Spatiotemporal Pattern Analysis of Scarlet Fever Incidence in Beijing, China, 2005–2014
Abstract
:1. Introduction
2. Methodology
2.1. Study Area
2.2. Data Source
2.3. Case Definition
2.3.1. Ethical Approval
2.3.2. Human Participant Protection
2.4. Statistical Analysis
2.4.1. Spatial Autocorrelation Analysis
2.4.2. Hot Spot Analysis
2.4.3. Retrospective SaTScan Analysis
3. Results
3.1. Demographic Characteristics
| Year | No. of Cases | Incidence Rate (/105) |
|---|---|---|
| 2005 | 2164 | 14.7 |
| 2006 | 2268 | 14.16 |
| 2007 | 2392 | 14.27 |
| 2008 | 1798 | 10.15 |
| 2009 | 1258 | 6.76 |
| 2010 | 1665 | 8.50 |
| 2011 | 6466 | 32.03 |
| 2012 | 3367 | 16.27 |
| 2013 | 2170 | 10.26 |
| 2014 | 3321 | 15.43 |
| Total | 26,860 | 14.25 (Average) |
3.2. Spatial Pattern
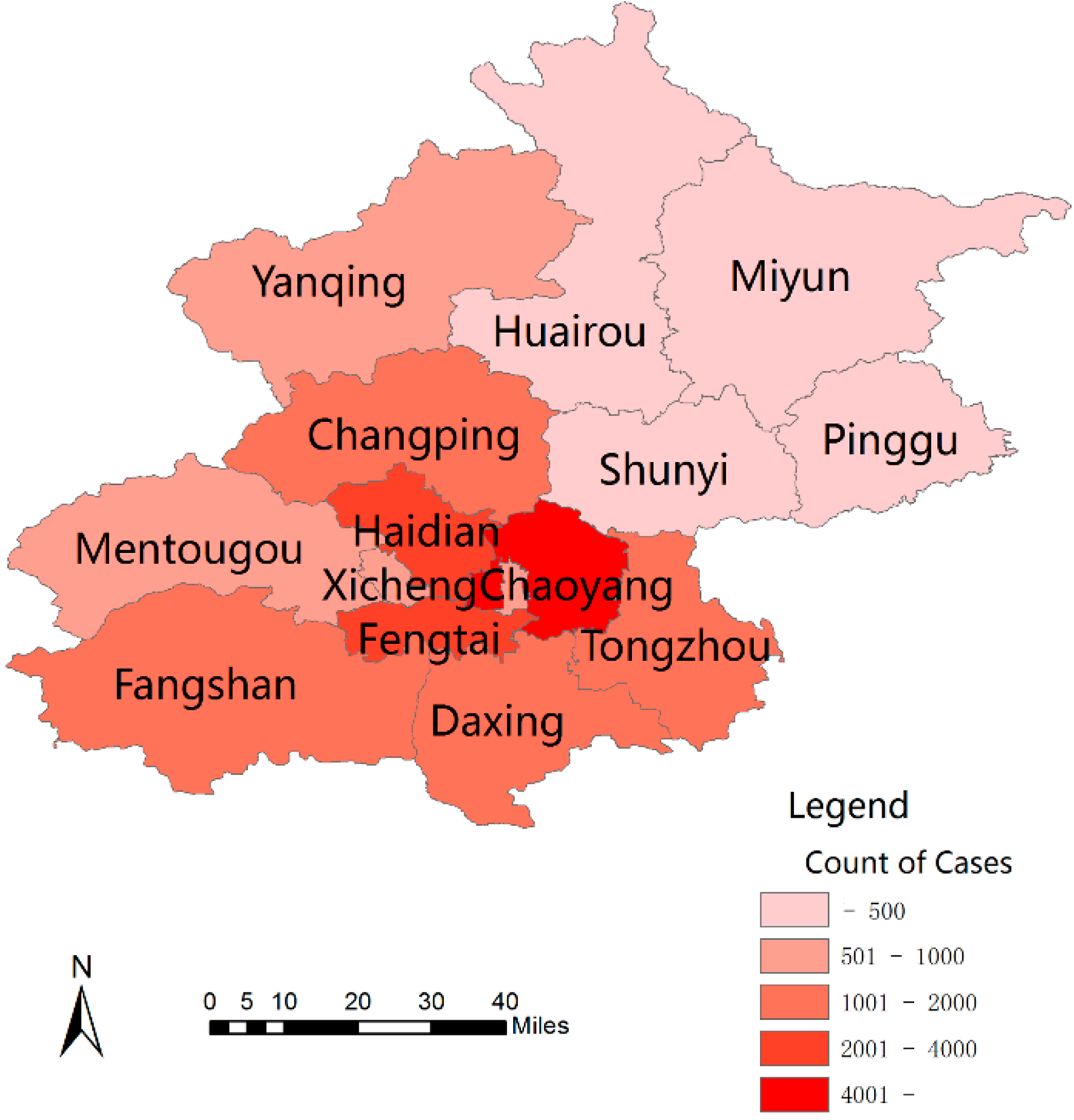
| Group | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | – | ||||||||||
| Male | 1358 (62.8) | 1419 (62.6) | 1523 (63.7) | 1111 (62.1) | 766 (60.9) | 1050 (63.1) | 4158 (64.3) | 2173 (64.5) | 1350 (62.2) | 2064 (62.1) | 16,972 (63.2) |
| Female | 806 (37.2) | 849 (37.4) | 869 (36.3) | 678 (37.9) | 492 (39.1) | 615 (36.9) | 2308 (35.7) | 1194 (35.5) | 820 (37.8) | 1257 (37.9) | 9888 (36.8) |
| Sex Ratio | 1.68 | 1.67 | 1.75 | 1.64 | 1.55 | 1.71 | 1.76 | 1.82 | 1.64 | 1.64 | 1.71 |
| Age group | – | ||||||||||
| Younger than 2 years | 48 (2.2) | 100 (4.4) | 115 (4.8) | 58(3.2) | 40 (3.2) | 81 (4.9) | 314 (4.9) | 163 (4.8) | 82 (3.8) | 152 (4.6) | 1153 (4.3) |
| 3–8 years | 1981 (91.5) | 1987 (87.6) | 2041 (85.3) | 1545(86.3) | 1101 (87.5) | 1448 (87.0) | 5357 (82.8) | 2637 (78.3) | 1675 (77.2) | 2739 (82.5) | 22,511 (83.8) |
| 9–15 years | 123 (5.7) | 167 (7.4) | 215 (9.0) | 163(9.1) | 112 (8.9) | 129 (7.7) | 668 (10.3) | 463 (13.8) | 350 (16.1) | 367 (11.1) | 2757 (10.3) |
| Older than 16 years | 12 (0.6) | 14 (0.6) | 21 (0.9) | 23(1.3) | 5 (0.4) | 7 (0.4) | 127 (2.0) | 104 (3.1) | 63 (2.9) | 63 (1.9) | 439 (1.6) |
| Districts | – | ||||||||||
| Urban | 1803 (83.3) | 1950 (86.0) | 1956 (85.7) | 1533(85.7) | 1076 (85.7) | 958 (57.5) | 3460 (53.5) | 1896 (56.3) | 1117 (51.5) | 965 (29.1) | 16,716 (62.2) |
| Rural | 361 (16.7) | 318 (14.0) | 436 (18.2) | 256(14.3) | 180 (14.3) | 707 (42.5) | 3006 (46.5) | 1471 (43.7) | 1053 (48.5) | 2356 (70.9) | 10,144 (37.8) |
| Occupation | – | ||||||||||
| Kindergarten Children | 975 (45.1) | 975 (43.0) | 1024 (42.8) | 919(51.1) | 518 (41.2) | 840 (50.5) | 2595 (40.1) | 1175 (34.9) | 669 (30.8) | 1173 (35.3) | 10863 (40.4) |
| Scattered children | 150 (6.9) | 274 (12.1) | 267 (11.2) | 164(9.2) | 107 (8.5) | 218 (13.1) | 673 (10.4) | 310 (9.2) | 190 (8.8) | 397 (12.0) | 2750 (10.2) |
| Student | 1036 (47.9) | 1015 (44.8) | 1097 (45.9) | 696(38.9) | 632 (50.2) | 604 (36.3) | 3142 (48.6) | 1846 (54.8) | 1280 (59.0) | 1726 (52.0) | 13,074 (48.7) |
| Others | 3 (0.1) | 4 (0.2) | 4 (0.2) | 10(0.6) | 1 (0.1) | 3 (0.2) | 56 (0.9) | 36 (1.1) | 31 (1.4) | 25 (0.8) | 173 (0.6) |
3.3. Temporal Pattern
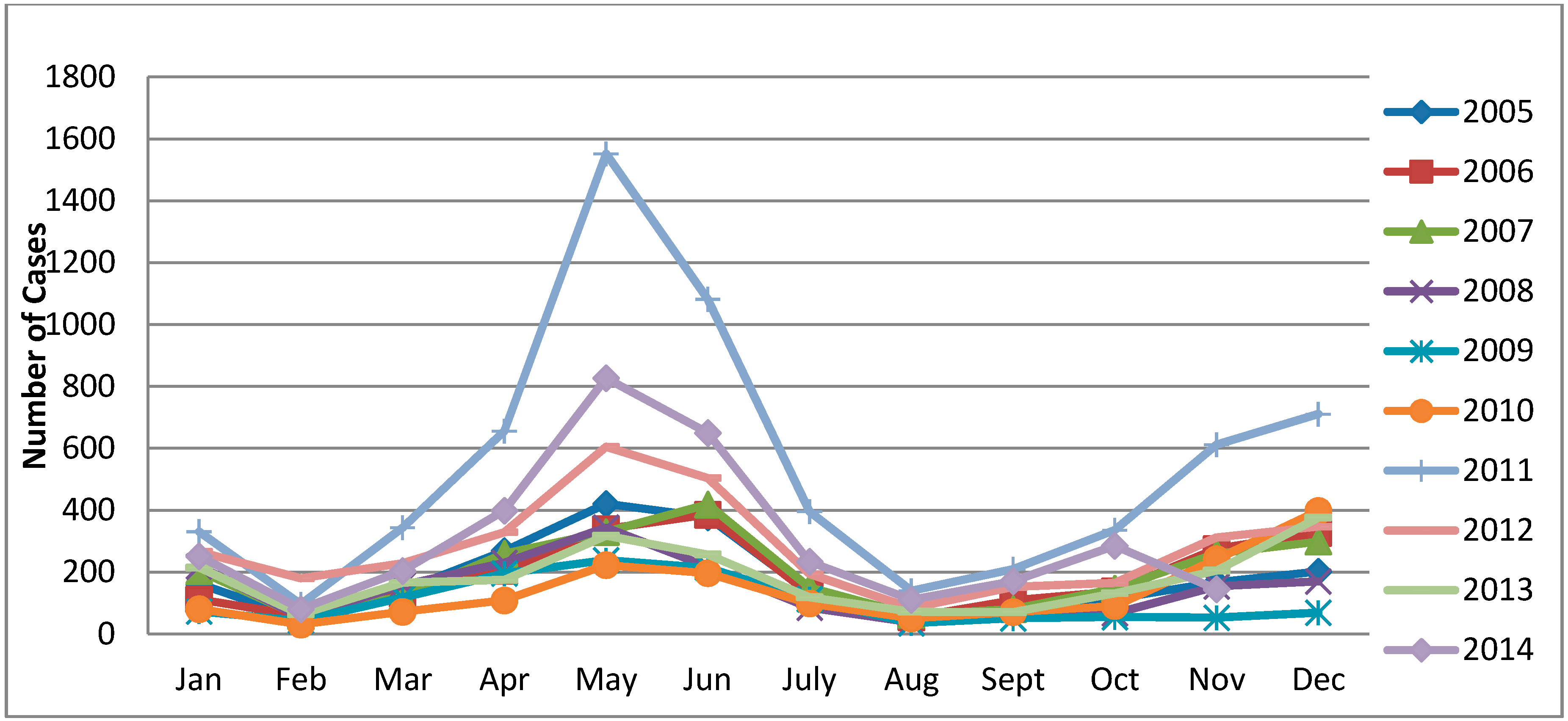
3.4. Spatial Autocorrelation Analysis
| Years | Moran I | Z-Score | p-Value |
|---|---|---|---|
| 2005 | 0.059 | 1.218 | 0.223 |
| 2006 | −0.004 | 0.629 | 0.529 |
| 2007 | 0.053 | 1.228 | 0.220 |
| 2008 | 0.100 | 1.703 | 0.088 |
| 2009 | 0.141 | 2.063 | 0.039 |
| 2010 | 0.095 | 1.641 | 0.101 |
| 2011 | 0.064 | 1.412 | 0.158 |
| 2012 | 0.040 | 1.142 | 0.253 |
| 2013 | 0.040 | 1.172 | 0.241 |
| 2014 | 0.019 | 0.946 | 0.344 |
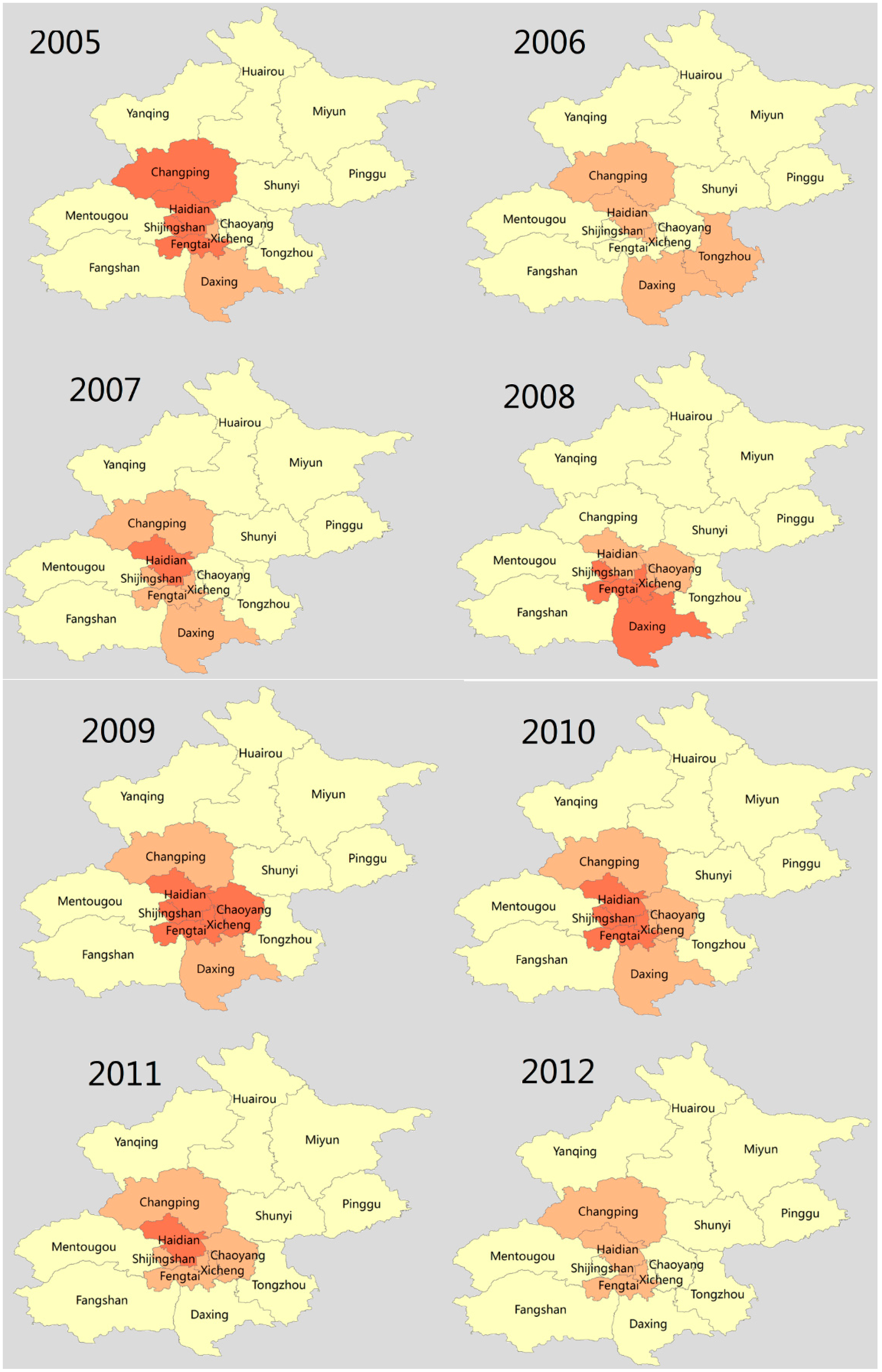
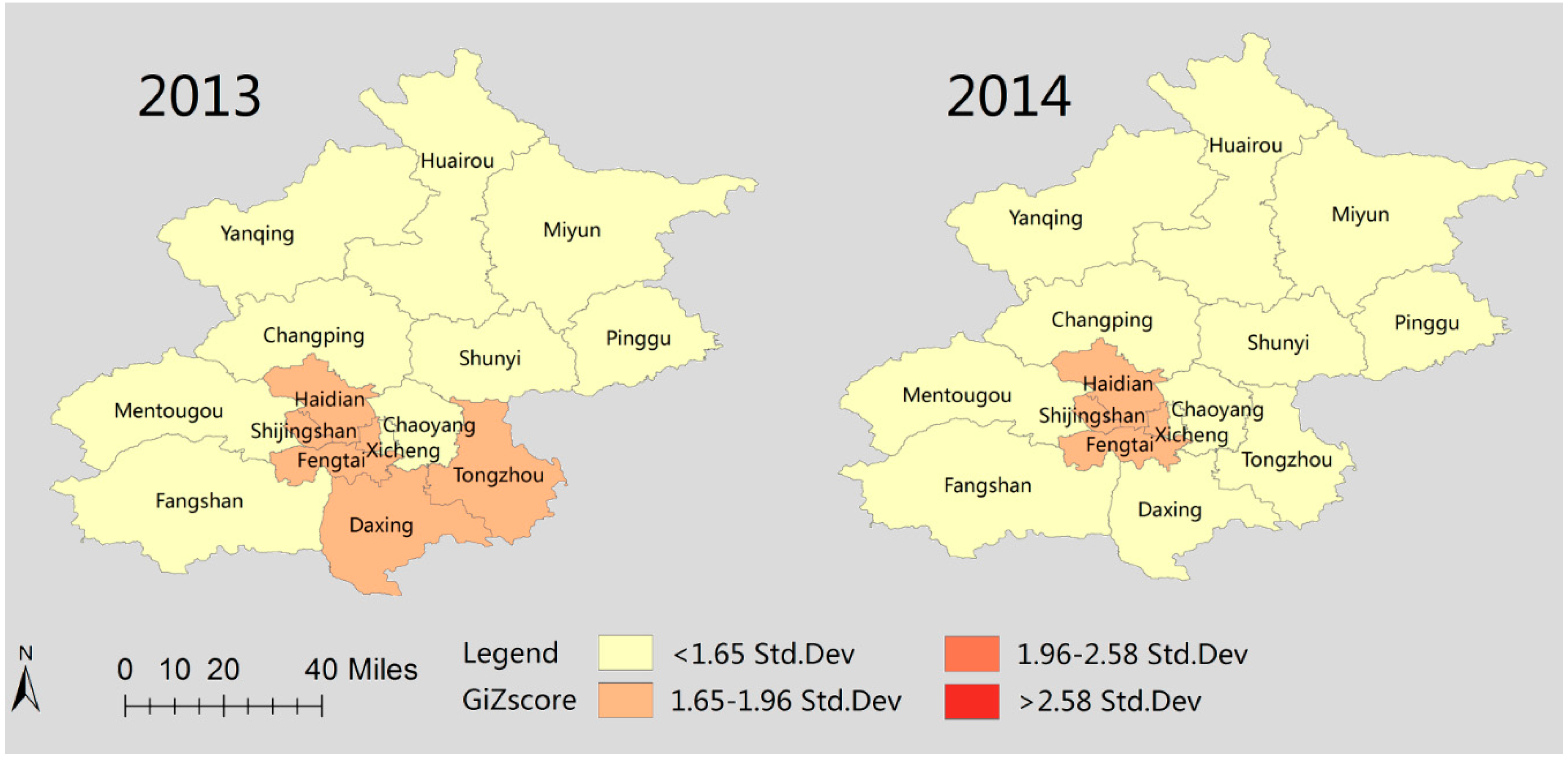
3.5. Spatial Clusters of Scarlet Fever Disease
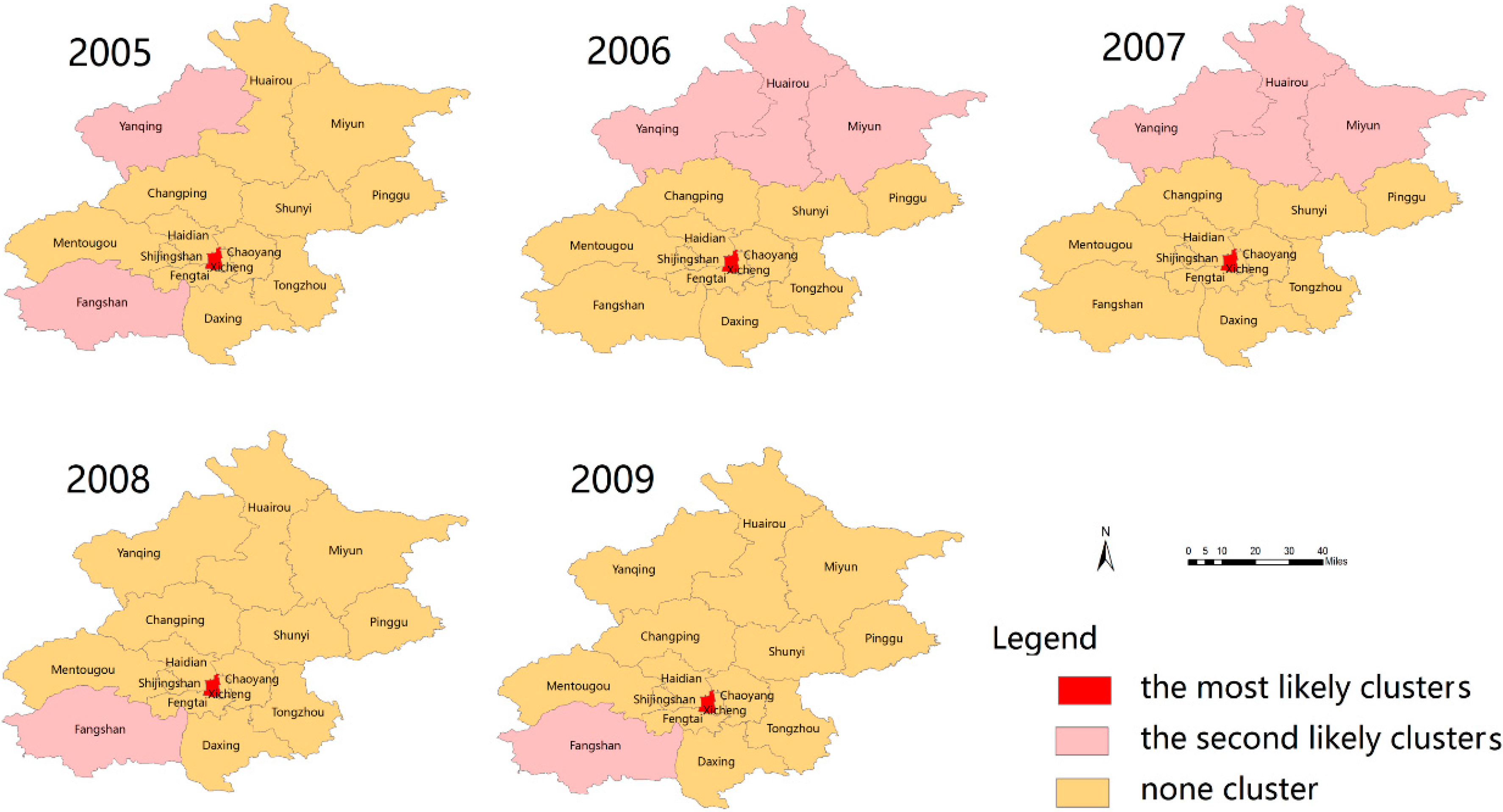
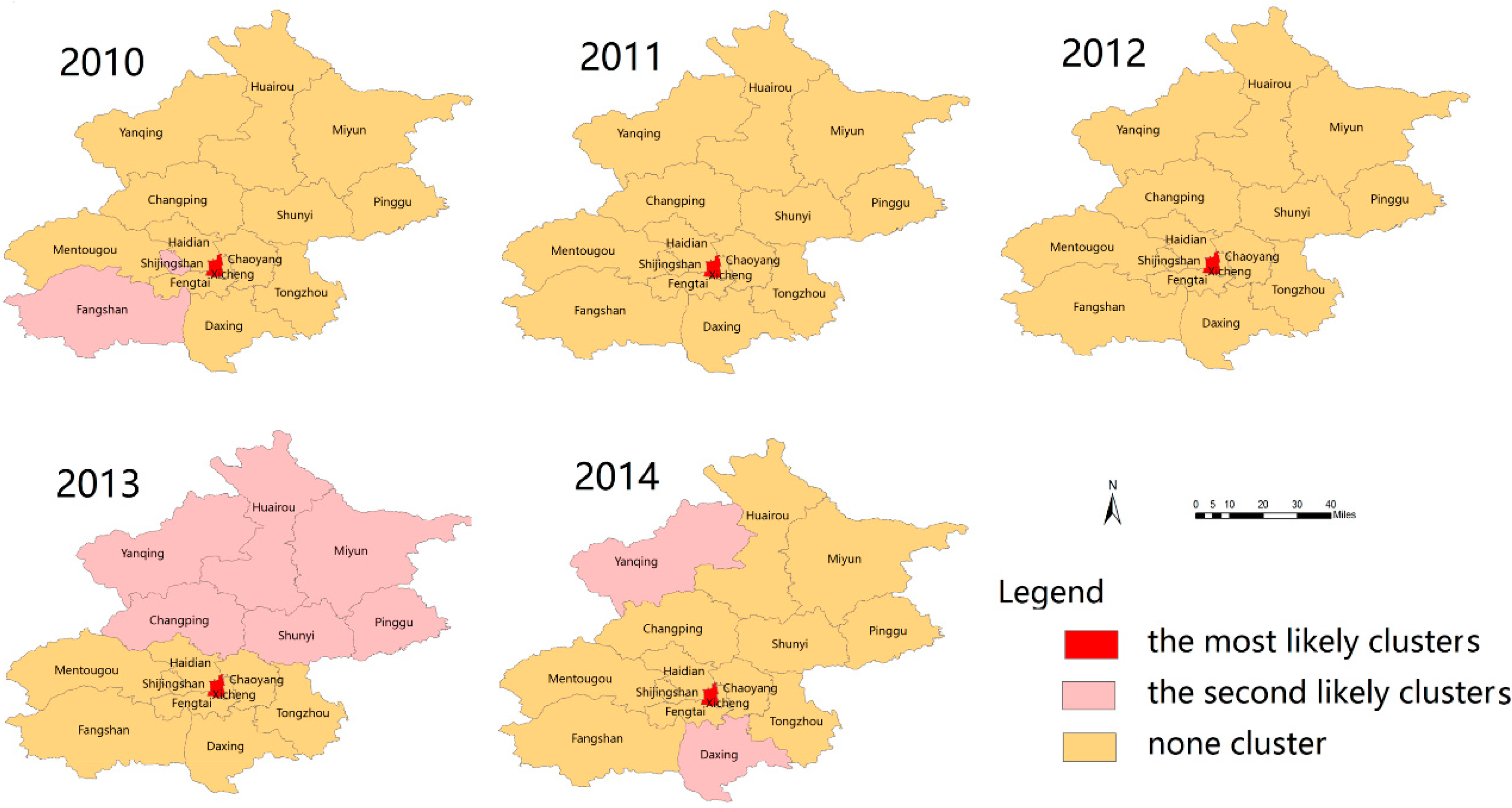
3.6. Space-Time Clusters
| Scan Year | Cluster Time Frame | Center(Latitude, Longitude)/Radius (km) | Cluster Districts | Relative Risk | p-Value |
|---|---|---|---|---|---|
| 2005 | 1 January to 30 April | 116.156 E, 40.537 N/0 | 1 | 1.90 | 0.014 |
| 2006 | 1 May to 31 August | 117.135 E, 40.205 N/0 | 1 | 1.97 | 0.000049 |
| 2007 | 1–31 January | 116.728 E, 39.801 N/35 | 4 | 1.55 | 0.012 |
| 2008 | 1 January to 29 February | 116.507 E, 39.949 N/0 | 1 | 1.82 | 0.00029 |
| 2009 | 1 January to 28 February | 39.99 N, 115.78 E/30.98 | 2 | 2.22 | 0.109 |
| 2010 | 1–31 December | 116.410 E, 39.911 N/4.34 | 2 | 1.28 | 0.081 |
| 2011 | 1 September to 31 December | 116.156 E, 40.537 N/0 | 1 | 2.97 | <0.001 |
| 2012 | 1 April to 30 June | 117.135 E, 40.205 N/56.68 | 4 | 1.49 | 0.0000014 |
| 2013 | 1 July to 30 September | 116.579 E, 40.628 N/55.86 | 5 | 1.87 | 0.017 |
| 2014 | 1 September to 30 November | 39.991 N, 115.787 E/33.31 | 3 | 1.43 | 0.107 |
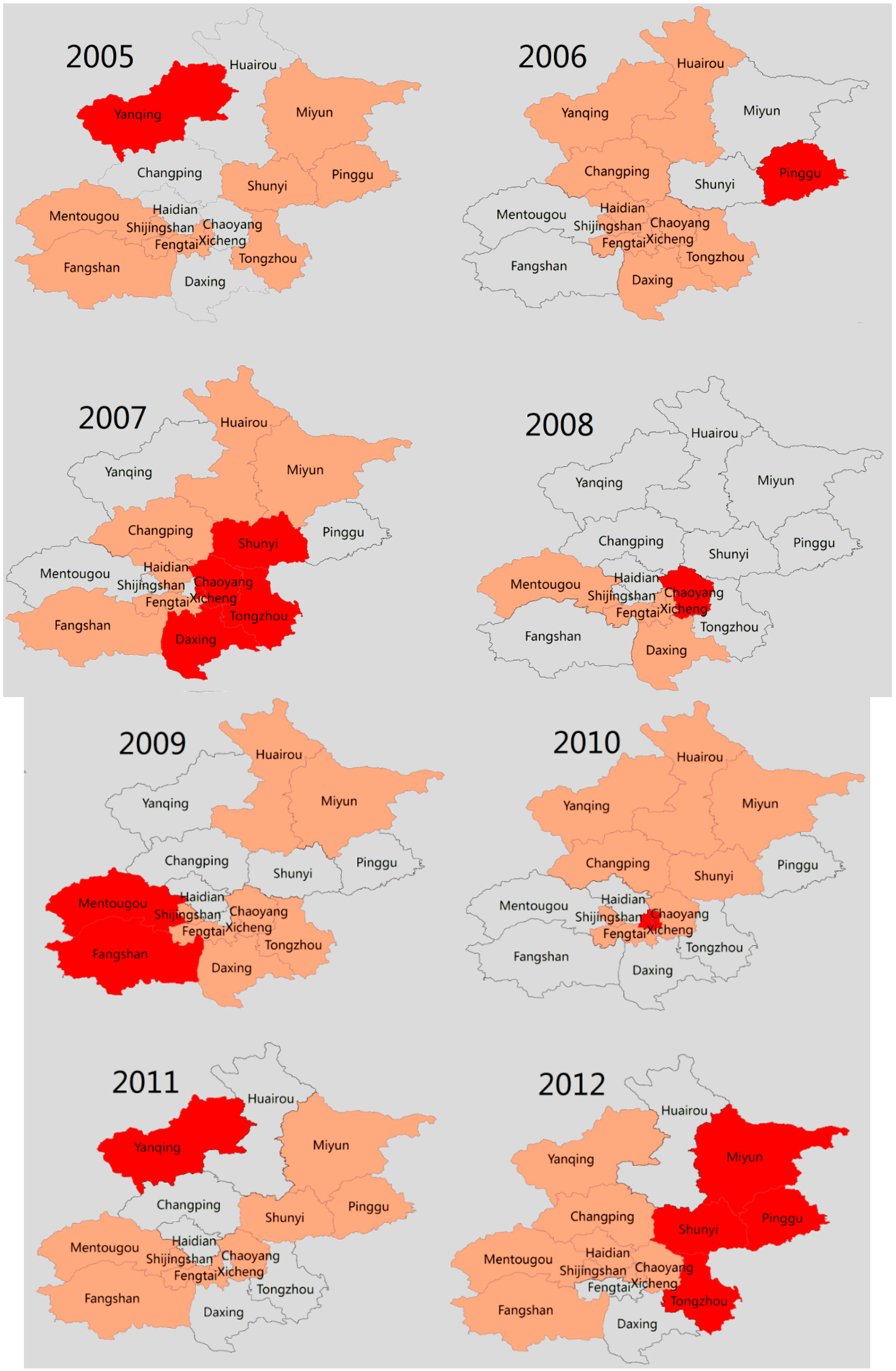
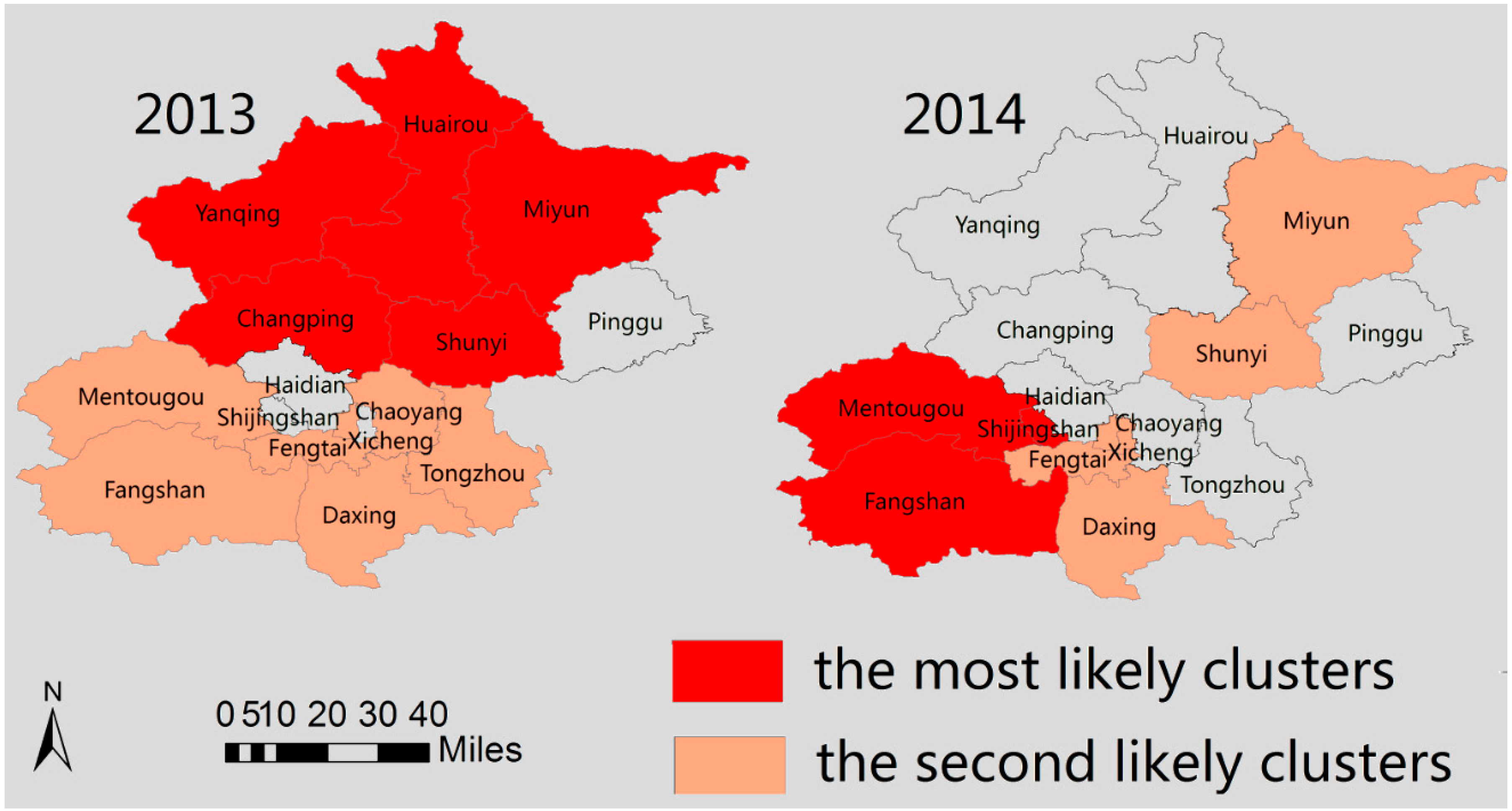
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edward, Z., Jr. Scarlet Fever. Medscape.com. 2014. Available online: http://emedicine.medscape.com/article/1053253-overview (accessed on 7 July 2015).
- World Health Organization. Western Pacific Region. Scarlet Fever: Factsheet of Scarlet Fever in China. Available online: http://www.wpro.who.int/china/mediacentre/factsheets/scarlet_fever/en/ (accessed on 5 June 2015).
- Yang, S.G.; Dong, H.J.; Li, F.R.; Xie, S.Y.; Cao, H.C.; Xia, S.C.; Yu, Z.; Li, L.J. Report and analysis of a scarlet fever outbreak among adults through food-borne transmission in China. J. Infect. 2007, 55, 419–424. [Google Scholar] [CrossRef]
- Dong, H.; Xu, G.; Li, S.; Song, Q.; Liu, S.; Lin, H.; Chai, Y.; Zhou, A.; Fang, T.; Zhang, H.; et al. Beta-haemolytic group A streptococci emm75 carrying altered pyrogenic exotoxin A linked to scarlet fever in adults. J. Infect. 2008, 56, 261–267. [Google Scholar] [CrossRef] [PubMed]
- CDC, Chinese Center for Disease Control and Prevention Beijing. Available online: http://www.chinacdc.cn/en/aboutus/200506/t20050604_22209.html (accessed on 1 June 2015).
- Eyler, J.M. Epidemiology of milk-born scarlet fever: The case of Edwardian Brighton. Am. J. Public Health 1986, 76, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.L.; Kozma, L.; Martin, A.; Landeros, A.; Katsovich, L.; King, R.A.; Leckman, J.F. Neuropsychiatric disorders associated with streptococcal infection: A case-control study among privately insured children. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Scarlet Fever: A Group A Streptococcal Infection. Available online: http://www.cdc.gov/features/scarletfever/ (accessed on 9 October 2015).
- Gómez-Carrasco, J.A.; Lassaletta, A.; Ruano, D. Acute hepatitis may form part of scarlet fever. An. Pediatr. 2004, 60, 382–383. [Google Scholar] [CrossRef]
- Güven, A. Hepatitis and hematuria in scarlet fever. Indian J. Pediatr. 2002, 69, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Woo, P.C.; Yuen, K.Y. Toxic scarlet fever complicating cellulites: Early clinical diagnosis is crucial to prevent a fatal outcome. New Microbiol. 2004, 27, 203–206. [Google Scholar] [PubMed]
- Rolleston, J.D. The history of scarlet fever. BMJ 1928, 2, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.T.; Dowse, G.K.; Keil, A.D.; Mackaay, C.; McLellan, D. Epidemiological features and control of an outbreak of scarlet fever in a Perth primary school. Commun. Dis. Intell. 2005, 29, 386–390. [Google Scholar]
- Hsieh, Y.C.; Huang, Y.C. Scarlet fever outbreak in Hong Kong, 2011. J. Microbiol. Immunol. Infect. 2011, 44, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Liu, C.C.; Ko, W.C.; Hsu, S.Y.; Wu, H.M.; Lin, Y.S.; Lin, M.T.; Chuang, W.J.; Wu, J.J. Molecular analysis of group A streptococcal isolates associated with scarlet fever in Southern Taiwan between 1993 and 2002. J. Clin. Microbiol. 2003, 41, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Peng, X.; Zhang, D.; Wu, S.; Liu, Y.; Cui, S.; Lu, G.; Duan, W.; Shi, W.; Liu, S.; et al. Group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China-2011. Emerg. Infect. Dis. 2013, 19, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, M.; Xiao, X.; Feng, Z.; Li, C.; Zhou, Z.; Ren, Q.; Li, X. Spectral analysis based on fast Fourier transformation (FFT) of surveillance data: The case of scarlet fever in China. Epidemiol. Infect. 2014, 142, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Daily News Paper of England. Available online: http://www.dailymail.co.uk/health/article-2994236/Scarlet-fever-England-doubles-one-year-doctors-saying-300-cases-reported-week-alone.html (accessed on 10 August 2015).
- Staszewska, E.; Kondej, B.; Czarkowski, M.P. Scarlet fever in Poland in 2012. Prz. Epidemiol. 2014, 68, 209–212. [Google Scholar]
- Guy, R.; Williams, C.; Irvine, N.; Reynolds, A.; Coelho, J.; Saliba, V.; Thomas, D.; Doherty, L.; Chalker, V.; von Wissmann, B.; et al. Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014, 19. [Google Scholar] [CrossRef]
- Luk, E.Y.Y.; Lo, J.Y.C.; Li, A.Z.L.; Lau, M.C.K.; Cheung, T.K.M.; Wong, A.Y.M.; Wong, M.M.H.; Wong, C.W.; Chuang, S.; Tsang, T. Scarlet fever epidemic, Hong Kong, 2011. Emerg. Infect. Dis. 2012, 18, 1658–1661. [Google Scholar]
- Chen., Y.Y.; Huang, C.T.; Yao, S.M.; Chang, Y.C.; Shen, P.W.; Chou, C.Y.; Li, S.Y. Molecular epidemiology of group A Streptococcus causing scarlet fever in northern Taiwan, 2001–2002. Diagn. Microbiol. Infect. Dis. 2007, 58, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prada, M.; Martínez-Diz, S.; López, A.C.; Nievas., D.A.; Romero, B.M.; Martínez, J.H. Scarlet fever outbreak in a Public school in Granada in 2012. An. Pediatr. 2014, 80, 249–253. [Google Scholar] [CrossRef]
- You, Y.H.; Song, Y.Y.; Yan, X.M.; Wang, H.B.; Zhang, M.H.; Tao, X.X.; Li, L.L.; Zhang, Y.X.; Jiang, X.H.; Zhang, B.H.; et al. Molucular epidemiology characteristics of Streptococcus pyogenes strains involved in an outbreak of scarlet fever in China, 2011. Biomed. Environ. Sci. 2013, 26, 877–885. [Google Scholar] [PubMed]
- Quian, H.K.; Yang, P.; Zhang, Y.; Wang, X.L.; Duan, W.; Wang, Q.Y. Spatial-Temporal scan statistics on scarlet fever cases in Beijing from 2005–2010. Dis. Surveill. 2011, 26, 435–238. [Google Scholar]
- Liu, Z.; Wang, B.X.; Wang, S.C. The analysis of dynamics of scarlet fever cases in China, 2005–2008. J. Public Health Prev. Med. 2009, 20, 21–22. [Google Scholar]
- Wang, J.; Zhang, J.Q.; Pan, H.F.; Zhu, Y.; He, Q. Epidemiological investigation of scarlet fever in Hefei City, China, from 2004 to 2008. Trop. Dr. 2010, 40, 4225–4226. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yao, W.; Wang, X.; Li, Y.; Chen, M.; Wang, G.; Zhang, X.; Pan, H.; Hu, J.; Zeng, M. Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr. Infect. Dis. J. 2012, 31, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.B. Statistical Methods in Spatial Epidemiology, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Liu, L.; Zhao, X.; Yin, F.; Lv, Q. Spatio-temporal clustering of hand, foot and mouth disease at the county level in Sichuan province, China, 2008–2013. Epidemiol. Infect. 2015, 143, 831–838. [Google Scholar] [CrossRef] [PubMed]
- BMBS. Beijing Municipal Bureau of Statistics. Available online: http://www.bjstats.gov.cn/esite/ (accessed on 15 September 2015).
- Data Sharing Infrastructure of Earth System Science. Base Map of Beijing City, China. Available online: http://www.geodata.cn/Portal/ (accessed on 17 July 2015).
- Ministry of Health of China. Incidence of Notifiable Infectious Disease in China. Available online: www.moh.gov.cn/public files (accessed on 27 September 2015).
- GIS Geography. Spatial Autocorrelation and Moran’s I in GIS. Available online: http://gisgeography.com/spatial-autocorrelation-moran-i-gis/ (accessed on 27 August 2015).
- Getis, A. Spatial interaction and spatial autocorrelation: Across-product approach. Environ. Plan. A 1991, 23, 1269–1277. [Google Scholar] [CrossRef]
- How Spatial Autocorrelation: Moran’s I (Spatial Statistics) Works. Available online: http://webhelp.esri.com/arcgisdesktop/9.3/index.cfm?TopicName=How%20Spatial%20Autocorrelation:%20Moran’s%20I %20(Spatial%20Statistics)%20works (accessed on 28 January 2015).
- Spatial Autocorrelation Statistic (Moran’s I) (Spatial Statistic). Available online: webhelp.esri.com/arcgisdesktop/9.2/index.cfm?TopicName=Spatial Autocorrelation (accessed on 23 November 2015).
- Anselin, L. Local indicators of spatial association-LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- ArcGIS Software for Geographic Study Version 10.1. Available online: www.ersi.com (accessed on 27 August 2015).
- Zulu, L.C.; Kalipeni, E.; Johannes, E. Analyzing spatial clustering and the spatiotemporal nature and trends of HIV/AIDS prevalence using GIS: The case of Malawi, 1994–2010. BMC Infect. Dis. 2014, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L.; Getis, O. Spatial statistical analysis and geographic information systems. Ann. Reg. Sci. 1992, 26, 19–33. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. Local spatial statistics: An overview. In Spatial Analysis: Modeling in GIS Environment; Longley, P., Batty, M., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 1996; pp. 261–278. [Google Scholar]
- Mitchell, A. Spatial measurements & statistics. In The ESRI Guide to GIS Analysis; ESRI Press: Redlands, CA, USA, 2005; Volume 2. [Google Scholar]
- Peeters, A.; Zude, M.; Käthner, J.; Ünlü, M.; Kanber, R.; Hetzroni, A. Getis-ord’s hot- and cold-spot statistics as a basis for multivariate spatial clustering of orchard tree data. Comput. Electron. Agric. 2015, 111, 140–150. [Google Scholar] [CrossRef]
- Saxena, R.; Nagpal, B.N.; Das, M.K.; Srivastava, A.; Gupta, S.K. A spatial statistical approach to analyze malaria situation at micro level for priority control in Ranchi district, Jharkhand. Indian J. Med. Res. 2012, 136, 776–782. [Google Scholar] [PubMed]
- Kulldorff, M.; Feuer, E.J.; Miller, B.A.; Freedman, L.S. Breast cancer clusters in the northeast United States: A geographic analysis. Am. J. Epidemiol. 1997, 146, 161–170. [Google Scholar] [CrossRef] [PubMed]
- SaTScan. Available online: www.satscan.org (accessed on 23 November 2015).
- Zhang, W.; Wang, L.; Fang, L.; Ma, J.; Xu, Y.; Jiang, J.; Hui, F.; Wang, J.; Liang, S.; Yang, H.; et al. Spatial analysis of malaria in Anhui province, China. Malar. J. 2008, 7. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, K.P.; Abrams, A.M.; Kulldorff, M.; Platt, R. A model-adjusted space-time scan statistic with an application to syndromic surveillance. Epidemiol. Infect. 2005, 133, 409–419. [Google Scholar] [CrossRef] [PubMed]
- SaTScan Software for Spatial Temporal Analysis. Software for the Spatial and Space-Time Scan Statistics. Available online: www.satscan.org (accessed on 9 June 2015).
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Kulldorff, M. SaTScan User Guide for Version 9.4. Available online: http://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download (accessed on 23 November 2015).
- Deng, T.; Huang, Y.; Yu, S.; Gu, J.; Huang, C.; Xiao, G.; Hao, Y. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS ONE 2013, 8, e56943. [Google Scholar] [CrossRef] [PubMed]
- Public Health England, United Kingdom. Scarlet Fever: Second Year of High Activity. Available online: https://www.gov.uk/government/news/scarlet-fever-second-year-of-high-activity (accessed on 9 July 2015).
- Lamagni, T.; Dennis, J.; George, R.; Efstratiou, A. Analysis of Epidemiological Patterns during a Century of Scarlet Fever. 2008. Available online: www.hpa.org.uk/webc/hpawebfile/hpaweb (accessed on 27 August 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahara, G.; Wang, C.; Huo, D.; Xu, Q.; Huang, F.; Tao, L.; Guo, J.; Cao, K.; Long, L.; Chhetri, J.K.; et al. Spatiotemporal Pattern Analysis of Scarlet Fever Incidence in Beijing, China, 2005–2014. Int. J. Environ. Res. Public Health 2016, 13, 131. https://doi.org/10.3390/ijerph13010131
Mahara G, Wang C, Huo D, Xu Q, Huang F, Tao L, Guo J, Cao K, Long L, Chhetri JK, et al. Spatiotemporal Pattern Analysis of Scarlet Fever Incidence in Beijing, China, 2005–2014. International Journal of Environmental Research and Public Health. 2016; 13(1):131. https://doi.org/10.3390/ijerph13010131
Chicago/Turabian StyleMahara, Gehendra, Chao Wang, Da Huo, Qin Xu, Fangfang Huang, Lixin Tao, Jin Guo, Kai Cao, Liu Long, Jagadish K. Chhetri, and et al. 2016. "Spatiotemporal Pattern Analysis of Scarlet Fever Incidence in Beijing, China, 2005–2014" International Journal of Environmental Research and Public Health 13, no. 1: 131. https://doi.org/10.3390/ijerph13010131







