Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data on Hospital Admissions
2.3. Environmental Data
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities Study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The effects of fine particle components on respiratory hospital admissions in children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.M. Ambiente e pulmão. J. Pneumol. 2002, 28, 261–269. [Google Scholar] [CrossRef]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Tiittanen, P.; Kulmala, M.; Pekkanen, J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology 2009, 20, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.; Johansson, C.; Forsberg, B. Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden. Environ. Health Perspect. 2012, 120, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and temporal variation in PM2.5 chemical composition in the United Statess for health effects studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Lee, J.T.; Kim, K.H.; Jung, K.; Bell, M.L. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ. Health Perspect. 2012, 120, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Franklin, M.; Koutrakis, P.; Schwartz, J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ. Health. 2009, 58, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Leaderer, B.P.; Gent, J.F.; Lee, H.J.; Koutrakis, P.; Wang, Y.; Dominici, F.; Peng, R.D. Associations of PM2.5 constituents and sources with hospital admissions: Analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥65 years of age. Environ. Health Perspect. 2014, 122, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, H.; Xu, Q.; Chen, B.; Kan, H. Fine particulate matter constituents and cardiopulmonary mortality in a heavily polluted Chinese city. Environ. Health Perspect. 2012, 120, 373–378. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans affairs normative aging study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Cassee, F.R.; Héroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Gouveia, N.; Cifuentes, L.A.; Leon, A.P.; Junger, W.; Vera, J.; Strappa, V.; Hurtado-Díaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Multicity study of air pollution and mortality in Latin America (the ESCALA Study). Res. Rep. Health Eff. Inst. 2012, 171, 5–86. [Google Scholar] [PubMed]
- O Vale, S. José Faz Cadastramento Para Restringir “Forasteiros” na Saúde. Available online: http://www.ovale.com.br/nossa-regi-o/s-jose-faz-cadastramento-para-restringir-forasteiros-na-saude-1.223717 (accessed on 8 August 2015).
- DATASUS. Departamento de Informática do SUS. Available online: http://www2.datasus.gov.br/DATASUS/index.php (accessed on 2 November 2013).
- Hopke, P.K.; Xie, Y.; Raunemma, T.; Biegalski, S.; Landsberger, S.; Maenhaut, W.; Artaxo, P.; Cohen, D. Characterization of the Gent stacked filter unit PM10 sampler. Aerosol Sci. Technol. 1997, 27, 726–735. [Google Scholar] [CrossRef]
- Ferreira, T.M.; Forti, M.C.; Alvalá, P.C. Protocolo Para Coleta de Material Particulado Atmosférico. Available online: http://urlib.net/8JMKD3MGP7W/3B9PGQL (accessed on 24 April 2014).
- Forti, M.C.; Alcaide, R.L.M. Validação de Métodos Analíticos do Laboratório de Aerossóis, Soluções Aquosas e Tecnologias—LAQUATEC. Available online: http://urlib.net/8JMKD3MGP7W/39QJ7P2 (accessed on 18 June 2014).
- Forti, M.C.; Alcaide, R.L.M. Protocolo de Determinação de ânions Inorgânicos em Soluções Aquosas Por Cromatografia iônica. Available online: http://urlib.net/8JMKD3MGP7W/3B86KQ5 (accessed on 18 June 2014).
- Forti, M.C.; Alcaide, R.L.M. Protocolo de Determinação de Cátions Inorgânicos em Soluções Aquosas Por Cromatografia Liquída de íons. Available online: http://urlib.net/8JMKD3MGP7W/3FJFPGL (accessed on 2 July 2014).
- Junger, W.L.; Ponce de Leon, A. Imputation of missing data in time series for air pollutants. Atmos. Environ. 2015, 102, 96–104. [Google Scholar] [CrossRef]
- Schwartz, J.; Spix, C.; Touloumi, G.; Bachárová, L.; Barumamdzadeh, T.; Tertre, A.L.; Piekarksi, T.; Ponce de Leon, A.; Ponka, A.; Rossi, G.; et al. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J. Epidemiol. Community Health. 1996, 50, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Wand, M.P.; Schwartz, J.; Ryan, L.M. Generalized additive distributed lag models: Quantifying mortality displacement. Biostatistics 2000, 1, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.L. Análise, Imputação de Dados e Interfaces Computacionais em Estudos de Séries Temporais Epidemiológicas. Doutorado Thesis, Universidade Estadual do Rio de Janeiro, Rio de Janeiro, Brazil, April 2008. [Google Scholar]
- World Health Organization (WHO). Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Global Update 2005. Summary of Risk Assessment; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nardocci, A.C.; Freitas, C.U.; Leon, A.C.M.P.; Junger, W.L.; Gouveia, N.C. Poluição do ar e doenças respiratórias e cardiovasculares: Estudo de séries temporais em Cubatão, São Paulo, Brasil. Cad. Saude Publica. 2013, 29, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.F.C.; Francisco, J.B.; Patto, M.B.R.; Antunes, A.M. Environmental pollutants and stroke-related hospital admissions. Cad. Saude Publica. 2012, 28, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Malig, B.J.; Green, S.; Basu, R.; Broadwin, R. Coarse particles and respiratory emergency department visits in California. Am. J. Epidemiol. 2013, 178, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Samoli, E.; Alessandrini, E.; Cadum, E.; Ostro, B.; Berti, G.; Faustini, A.; Jacquemin, B.; Linares, C.; Pascal, M.; et al. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: Results from the MED-PARTICLES Project. Environ. Health Perspect. 2013, 121, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Yu, I.T.; Qiu, H.; Ho, K.-F.; Sun, Z.; Louie, P.K.K.; Wong, T.W.; Tian, L. Short-term associations of cause-specific emergency hospitalizations and particulate matter chemical components in Hong Kong. Am. J. Epidemiol. 2014, 179, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittlemanet, M.A.; et al. Statement from the American Heart Association particulate matter air pollution and cardiovascular disease: An update to the scientific. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Mathes, R.; Ross, Z.; Nádas, A.; Thurston, G.; Matte, T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ. Health Perspect. 2011, 119, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Peel, J.L.; Hannigan, M.P.; Dutton, S.J.; Sheppard, L.; Clark, M.L.; Veda, S. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ. Health Perspect. 2012, 120, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.R.; Anderson, G.B.; Dominici, F.; Bell, M.L.; Peng, R.D. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ. Health Perspect. 2013, 121, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.F.; Miranda, R.M.; Fornaro, A.; Kerr, A.; Oyama, B.; Andre, P.A.; Saldiva, P. Vehicle emissions and PM2.5 mass concentrations in six Brazilian cities. Air Qual. Atmos. Health. 2010, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.A.; Mello, W.Z.; Mariani, R.L.; Sella, S.M. Caracterização do material particulado fino e grosso e composição da fração Inorgânica solúvel em água em São José dos Campos (SP). Quim. Nova. 2010, 33, 1247–1253. [Google Scholar] [CrossRef]
- Gan, W.Q.; FitzGerald, J.M.; Carlsten, C.; Sadatsafavi, M.; Brauer, M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care Med. 2013, 187, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Yu, I.T.; Ho, K.-F.; Qiu, H.; Sun, Z.; Tian, L. Differential effects of source-specific particulate matter on emergency hospitalizations for ischemic heart disease in Hong Kong. Environ. Health Perspect. 2014, 122, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, U.S.; Rastogi, N.; McWhinney, R.D.; Urch, B.; Chow, C.-W.; Evans, G.J.; Scott, J.A. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicol. Rep. 2014, 1, 145–156. [Google Scholar] [CrossRef]

| Parameter | Mean | SD | Min | P25 | P50 | P75 | Max | T |
|---|---|---|---|---|---|---|---|---|
| Inhalable Particulate Matter (μg/m3) | ||||||||
| PM2.5 | 4.4 | 2.4 | 0.2 | 2.6 | 3.9 | 5.7 | 15.1 | |
| PM2.5–10 | 8.4 | 5.6 | 0.1 | 4.3 | 7.1 | 11.2 | 29.7 | |
| PM10 | 12.7 | 7.2 | 0.7 | 7.4 | 11.2 | 16.7 | 36.8 | |
| PM10 a | 24.5 | 13.6 | 6.2 | 15.2 | 21.0 | 30.3 | 93.6 | |
| Weather variables | ||||||||
| Minimum temperature (°C) | 17.5 | 3.5 | 8.3 | 15.1 | 17.9 | 20.8 | 23.7 | |
| Average temperature (°C) | 21.9 | 3.3 | 13.0 | 19.3 | 22.3 | 24.4 | 29.0 | |
| Maximum temperature (°C) | 28.6 | 4.6 | 17.1 | 25.3 | 29.0 | 32.5 | 37.3 | |
| Minimum humidity (%) | 56.9 | 16.4 | 20.1 | 45.9 | 55.9 | 66.0 | 99.1 | |
| Average humidity (%) | 81.1 | 10.0 | 49.1 | 75.3 | 81.9 | 87.1 | 99.2 | |
| Hospital admissions (cases/day) | ||||||||
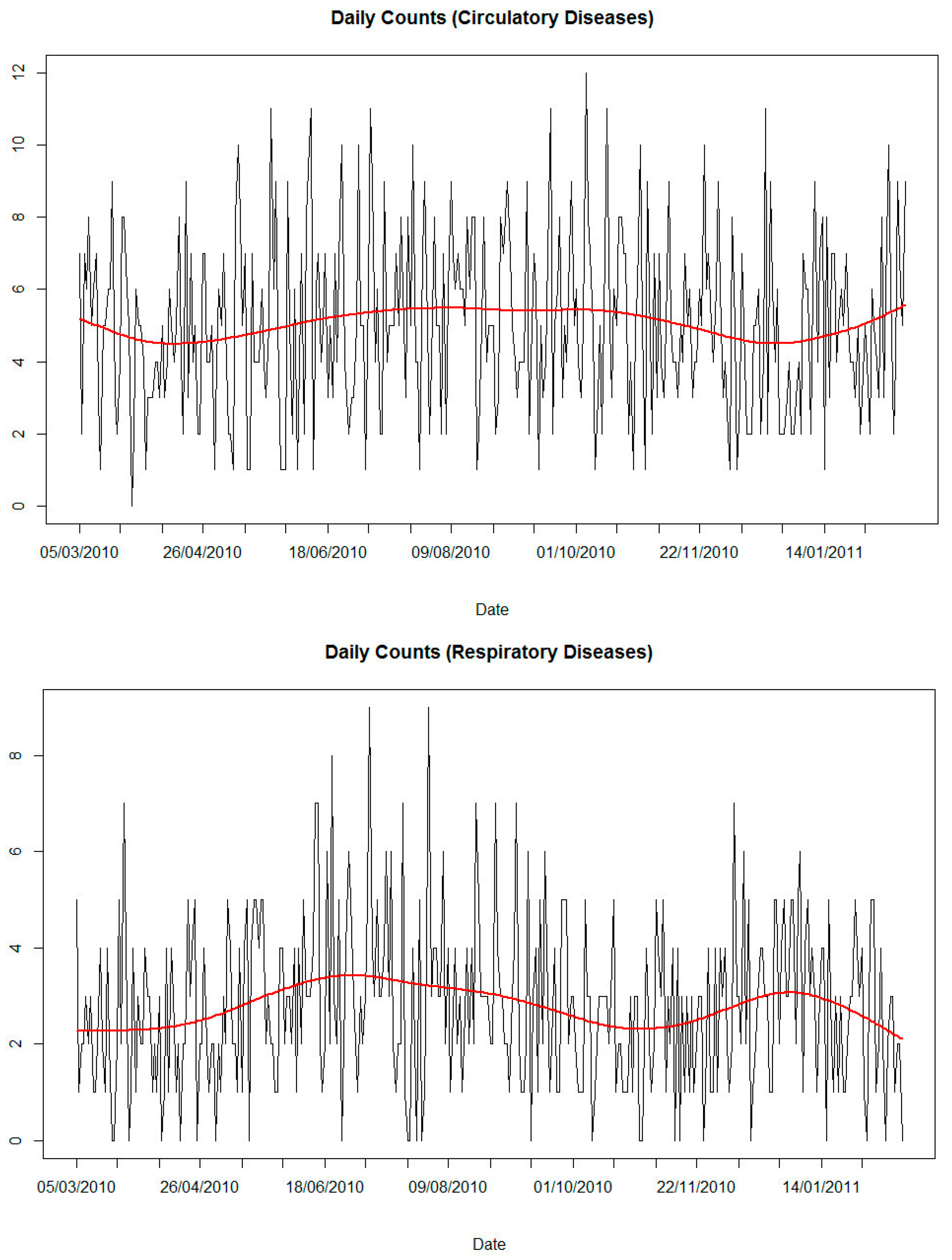
| Circulatory diseases | 5 | 2 | 0 | 3 | 5 | 7 | 12 | 1765 |
| Respiratory diseases | 3 | 2 | 0 | 1 | 3 | 4 | 9 | 972 |
| Parameter | Mean | SD | Min | P25 | P50 | P75 | Max |
|---|---|---|---|---|---|---|---|
| PM2.5 | |||||||
| Cl− | 38.2 | 24.0 | 0.7 | 21.8 | 38.8 | 43.1 | 188.0 |
| NO3- | 79.6 | 83.4 | 3.7 | 35.2 | 46.5 | 93.5 | 599.0 |
| SO42− | 399.2 | 314.4 | 0.6 | 186.4 | 320.3 | 530.3 | 1957.5 |
| Na+ | 40.4 | 39.4 | 1.4 | 20.6 | 25.1 | 44.6 | 285.6 |
| NH4+ | 98.3 | 94.8 | 0.3 | 43.7 | 63.4 | 120.7 | 636.8 |
| K+ | 26.7 | 24.9 | 0.1 | 9.0 | 23.0 | 31.4 | 208.6 |
| Ca2+ | 50.9 | 55.5 | 0.1 | 25.0 | 36.3 | 47.4 | 542.3 |
| Mg2+ | 14.2 | 14.1 | 0.1 | 3.5 | 8.3 | 28.3 | 88.4 |
| PM2.5–10 | |||||||
| Cl− | 82.7 | 119.3 | 2.1 | 31.3 | 44.2 | 90.8 | 1074.0 |
| NO3- | 284.7 | 295.0 | 5.2 | 106.4 | 202.7 | 384.3 | 3566.5 |
| SO42− | 158.7 | 149.9 | 0.2 | 64.4 | 110.5 | 212.9 | 1286.4 |
| Na+ | 79.2 | 101.2 | 1.2 | 25.8 | 50.7 | 96.6 | 1006.5 |
| NH4+ | 45.3 | 65.1 | 0.8 | 20.2 | 37.5 | 50.6 | 896.1 |
| K+ | 31.5 | 36.3 | 0.6 | 14.3 | 21.2 | 32.4 | 282.8 |
| Ca2+ | 167.9 | 176.4 | 7.6 | 62.2 | 121.4 | 216.9 | 2157.8 |
| Mg2+ | 28.5 | 23.6 | 0.2 | 13.0 | 24.4 | 38.4 | 307.4 |
| Pollutants | Respiratory Diseases %RR (LL, UL) | Circulatory Diseases %RR (LL, UL) |
|---|---|---|
| PM2.5 | 8.5 (−6.8, 26.3) | 19.6 (6.4, 34.6) |
| PM2.5–10 | 23.5 (13.5, 34.3) | 0.8 (−5.8, 7.7) |
| PM10 | 12.8 (6.0, 20.0) | 2.7 (−2.2, 7.9) |
| PM10 a | 8.9 (5.2, 12.8) | 1.2 (−1.7, 4.0) |
| Soluble ions (PM2.5) | ||
| SO42− | 0.0 (−0.1, 0.1) | 0.2 (0.1, 0.3) |
| NH4+ | −0.3 (−0.7, 0.1) | 1.2 (1.0, 1.5) |
| K+ | 2.7 (1.1, 4.3) | 1.6 (0.3, 2.8) |
| Soluble ions (PM2.5–10) | ||
| Cl− | −0.5 (−0.8, −0.2) | −0.7 (−0.9, −0.4) |
| NO3- | −0.1 (−0.2, 0.1) | 0.2 (0.1, 0.3) |
| SO42− | 0.4 (0.1, 0.6) | 0.8 (0.6, 1.0) |
| Na+ | −0.4 (−0.8, 0.0) | −0.3 (−0.6, 0.0) |
| K+ | −0.2 (-1.2, 0.8) | 1.0 (0.2, 1.8) |
| Ca2+ | 0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) |
| Mg2+ | 0.1 (−1.5, 1.8) | 0.4 (−0.9, 1.7) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.M.; Forti, M.C.; De Freitas, C.U.; Nascimento, F.P.; Junger, W.L.; Gouveia, N. Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases. Int. J. Environ. Res. Public Health 2016, 13, 947. https://doi.org/10.3390/ijerph13100947
Ferreira TM, Forti MC, De Freitas CU, Nascimento FP, Junger WL, Gouveia N. Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases. International Journal of Environmental Research and Public Health. 2016; 13(10):947. https://doi.org/10.3390/ijerph13100947
Chicago/Turabian StyleFerreira, Tatiane Morais, Maria Cristina Forti, Clarice Umbelino De Freitas, Felipe Parra Nascimento, Washington Leite Junger, and Nelson Gouveia. 2016. "Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases" International Journal of Environmental Research and Public Health 13, no. 10: 947. https://doi.org/10.3390/ijerph13100947
APA StyleFerreira, T. M., Forti, M. C., De Freitas, C. U., Nascimento, F. P., Junger, W. L., & Gouveia, N. (2016). Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases. International Journal of Environmental Research and Public Health, 13(10), 947. https://doi.org/10.3390/ijerph13100947






