Diabetes Mellitus, ArterialWall, and Cardiovascular Risk Assessment
Abstract
:1. Introduction
2. Hyperglycemia
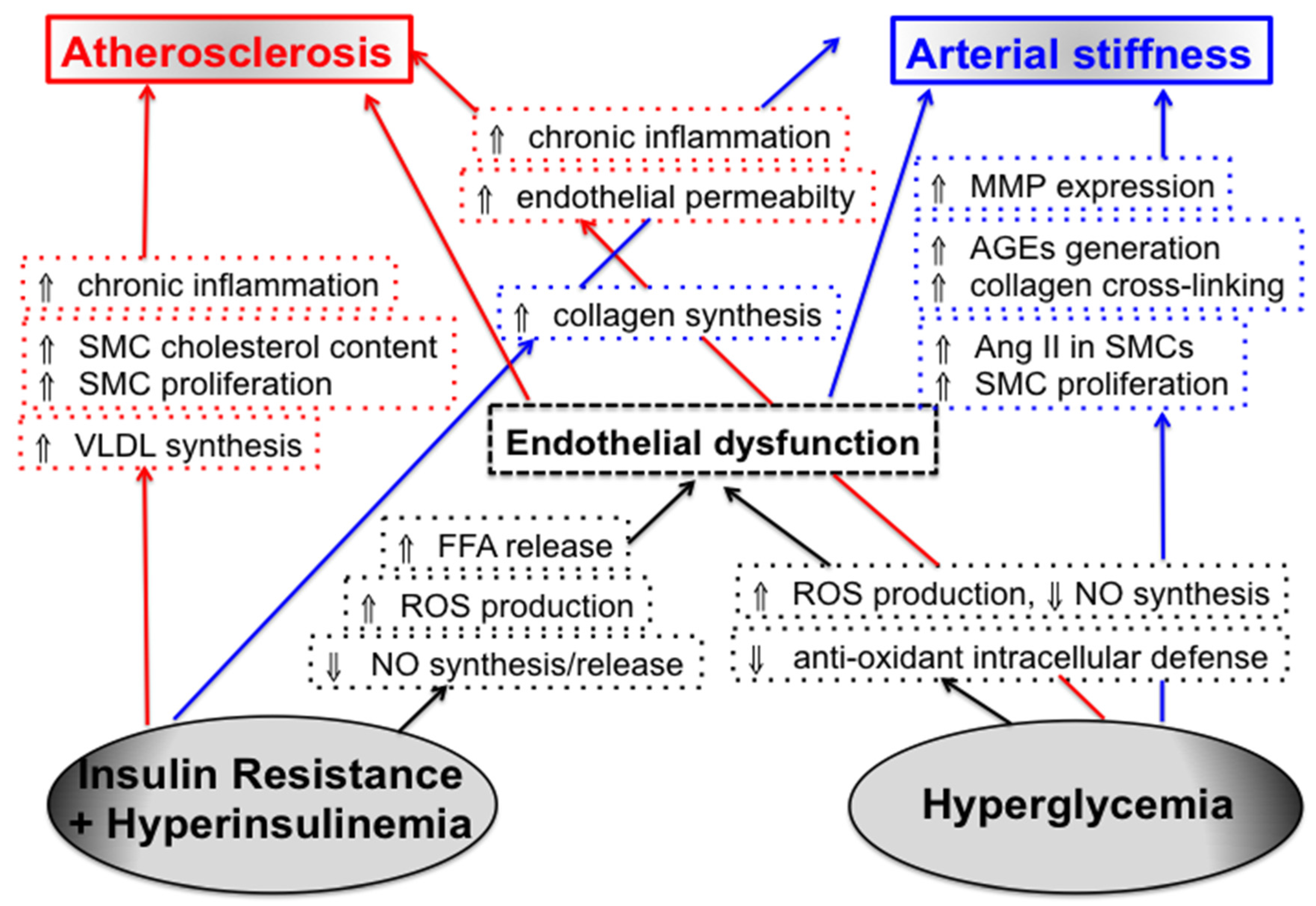
3. Insulin Resistance
4. Vascular Biomarkers
5. Endothelial Function
6. CIMT and Plaque Presence
7. Arterial Stiffness
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular risk factors: The Framingham study. Circulation 1979, 59, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.L.; Kapral, M.K.; Fung, K.; Tu, J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet 2006, 368, 29–36. [Google Scholar] [CrossRef]
- Whiteley, L.; Padmanabhan, S.; Hole, D.; Isles, C. Should diabetes be considered a coronary heart disease risk equivalent? Results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care 2005, 28, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.A.; Lopes-Virella, M.; Halushka, P.V. Pathogenesis of atherosclerosis in diabetes mellitus. Diabetes Care 1981, 4, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, C.D.; Henry, R.M.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Stratton, L.M.; Adler, A.J.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bonfeld, K.E.; Tabas, I. Insulin resistance, hyperglycemia and atherosclerosis. Cell Metabolism 2011, 14, 575–585. [Google Scholar]
- DeFronzo, R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, G.; Yuan, Z.; Chen, L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2012, 7, e42551. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Scirica, B.M.; Raz, I.; Steg, P.G.; McGuire, D.K.; Leiter, L.A.; Hirshberg, B.; Davidson, J.; Cahn, A.; Mosenzon, O.; et al. Cardiovascular outcomes of patients in SAVOR-TIMI 53 by baseline hemoglobin A1c. Am. J. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Paul, S.J.; Bethel, M.A.; Matthews, D.R.; Neil, A.W. 10-Year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Dluhy, R.G.; McHanon, G.T. Intensive glycemic control in the ACCORD and ADVANCE trials. N. Engl. J. Med. 2008, 358, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Vedel, P.; Larsen, N.; Jensen, G.V.; Parving, H.H.; Pedersen, O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Borges, M.; David, C.; Vaz Carneiro, A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: Meta-analysis of randomised controlled trials. BMJ 2006, 332, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998, 317, 703–713. [Google Scholar]
- Hansson, L.; Zanchetti, A.; Carruthers, S.G.; Dahlöf, B.; Elmfeldt, D.; Julius, S.; Ménard, J.; Rahn, K.H.; Wedel, H.; Westerling, S.; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998, 351, 1755–1762. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Monnier, V.M. Molecular basis of arterial stiffening: Role of glycation—A mini-review. Gerontology 2012, 58, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Death, A.K.; Fisher, E.J.; McGrath, K.C.; Yue, D.K. High glucose alters matrix metalloproteinase expression in two key vascular cells: Potential impact on atherosclerosis in diabetes. Atherosclerosis 2003, 168, 263–269. [Google Scholar] [CrossRef]
- Lavrentyev, E.N.; Estes, A.M.; Malik, K.U. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ. Res. 2007, 101, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wang, X.F.; Li, L.; Zhang, L.; Shen, D.L.; Li, D.H.; Jin, Q.S.; Zhang, J.Y. Effects of high glucose on human umbilical vein endothelial cell permeability and myosin light chain phosphorylation. Diabetol. Metab. Syndr. 2015, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Barbieri, M.; Marfella, R.; Paolisso, G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: Role of dipeptidyl peptidase-IV inhibition. Diabetes Care 2012, 35, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Kawamori, R.; Toyofuku, Y.; Kitahara, Y.; Sato, F.; Shimizu, T.; Miura, K.; Mine, T.; Tanaka, Y.; Mitsumata, M.; et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Atherosclerosis: Evolving vascular biology and clinical implication. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kinley, S.; Creager, M.A.; Fukumoto, M.; Hikita, H.; Fang, J.C.; Selwyn, A.P.; Ganz, P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 2001, 38, 1049–1053. [Google Scholar] [CrossRef]
- Hanley, A.J.; Williams, K.; Stern, M.P.; Haffner, S.M. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio heart study. Diabetes Care 2002, 25, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Meigs, J.B.; Bonadonna, R.; Muggeo, M. Homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population. The Bruneck Study. Diabetes Care 2007, 30, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zethelius, B.; Lithell, H.; Hales, C.N.; Berne, C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year-old men using the euglycemic glucose clamp. Diabetologia 2005, 48, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.J.R. Mechanisms of vascular insulin resistance. A substitute Akt? Circ. Res. 2009, 104, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Mathen, K.J.; Steinberg, H.O.; Baron, A.D. Insulin resistance in the vasculature. J. Clin. Invest. 2013, 123, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; DeFronzo, R.A. Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes Metab. Res. Rev. 2006, 22, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Zanetti, M.; Gortan Cappellari, G.; Semolic, A.; Boschelle, M.; Codarin, E.; Pirulli, A.; Cattin, L.; Guarnieri, G. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 2012, 55, 773–782. [Google Scholar] [PubMed]
- Nigro, J.; Osman, N.; Dart, A.M.; Little, P.J. Insulin resistance and atherosclerosis. Endocrine Review 2006, 27, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, S.J.; Kushwaha, R.S.; DeFronzo, R.A. Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis in conscious normal rats. Metabolism 1999, 48, 330–337. [Google Scholar] [CrossRef]
- Nakao, J.; Ito, H.; Kanayasu, T.; Murota, S. Stimulatory effect of insulin on aortic smooth muscle cell migration induced by 12-L-hydroxy-5, 8, 10, 14-eicosatetraenoic acid and its modulation by elevated extracellular glucose levels. Diabetes 1985, 34, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pfeifle, B.; Ditschuneit, H. Effect of insulin on the growth of cultured arterial smooth muscle cells. Diabetologia 1981, 20, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definition Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; de Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [PubMed]
- Lekakis, J.; Abraham, P.; Balbarini, A.; Blann, A.; Boulanger, C.M.; Cockcroft, J.; Cosentino, F.; Deanfield, J.; Gallino, A.; Ikonomidis, I.; et al. Methods for evaluating endothelial function: A position statement from the European society of cardiology working group on peripheral circulation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 775–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkimattila, S.; Liu, M.L.; Vakkilainen, J.; Schlenzka, A.; Lahdenperä, S.; Syvänne, M.; Mäntysaari, M.; Summanen, P.; Bergholm, R.; Taskinen, M.R.; et al. Impaired endothelium-dependent vasodilation in type 2 diabetes. Relation to LDL size, oxidized LDL, and antioxidants. Diabetes Care 1999, 22, 973–981. [Google Scholar]
- Watts, G.F.; O’Brien, W.J.; Silvester, W.; Millar, J.A. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes. Clin. Sci. 1996, 91, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E.; Arora, S.; Saouaf, R.; Lim, S.C.; Smakowski, P.; Park, J.Y.; King, G.L.; LoGerfo, F.W.; Horton, E.S.; Veves, A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 1999, 48, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Vehkavaara, S.; Seppala-Lindroos, A.; Westerbacka, J.; Groop, P.H.; Yki-Järvinen, H. In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care 1999, 22, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Balletshofer, B.M.; Rittig, K.; Enderle, M.D.; Volk, A.; Maerker, E.; Jacob, S.; Matthaei, S.; Rett, K.; Häring, H.U. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation 2000, 101, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okada, Y.; Mori, H.; Tanaka, Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Chow, W.S.; Ai, V.H. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care 2002, 25, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, X.M.; Sun, Y.M.; Jin, H.B.; Fu, R.; Wang, Y.Y.; Wu, Y.; Luan, Y. The relationship between endothelial dysfunction and oxidative stress in diabetes and prediabetes. Int. J. Clin. Pract. 2008, 62, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Vita, J.A. Effects of systemic inflammation on endothelium-dependent vasodilation. Trend. Cardiovasc. Med. 2006, 16, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Person, S.D.; Wei, G.S.; Godreau, A.; Jacobs, D.R., Jr.; Harrington, A.; Sidney, S.; O’Leary, D.H. Segment-specific associations of carotid intima-media thickness with cardiovascular risk factors: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke 2010, 41, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S.; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [PubMed]
- Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S. Reference Values for Arterial Measurements Collaboration. Reference intervals for common carotid intima-media thickness measured with echotracking: Relation with risk factors. Eur. Heart J. 2013, 34, 2368–2380. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.D.; Blake, C.C.; Tamayo, A.; Beletsky, V.; Fenster, A.; Spence, J.D. 3D ultrasound measurement of change in carotid plaque volume: A tool for rapid evaluation of new therapies. Stroke 2005, 36, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Brohall, G.; Odén, A.; Fagerberg, B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: A systematic review. Diabet. Med. 2006, 23, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Lahoz, C.; Salinero-Fort, M.A.; de Burgos-Lunar, C.; Laguna, F.; Estirado, E.; García-Iglesias, F.; González-Alegre, T.; Cornejo-Del-Río, V.; Sabín, C.; et al. Carotid atherosclerosis severity in relation to glycemic status: A cross-sectional population study. Atherosclerosis 2015, 242, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Pollex, R.L.; Spence, J.D.; House, A.A.; Fenster, A.; Hanley, A.J.G.; Zinman, B.; Harris, S.B.; Hegele, R.A. A comparison of ultrasound measurements to assess carotid atherosclerosis development in subjects with and without type 2 diabetes. Cardiovasc. Ult. 2005, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdoia, M.; Schaffer, A.; Cassetti, E.; Barbieri, L.; Di Ruocco, M.V.; Perrone-Filardi, P.; Marino, P.; de Luca, G.; On behalf of the Novara Atherosclerosis Study Group. Glycosylated hemoglobin and coronary artery disease in patients without diabetes mellitus. Am. J. Prev. Med. 2014, 47, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Baumeister, S.E.; Lieb, W.; von Sarnowski, B.; Volzke, H.; Felix, S.B.; Nauck, M. Glycated hemoglobin as a marker of subclinical atherosclerosis and cardiac remodeling among non-diabetic adults from the general population. Diabetes Res. Clin. Pract. 2014, 105, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, L.E.; D’Agostino, R., Jr.; Savage, P.J.; O’Leary, D.H.; Saad, M.F.; Haffner, S.M. Duration of diabetes and carotid wall thickness. The Insulin Resistance Atherosclerosis Study (IRAS). Stroke 1997, 28, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Dolan, L.M.; Kimball, T.R.; Gao, Z.; Khoury, P.R.; Daniels, S.R.; Urbina, E.M. Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and young adults with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009, 94, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Zhang, Y.; Shen, X.P.; Huang, Q.; Ma, H.; Huang, Y.L.; Zhang, W.Q.; Wu, H.J. Correlation between glucose fluctuations and carotid intima-media thickness in type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 90, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kowall, B.; Ebert, N.; Then, C.; Thiery, J.; Koenig, W.; Meisinger, C.; Rathmann, W.; Seissler, J. Association between blood glucose and carotid intima-media thickness disappears after adjustment for shared risk factors: The KORA F4 study. PLoS ONE 2012, 7, e52590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folsom, A.R.; Eckfeldt, J.H.; Weitzman, S.; Ma, J.; Chambless, L.E.; Barnes, R.W.; Cram, K.B.; Hutchinson, R.G. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke 1994, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; O’Leary, D.H.; Zaccaro, D.; Haffner, S.; Rewers, M.; Hamman, R.; Selby, J.V.; Saad, M.F.; Savage, P.; Bergman, R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996, 93, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Hedblad, B.; Nilsson, P.; Janzon, L.; Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet. Med. 2000, 17, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sourij, H.; Schmoelzer, I.; Dittrich, P.; Paulweber, G.; Iglseder, B.; Wascher, T.C. Insulin resistance as a risk factor for carotid atherosclerosis. A comparison of the homeostasis model assessment and short insulin tolerance test. Stroke 2008, 39, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Natali, A.; Dekker, J.; Beck-Nielsen, H.; Laakso, M.; Nilsson, P.; Balkau, B.; Ferrannini, E.; RISC Investigators. Insulin sensitivity and carotid intima-media thickness: Relationship between Insulin Sensitivity and Cardiovascular risk study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.A.; Hiremagular, B.; Haluska, B.A.; Campbell, S.B.; Hawley, C.M.; Marks, L.; Prins, J.; Johnson, D.W.; Isbel, N.M. Free fatty acids are associated with obesity, insulin resistance, and atherosclerosis in renal transplant recipients. Transplantation 2005, 80, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Hayaishi-Okano, R.; Yamasaki, Y.; Katakami, N.; Ohtoshi, K.; Gorogawa, S.; Kuroda, A.; Matsuhisa, M.; Kosugi, K.; Nishikawa, N.; Kajimoto, Y.; et al. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 2002, 25, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Sjoberg, C.; Crisby, M.; Goldberg, R.; Mendez, A.; Wright, C.B.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Adiponectin and carotid intima-media thickness in the northern Manhattan study. Stroke 2012, 43, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.M.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Kamp, O.; Bouter, L.M.; Stehouwer, C.D.A. Carotid arterial remodeling: A maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism: The Hoorn study. Stroke 2004, 35, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Morizzo, C.; Bianchi, C.; Di Filippi, M.; Miccoli, R.; Paterni, M.; Di Bello, V.; Palombo, C. Glucose-related arterial stiffness and carotid artery remodeling: A study in normal subjects and type 2 diabetes patients. J. Clin. Endocrinol. Metab. 2014, 99, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Gillebert, T.C.; de Buyzere, M.L.; van Daele, C.M.; Khan, Z.A.; Khawar, U.; de Bacquer, D.; Rietzschel, E.R.; On behalf of the Asklepios Investigators. Central pulse pressure and its hemodynamic determinants in middle-aged adults with impaired fasting glucose and diabetes: The Asklepios study. Diabetes Care 2013, 36, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Schram, M.T.; Henry, R.M.; van Dijk, R.A.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Westerhof, N.; Stehouwer, C.D. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: The Hoorn Study. Hypertension 2004, 43, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.; Gariepy, J.; Denarie, N.; Balice, M.; Megnien, J.L.; Levenson, J.; Simon, A. Influence of hypertension on early carotid artery remodeling. Atheroscler. Thromb. Vasc. Biol. 2003, 23, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C.; Paterni, M.; Anderwald, C.H.; Konrad, T.; Colgan, M.P.; Flyvbjerg, A.; Dekker, J. Body composition and common carotid artery remodeling in a healthy population. J. Clin. Endocrinol. Metab. 2008, 93, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Winston, G.J.; Palmas, W.; Lima, J.; Polak, J.F.; Bertoni, A.G.; Burke, G.; Eng, J.; Gottesman, R.; Shea, S. Pulse pressure and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am. J. Hypertens. 2013, 26, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Bussy, C.; Hayoz, D.; Hengstler, J.; Dartois, N.; Laloux, B.; Brunner, H.; Laurent, S. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 2000, 101, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Morizzo, C.; Guarino, D.; Federico, G.; Miccoli, M.; Giannattasio, C.; Palombo, C. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J. Hypertens. 2015, 33, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- The Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values”. Eur. Heart. J. 2010, 31, 2338–2350. [Google Scholar]
- Engelen, L.; Bossuyt, J.; Ferreira, I.; van Bortel, L.M.; Reesink, K.; Segers, P.; Stehouwer, C.D.; Laurent, S.; Boutouyrie, P.; On behalf of the Reference Values for Arterial Measurements Collaboration. Reference values for local arterial stiffness. Part A: Carotid artery. J. Hypertens. 2015, 33, 1981–1996. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, J.; Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S.; Segers, P.; Reesink, K.; van Bortel, L.M.; On behalf of the Reference Values for Arterial Measurements Collaboration. Reference values for local arterial stiffness. Part B: Femoral artery. J. Hypertens. 2015, 33, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M. Hemodynamic aging as the consequence of structural changes associated with Early Vascular Aging (EVA). Aging Dis. 2014, 5, 109–113. [Google Scholar] [PubMed]
- Najjar, S.S.; Scuteri, A.; Lakatta, E.G. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension 2005, 46, 454–462. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, L.; Millasseau, S.C.; Smith, A.; Viberti, G.; Jones, R.H.; Ritter, J.M.; Chowienczyk, P.J. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension 2004, 44, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wilson, P.W. Risk factors that attenuate the female coronary disease advantage. Arch. Intern. Med. 1995, 155, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Van Sloten, T.T.; Schram, M.T.; van den Hurk, K.; Dekker, J.M.; Nijpels, G.; Henry, R.M.; Stehouwer, C.D. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: The Hoorn study. J. Am. Coll. Cardiol. 2014, 63, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.D.; Bulpitt, C.J.; Pinto, E.S.; Rajkumar, C. The aging elastic arteries. A comparison of diabetic and nondiabetic subjects. Diabetes Care 2003, 26, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.S.; Brien, O.; Stanley, D.G.; Deware, A.G.; Swales, P.P.R.; Williams, B. Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J. Hypertens. 2004, 22, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Paini, A.; Boutouyrie, P.; Calvet, D.; Tropeano, A.I.; Laloux, B.; Laurent, S. Carotid and aortic stiffness. Determinants of discrepancies. Hypertension 2006, 47, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.R.; Khunti, K.; Silverman, R.; Gray, L.J.; Srinivasan, B.; Lacy, P.S.; Williams, B.; Davies, M.J. Impact of metabolic indices on central artery stiffness: Independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010, 53, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Nambi, V.; Chambless, L.E.; Steffes, M.W.; Juraschek, S.P.; Coresh, J.; Sharrett, A.R.; Selvin, E. Hyperglycemia and arterial stiffness: The atherosclerosis risk in communities study. Atherosclerosis 2012, 225, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Salomaa, V.; Riley, W.; Kark, J.D.; Nardo, C.; Folsom, A.R. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentration are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation 1995, 91, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, N.; Teng, F.; Zou, C.; Xue, Y.; Yang, M.; Song, H.; Qi, L. Hemoglobin A1c levels and aortic arterial stiffness: The Cardiometabolic Risk in Chinese (CRC) study. PLoS ONE 2012, 7, e38485. [Google Scholar] [CrossRef] [PubMed]
- Gottsäter, M.; Östling, G.; Persson, M.; Engström, G.; Melander, O.; Nilsson, P.M. Non-hemodynamic predictors of arterials stiffness after 17 years follow-up: Malmö diet and cancer study. J. Hypertens. 2015, 33, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.; Nishizawa, Y.; Kawagishi, T.; Maekawa, K.; Hiura, Y.; Kanda, H.; Izumotani, K.; Shoji, T.; Ishimura, E.; Inaba, M.; et al. Stiffness indexes beta of the common carotid and femoral arteries are associated with insulin resistance in NIDDM. Diabetes Care 1998, 21, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Zhou, Y.; Wang, C.; Hu, H.; Hoff, K.; Guo, Y.; Gao, X.; Wang, A.; Wu, S.; et al. Increased fasting glucose and the prevalence of arterial stiffness: A cross-sectional study in Chinese adults. Neurol. Res. 2014, 36, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Lee, H.R.; Lee, D.C. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc. Diabetol. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Niijima, K.; Muranaka, Y.; Ando, T.; Okada, S.; Niijima, Y.; Hashimoto, K.; Yamada, M.; Ohshima, K.; Mori, M.; Ono, K. Elevated 1-h plasma glucose following 75-g oral glucose load is a predictor of arterial stiffness in subjects with normal glucose tolerance. Diabet. Med. 2012, 29, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Lin, C.C.; Hsu, H.S.; Liu, C.S.; Davidson, L.E.; Li, T.C.; Li, C.I.; Lin, W.Y. Arterial stiffness is strongly associated with insulin resistance in Chinese—A population-based study (Taichung Community Health Study, TCHS). J. Atheroscler. Thromb. 2011, 18, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Kang, T.S.; Park, S.; Park, H.Y.; Ko, Y.G.; Choi, D.; Jang, Y.; Chung, N. Insulin resistance is associated with arterial stiffness in nondiabetic hypertensives independent of metabolic status. Hypertens. Res. 2005, 28, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Strazhesko, I.; Tkacheva, O.; Boytsov, S.; Akasheva, E.; Vygodin, V.; Skvortsov, D.; Nilsson, P. Association of insulin resistance, arterial stiffness and telomere length in adults free of cardiovascular diseases. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Makavos, G.; Lekakis, J. Arterial stiffness and coronary artery disease. Curr. Opin. Cardiol. 2015, 30, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Agirbasli, M.; Tanrikulu, A.M.; Berenson, G.S. Metabolic syndrome: Bridging the gap from childhood to adulthood. Cardiovasc. Ther. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Morizzo, C.; Bianchi, V.; Marchetti, S.; Federico, G.; Palombo, C. Hedmodynamic overload and intra-abdominal adiposity in obese children: Relationship with cardiovascular structure and function. Nutr. Metabol. Cardiovasc. Dis. 2016, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Jounala, M.; Raitakari, M.; Viikari, J.S.A.; Raitakari, O.T. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2006, 185, 388–393. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakova, M.; Palombo, C. Diabetes Mellitus, ArterialWall, and Cardiovascular Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 201. https://doi.org/10.3390/ijerph13020201
Kozakova M, Palombo C. Diabetes Mellitus, ArterialWall, and Cardiovascular Risk Assessment. International Journal of Environmental Research and Public Health. 2016; 13(2):201. https://doi.org/10.3390/ijerph13020201
Chicago/Turabian StyleKozakova, Michaela, and Carlo Palombo. 2016. "Diabetes Mellitus, ArterialWall, and Cardiovascular Risk Assessment" International Journal of Environmental Research and Public Health 13, no. 2: 201. https://doi.org/10.3390/ijerph13020201





