The Genetic Diversity and Evolution of HIV-1 Subtype B Epidemic in Puerto Rico
Abstract
:1. Introduction
2. Methods
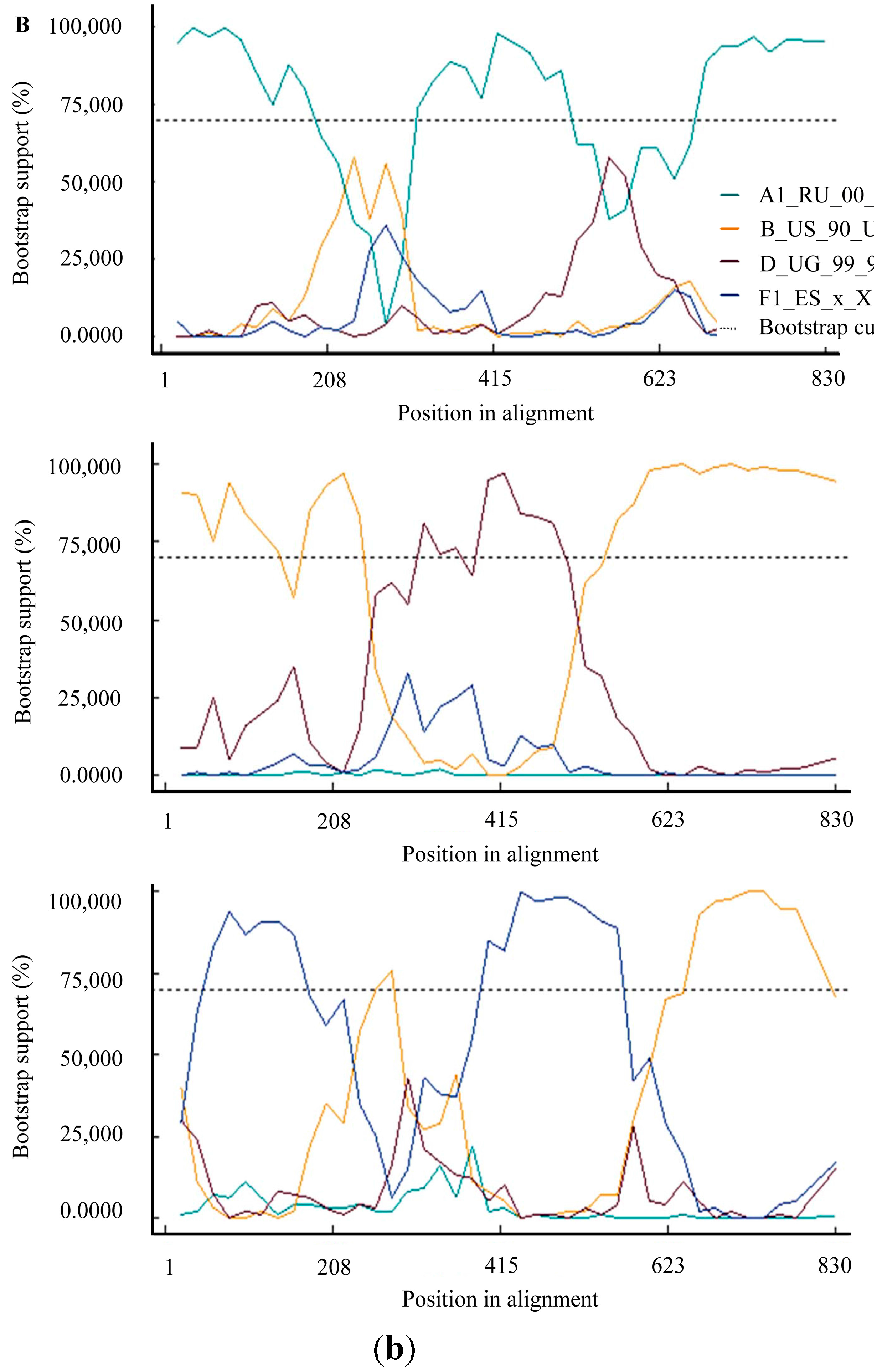
3. Results and Discussion
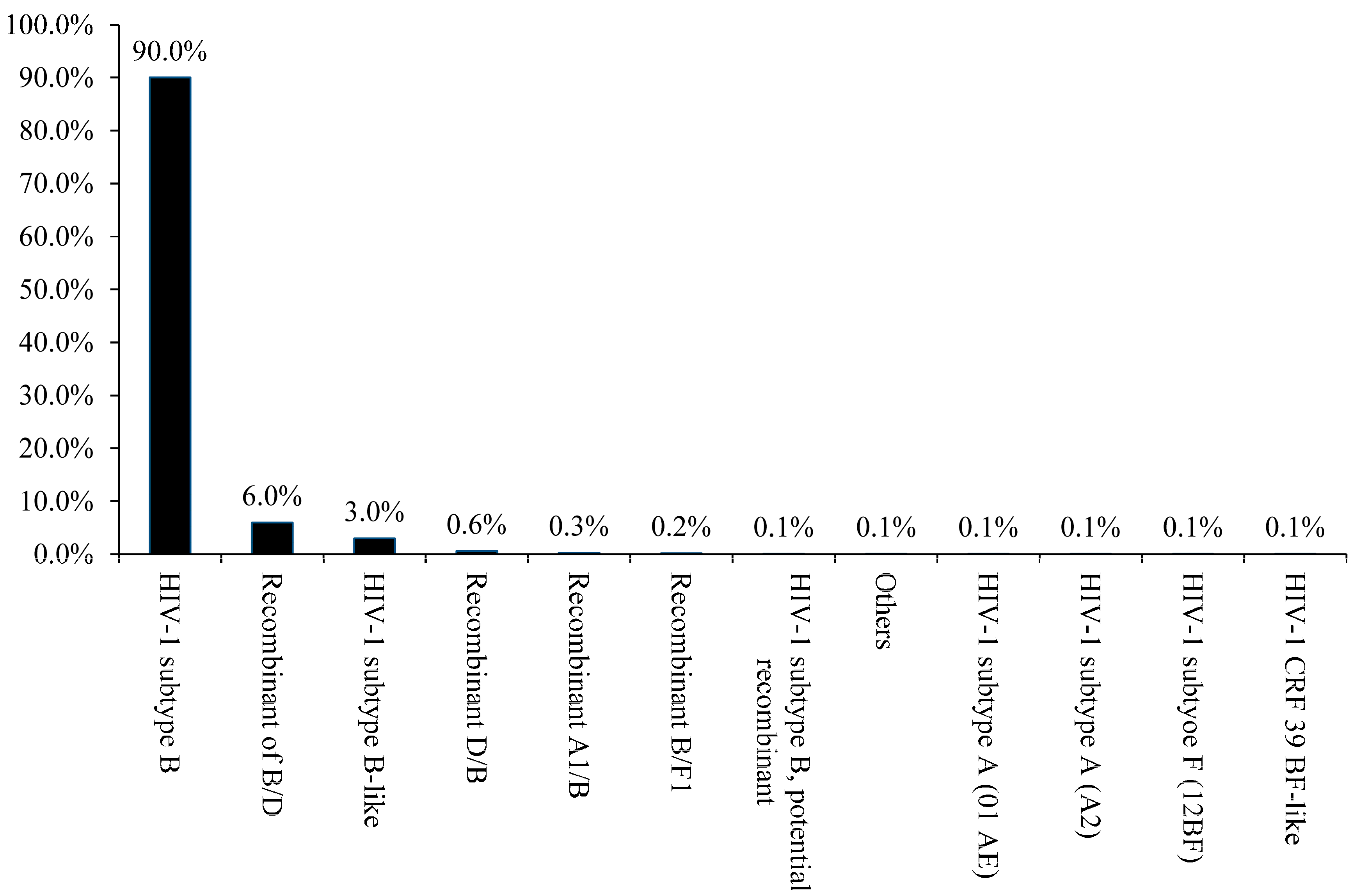
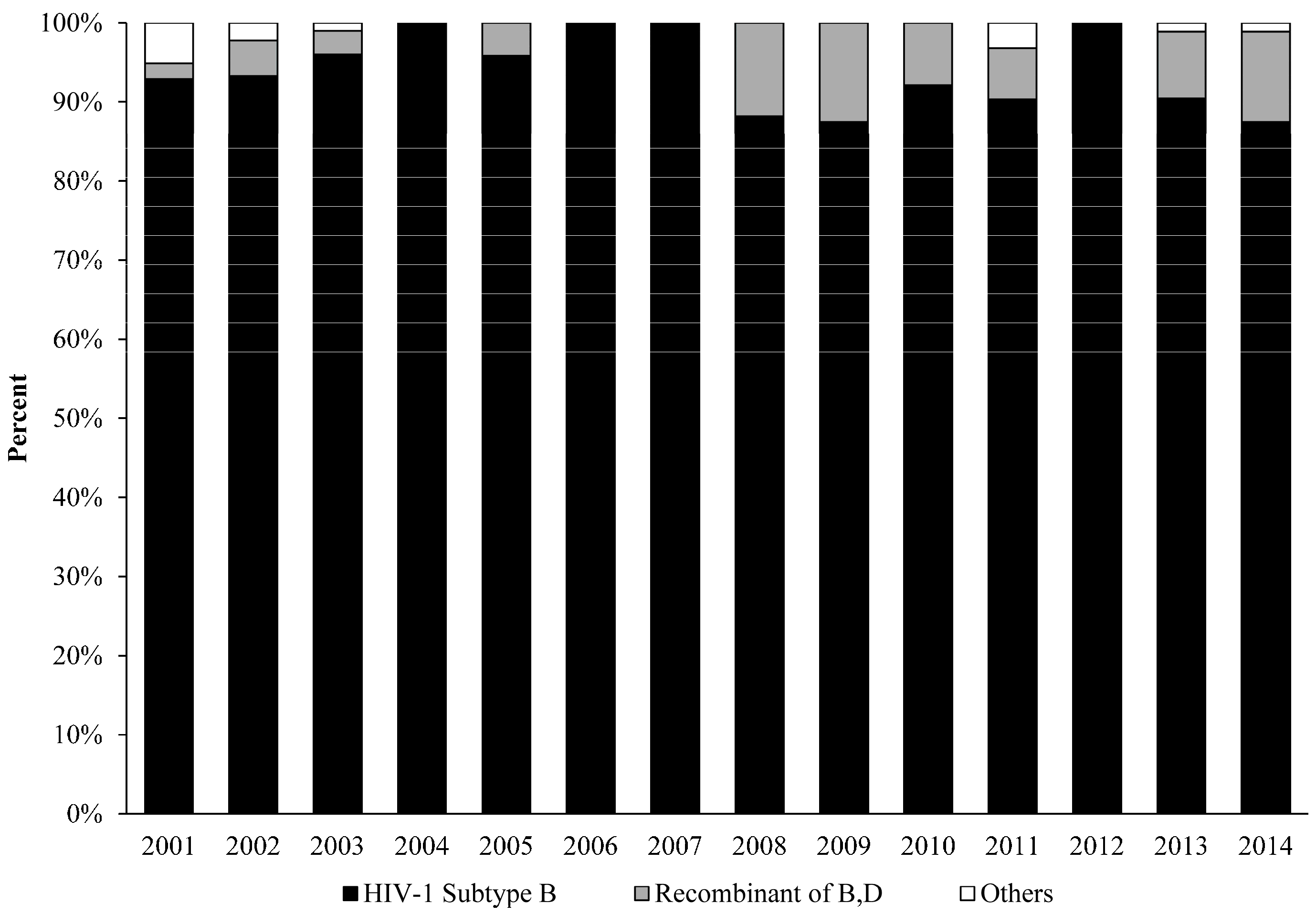
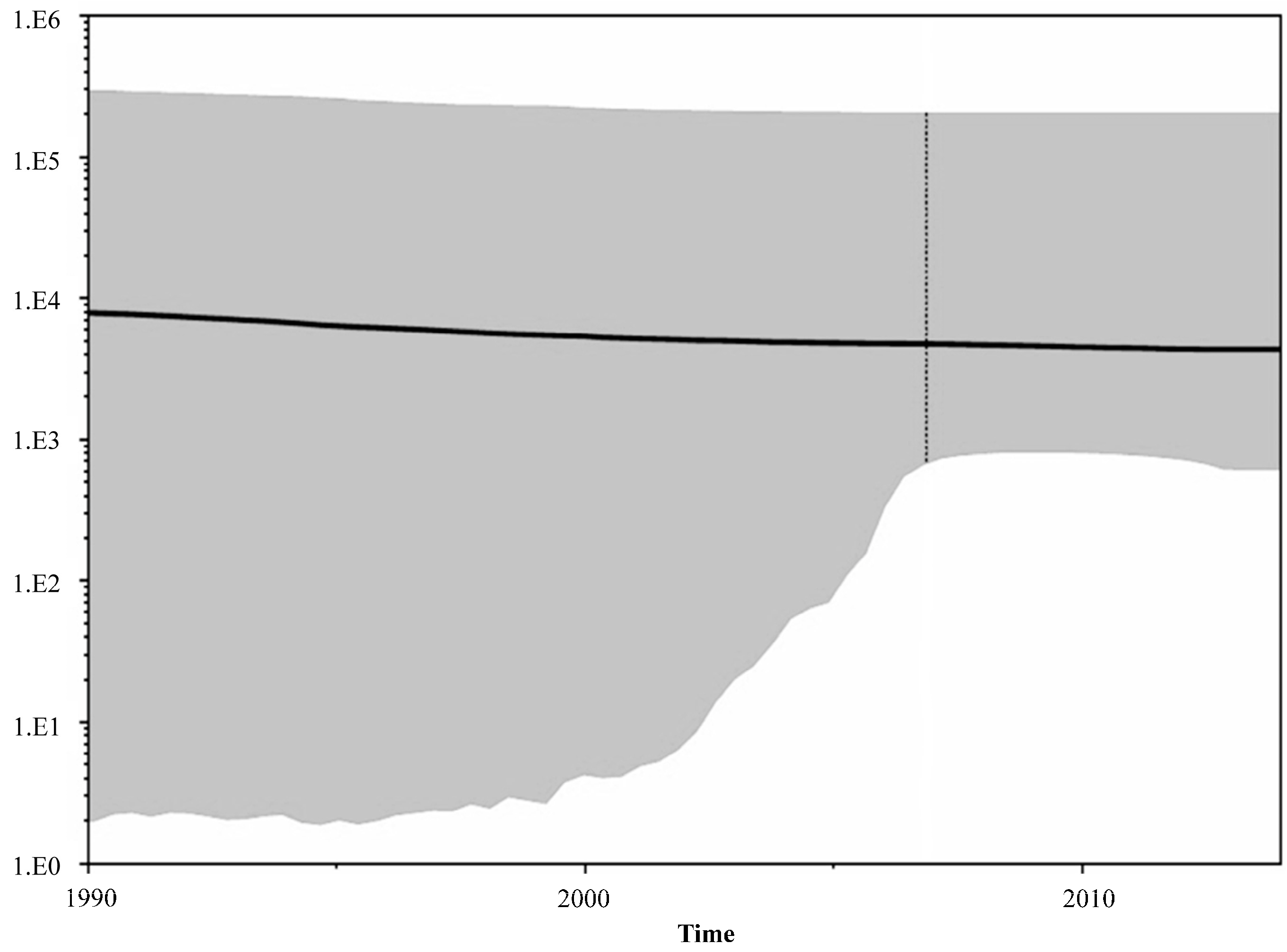
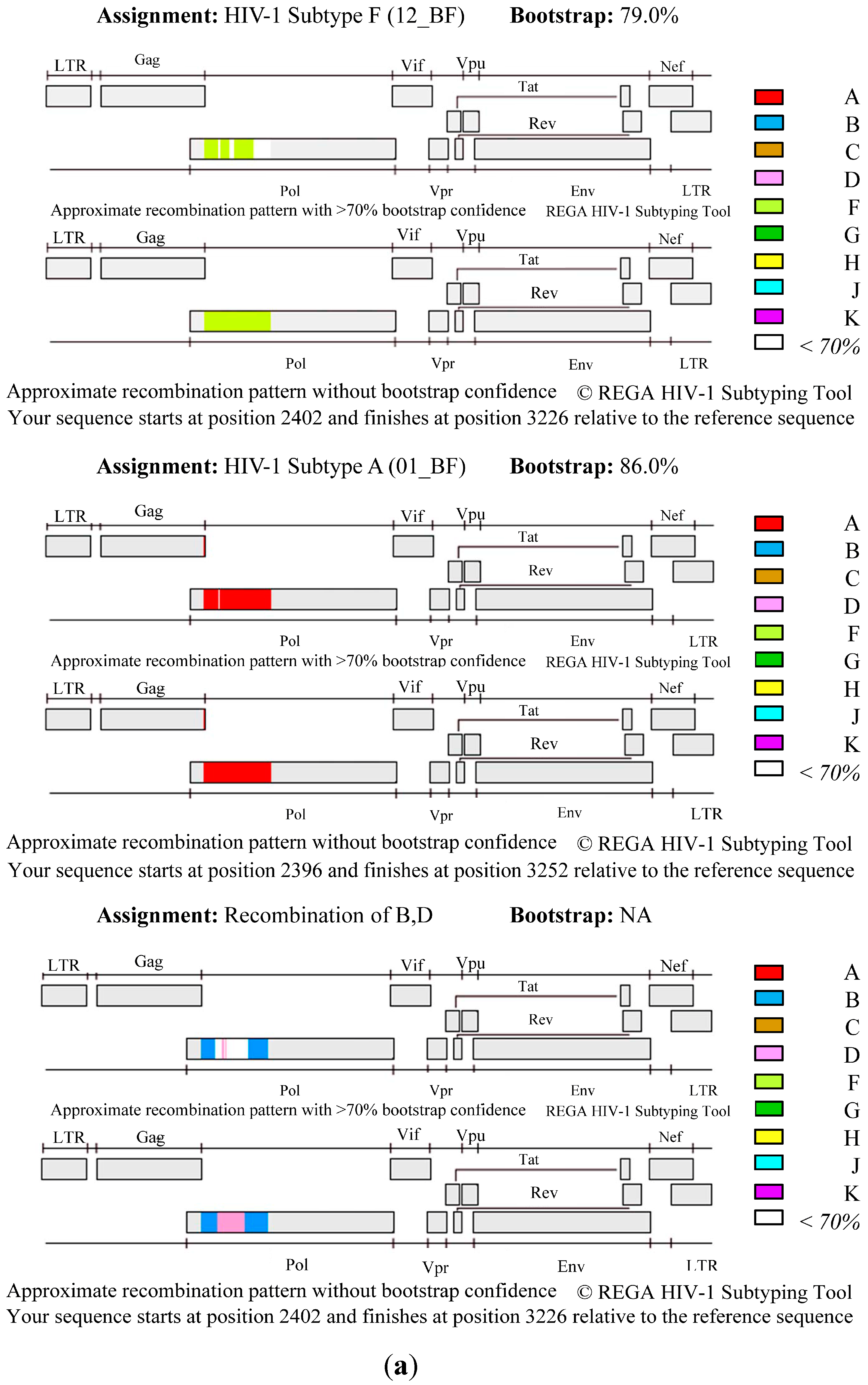
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seillier-Moiseiwitsch, F.; Margolin, B.H.; Swanstrom, R. Genetic variability of the human immunodeficiency virus: Statistical and biological issues. Ann. Rev. Genet. 1994, 28, 559–596. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Sharp, P.M. Genetic diversity of HIV-1: The moving target. AIDS 2000, 14, S129–S140. [Google Scholar] [PubMed]
- Taylor, B.S.; Sobieszczyk, M.E.; McCutchan, F.E.; Hammer, S.M. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 2008, 358, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- HIV Databases. Available online: http://www.hiv.lanl.gov/ (accessed on 14 August 2015).
- Lynch, R.M.; Shen, T.; Gnanakaran, S.; Derdeyn, C.A. Appreciating HIV type 1 diversity: Subtype differences in Env. AIDS Res. Hum. Retrovir. 2009, 25, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Pieniazek, D.; Moran, N.; Soler, A.; Rodriguez, N.; Alegria, M.; Vera, M.; Janini, L.M.; Bandea, C.I.; Ramos, A.; et al. HIV-1 subtype F in single and dual infections in Puerto Rico: A potential sentinel site for monitoring novel genetic HIV variants in North America. Emerg. Infect. Dis. 1999, 5, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2759. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Rivera-Amill, V.; Paulino-Ramirez, R.; Yamamura, Y. Short communication: HIV-1 subtype B in the dominican republic: evolution and molecular epidemiology. AIDS Res. Hum. Retrovir. 2015, 31, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Pybus, O.; Magiorkinis, G.; Hatzakis, A.; Wensing, A.M.; van de Vijver, D.A.; Albert, J.; Angarano, G.; Asjo, B.; Balotta, C.; et al. Tracing the HIV-1 subtype B mobility in Europe: A phylogeographic approach. Retrovirology 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Deforche, K.; Cassol, S.; Salminen, M.; Paraskevis, D.; Seebregts, C.; Snoeck, J.; van Rensburg, E.J.; Wensing, A.M.; van de Vijver, D.A.; et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics 2005, 21, 3797–3800. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with beauti and the beast 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; de Oliveira, T.; Ciccozzi, M.; Rezza, G.; Goodenow, M.M. High-resolution molecular epidemiology and evolutionary history of HIV-1 subtypes in Albania. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; Khudyakov, Y.E. Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Esbjornsson, J.; Mild, M.; Mansson, F.; Norrgren, H.; Medstrand, P. HIV-1 molecular epidemiology in Guinea-Bissau, West Africa: Origin, demography and migrations. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Pybus, O.G.; Charleston, M.A.; Gupta, S.; Rambaut, A.; Holmes, E.C.; Harvey, P.H. The epidemic behavior of the hepatitis C virus. Science 2001, 292, 2323–2325. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Tracer. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 14 August 2015).
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1. [Google Scholar] [CrossRef]
- Novitsky, V.; Smith, U.R.; Gilbert, P.; McLane, M.F.; Chigwedere, P.; Williamson, C.; Ndung’u, T.; Klein, I.; Chang, S.Y.; Peter, T.; et al. Human immunodeficiency virus type 1 subtype C molecular phylogeny: Consensus sequence for an AIDS vaccine design? J. Virol. 2002, 76, 5435–5451. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Castillo, L.; Leon-Bratti, M.P.; Solano-Chinchilla, A.; Herrera-Martinez, G.; Boza-Cordero, R.; Leon, B.; Luftig, R.B.; Visona, K. Variability in HIV-1 partial genomic sequences in Costa Rican patients: analysis with different bioinformatics tools. Rev. Panam. Salud Publica 2010, 27, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Maldarelli, F. Clinical management of HIV drug resistance. Viruses 2011, 3, 347–378. [Google Scholar] [CrossRef] [PubMed]
- Kouri, V.; Aleman, Y.; Perez, L.; Perez, J.; Fonseca, C.; Correa, C.; Aragones, C.; Campos, J.; Alvarez, D.; Schrooten, Y.; et al. High frequency of antiviral drug resistance and non-B subtypes in HIV-1 patients failing antiviral therapy in Cuba. J. Clin. Virol. 2012, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Ripamonti, D.; Arici, C.; Gregis, G.; Quinzan, G.; Camacho, G.A.; Ravasio, L.; Suter, F. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin. Trials 2002, 3, 371–378. [Google Scholar] [PubMed]
- Julg, B.; Bogner, J.R. Atriplatrade mark—HIV therapy in one pill. Ther. Clin. Risk Manag. 2008, 4, 573–577. [Google Scholar] [PubMed]
- Rivero-Mendez, M.; Dawson-Rose, C.S.; Solis-Baez, S.S. A Qualitative Study of Providers’ Perception of Adherence of Women Living with HIV/AIDS in Puerto Rico. Qual. Rep. 2010, 15, 232–251. [Google Scholar] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Lessells, R.J.; Katzenstein, D.K.; de Oliveira, T. Are subtype differences important in HIV drug resistance? Curr. Opin. Virol. 2012, 2, 636–643. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, P.; Rivera-Amill, V.; Rodríguez, N.; Vargas, F.; Yamamura, Y. The Genetic Diversity and Evolution of HIV-1 Subtype B Epidemic in Puerto Rico. Int. J. Environ. Res. Public Health 2016, 13, 55. https://doi.org/10.3390/ijerph13010055
López P, Rivera-Amill V, Rodríguez N, Vargas F, Yamamura Y. The Genetic Diversity and Evolution of HIV-1 Subtype B Epidemic in Puerto Rico. International Journal of Environmental Research and Public Health. 2016; 13(1):55. https://doi.org/10.3390/ijerph13010055
Chicago/Turabian StyleLópez, Pablo, Vanessa Rivera-Amill, Nayra Rodríguez, Freddie Vargas, and Yasuhiro Yamamura. 2016. "The Genetic Diversity and Evolution of HIV-1 Subtype B Epidemic in Puerto Rico" International Journal of Environmental Research and Public Health 13, no. 1: 55. https://doi.org/10.3390/ijerph13010055




