity of filamentous fungi and yeasts in groundwater and their correlation to faecal pollution indicators and physicochemical parameters. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 905–919. [Google Scholar]
- Wingender, J.; Flemming, H.C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Environ. Res. Public Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.J.; Stratton, S.L.; Dignani, M.C.; Summerbell, R.C.; Rex, J.H.; Monson, T.P.; Spencer, T.; Kasai, M.; Francesconi, A.; Walsh, T.J. Pathogenic Aspergillus species recovered from a hospital water system: A 3-year prospective study. Clin. Infect. Dis. 2002, 24, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.D.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—State of the art: Cystic fibrosis foundation consensus conference. Clin. Infect. Dis. 2003, 37 (Suppl. 3), S225–S264. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- White, D.A. Aspergillus pulmonary infections in transplant recipients. Clin. Chest Med. 2005, 26, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M.; Kelley, J.; Gallagher, M. Natural occurrence of aflatoxin and Aspergillus flavus (Link) in water. Lett. Appl. Microbiol. 1997, 25, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M.; Lima, N. Penicillium—Mycosis and mycotoxicosis. In Molecular Detection of Human Fungal Pathogens; Liu, D., Ed.; CRC Press: Boca Rotan, FL, USA, 2011; pp. 323–337. [Google Scholar]
- Ma, X.; Baron, J.L.; Vikram, A.; Stout, J.E.; Bibby, K. Fungal diversity and presence of potentially pathogenic fungi in a hospital hot water system treated with on-site monochloramine. Water Res. 2015, 71, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Galgóczy, L.; Selvam, K.P.; Shobana, C.N.; Kocsubé, S.; Vágvölgyi, P.; Narendran, V.; Kredics, L. Fusarium . In Molecular Detection of Human Fungal Pathogens; Liu, D., Ed.; CRC Press: Boca Rotan, FL, USA, 2011; pp. 417–433. [Google Scholar]
- De Lucca, A.; Walsh, T.J. Mycotoxins of Fusarium spp. Biochemistry and toxicology. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 323–353. [Google Scholar]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Grant, G.B.; O’Donnell, K.; Wannemuehler, K.A.; Noble-Wang, J.; Rao, C.Y.; Jacobson, L.M.; Crowell, C.S.; Sneed, R.S.; Lewis, F.M.T.; et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 2006, 296, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Short, P.G.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geiser, D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Sutton, A.D.; Cano-Lira, J.F.; Gené, J.; Fothergill, A.W.; Wiederhold, N.P.; Guarro, J. Phylogeny of the clinically relevant species of the emerging fungus Trichoderma and their antifungal susceptibilities. J. Clin. Microbiol. 2014, 52, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Druzhinina, I.S. Trichoderma mycoses and mycotoxins. In Molecular Biology of Food and Water Born Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; Food Microbiology Series; CRC Press: Boca Rotan, FL, USA, 2015; pp. 521–537. [Google Scholar]
- Webster, J.; Weber, R.W.S. Introduction to Fungi, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
| Parameters | TP | UR | PEC | UH | ||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | |
| Residual chlorine (mg/L) | - | - | 0.0 * | 1.5 * | 0.0 | 0.0 | 0.0 * | 1.0 * |
| Temperature (°C) | 26.4 | 28.0 | 26.5 | 27.6 | 26.6 | 27.2 | 26.3 | 27.5 |
| pH | 5.0 | 6.6 | 4.1 | 6.4 | 5.1 | 7.3 | 5.6 | 6.7 |
| Turbidity (UT) | 5.7 | 13.0 | 7.5 | 13.6 | 7.9 | 16.5 | 5.2 | 8.3 |
| Conductivity (mS/cm) | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Dissolved oxygen (mg/L) | 5.4 | 12.3 | 9.2 | 13.2 | 8.1 | 12.6 | 8.2 | 18.2 |
| Total organic carbon (mgC/L) | 2.8 | 3.6 | 2.8 | 4.2 | 2.9 | 4.2 | 3.7 | 4.0 |
| Fungi | TP | UR | PEC | UH |
|---|---|---|---|---|
| Relative frequency (%) | ||||
| Aspergillus alliaceus | 0.2 | 0.1 | 0.6 | |
| Aspergillus chevalieri | 0.1 | |||
| Aspergillus flavus | 7.3 | 0.1 | 3.4 | 2.7 |
| Aspergillus fumigatus | 0.2 | 0.2 | 0.2 | |
| Aspergillus neoniveus | 0.2 | |||
| Aspergillus niger complex | 5.5 | 1.4 | 1.3 | 0.8 |
| Aspergillus violaceofuscus | 0.2 | |||
| Aspergillus parasiticus | 1.7 | 1.6 | 1.9 | 0.7 |
| Aspergillus terreus | 0.6 | 0.4 | 1.4 | |
| Aspergillus versicolor | 0.2 | 3.3 | 0.6 | 0.7 |
| Penicillium citrinum | 6.8 | 5.7 | 2.7 | 5.2 |
| Penicillium corylophilum | 0.2 | |||
| Penicillium janczewskii | 0.9 | 0.5 | ||
| Penicillium janthinellum | 0.1 | |||
| Penicillium oxalicum | 0.1 | 1.0 | 0.8 | |
| Penicillium waksmanii | 0.1 | 1.0 | ||
| Acremonium sp. | 0.4 | 1.0 | ||
| Chaetomium sp. | 0.2 | |||
| Cladosporium cladosporioides | 0.9 | 0.1 | ||
| Cladosporium macrocarpum | 0.4 | |||
| Colletotrichum sp. | 0.1 | |||
| Cunninghamella sp. | 0.9 | 0.2 | ||
| Curvularia pallescens | 1.3 | 2.0 | 0.2 | 1.8 |
| Fusarium solani | 0.7 | 2.2 | 1.4 | 4.5 |
| Humicola grisea | 0.6 | |||
| Humicola fuscoatra | 0.1 | |||
| Leptodontium sp. | 0.4 | 0.6 | ||
| Lichtheimia hyalospora | 0.2 | |||
| Myrothecium sp. | 0.1 | |||
| Paecilomyces aerugineus | 0.1 | |||
| Paecilomyces variotii | 0.4 | |||
| Pestalotiopsis karstenii | 1.0 | 0.1 | 0.7 | 0.5 |
| Phaeoacremonium sp. | 0.2 | 0.1 | ||
| Phialophora richardsiae | 0.1 | |||
| Phoma leveillei | 0.1 | |||
| Ramichloridium matsushimae | 1.0 | 0.1 | ||
| Scolecobasidium humicola | 0.1 | |||
| Talaromyces purpurogenus | 0.1 | |||
| Trichoderma aureoviride | 0.7 | |||
| Trichoderma harzianum | 0.6 | 2.6 | 1.0 | 3.1 |
| Trichoderma viride | 1.4 | |||
| Verticillium sp. | 0.4 | |||
| Unidentified arthrosporic fungi | 0.7 | 0.7 | 0.2 | 0.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, H.M.B.; Santos, C.; Paterson, R.R.M.; Gusmão, N.B.; Lima, N. Fungi from a Groundwater-Fed Drinking Water Supply System in Brazil. Int. J. Environ. Res. Public Health 2016, 13, 304. https://doi.org/10.3390/ijerph13030304
Oliveira HMB, Santos C, Paterson RRM, Gusmão NB, Lima N. Fungi from a Groundwater-Fed Drinking Water Supply System in Brazil. International Journal of Environmental Research and Public Health. 2016; 13(3):304. https://doi.org/10.3390/ijerph13030304
Chicago/Turabian StyleOliveira, Helena M.B., Cledir Santos, R. Russell M. Paterson, Norma B. Gusmão, and Nelson Lima. 2016. "Fungi from a Groundwater-Fed Drinking Water Supply System in Brazil" International Journal of Environmental Research and Public Health 13, no. 3: 304. https://doi.org/10.3390/ijerph13030304








