Association between UGT1A1 Polymorphism and Risk of Laryngeal Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Experimental Section
2.1. Statistical Analysis
2.2. Ethical Considerations
3. Results
| Variables | Laryngeal Cancer Cases (n = 103) N (%) | Controls (n = 220) N (%) | p-Value a |
|---|---|---|---|
| Demographic characteristics | |||
| Gender | |||
| Male | 99 (96.12) | 211 (95.91) | |
| Female | 4 (3.88) | 9 (4.09) | 1.000 |
| Age | |||
| <60 | 25 (24.27) | 64 (29.09) | |
| ≥60 | 78 (75.73) | 156 (70.91) | 0.366 |
| Clinical stages | |||
| 0 | 2 (1.94) | ||
| I | 30 (29.13) | ||
| II | 38 (36.89) | ||
| III | 27 (26.21) | ||
| IV | 6 (5.83) | ||
| Histological grades | |||
| Low-differentiation | 26 (25.24) | ||
| Moderate-differentiation | 40 (38.83) | ||
| High-differentiation | 37 (35.92) | ||
| Exposure Status | |||
| Smoking status | |||
| Never-smokers | 22 (21.36) | 108 (49.09) | |
| Ever-smokers | 81 (78.64) | 112 (50.91) | <0.001 |
| Alcohol consumption | |||
| Never-drinkers | 36 (34.95) | 134 (60.91) | |
| Ever-drinkers | 67 (65.05) | 86 (39.09) | <0.001 |
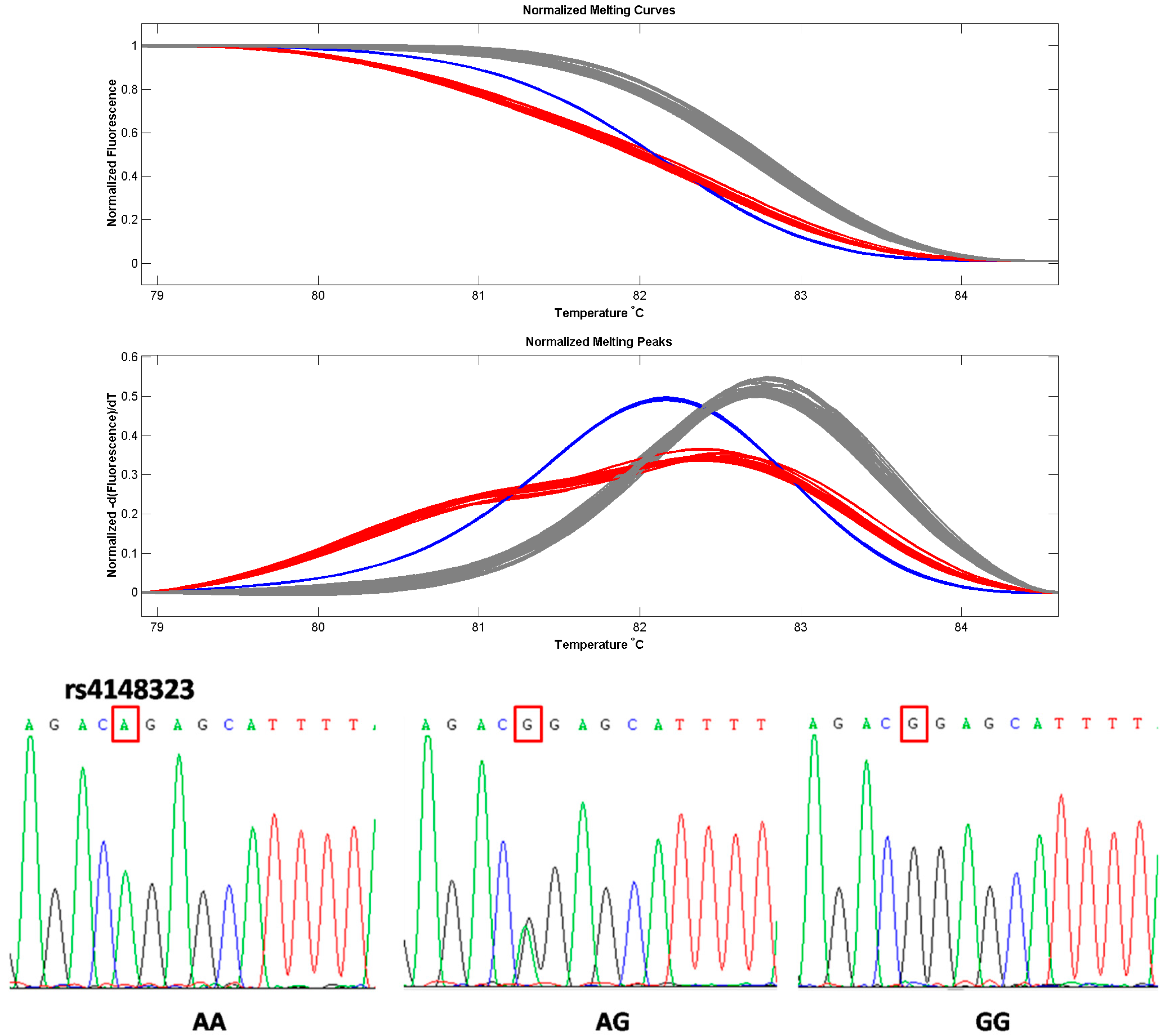
| Genotype | Patients (n = 103) Number (%) | Controls (n = 220) Number (%) | OR (95% CI) a | p |
|---|---|---|---|---|
| rs4148323 | ||||
| AA | 6 (5.8) | 29 (13.2) | 1.00 (reference) | |
| AG | 21 (20.4) | 62 (28.2) | 1.142 (0.893–1.460) | 0.335 |
| GG | 76 (73.8) | 129 (58.6) | 1.135 (1.032–1.249) | 0.022 * |
| rs4148323 Allele | Patients (Gene Frequency) | Controls (Gene Frequency) | OR (95% CI) a | p | MAF [22] | |||
|---|---|---|---|---|---|---|---|---|
| EA or AA | KR | JP | HC | |||||
| A | 0.16 | 0.27 | 1.00 (reference) | - | 0.172 | 0.106 | 0.223 | |
| G | 0.84 | 0.73 | 1.149 (1.052–1.254) | 0.004 * | ||||
| Patients | AA Number (%) | AG+GG Number (%) | OR (95% CI) a | p |
|---|---|---|---|---|
| Histological grade | ||||
| Moderate or low-differentiation | 3(4.6) | 63 (95.5) | 1.0 (reference) | |
| High-differentiation | 3 (8.1) | 34 (91.9) | 1.039 (0.931–1.159) | 0.664 |
| Clinical stage | ||||
| 0–II | 4 (5.7) | 66 (94.3) | 1.00 (reference) | |
| III–IV | 2 (6.1) | 31 (93.9) | 1.004 (0.904–1.114) | 1.000 |
| Variables | Patients/Control | AA | AG+GG | p | OR (95% CI) a |
|---|---|---|---|---|---|
| Smoking | |||||
| Ever | 81/112 | 4/16 | 77/96 | 0.035 * | 1.109 (1.013–1.214) a |
| Never | 22/108 | 2/13 | 20/95 | 1.000 | 1.033 (0.890–1.200) b |
| Drinking | |||||
| Ever | 67/86 | 2/14 | 65/72 | 0.008 ** | 1.159 (1.046–1.284) a |
| Never | 36/134 | 4/15 | 32/119 | 1.000 | 1.001 (0.879–1.140) b |
| Variables | Genotype | Patients | Control | OR (95% CI) | p |
|---|---|---|---|---|---|
| Smoking | |||||
| Never | AA | 2 | 13 | 1.00 (reference) | |
| Ever | AA | 4 | 16 | 1.208 (0.628–2.324) a | 0.680 |
| Never | AG+GG | 20 | 95 | 1.00 (reference) | |
| Ever | AG+GG | 77 | 96 | 1.579 (1.327–1.879) b | <0.001 |
| Drinking | |||||
| Never | AA | 4 | 15 | 1.00 (reference) | |
| Ever | AA | 2 | 14 | 0.690 (0.210–2.276) a | 0.666 |
| Never | AG+GG | 32 | 119 | 1.00 (reference) | |
| Ever | AG+GG | 65 | 72 | 1.778 (1.413–2.237) b | <0.001 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, W.Y.; Olshan, A.F.; Schwartz, S.M.; Berndt, S.I.; Chen, C.; Llaca, V.; Chanock, S.J.; Fraumeni, J.F.; Hayes, R.B. Selected genetic polymorphisms in MGMT, XRCC1, XPD, and XRCC3 and risk of head and neck cancer: Apooled analysis. Cancer Epidemol. Biomark. Prev. 2005, 14, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Eng, C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res. 2003, 114, 15–60. [Google Scholar] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Tukey, R.H.; Strassburg, C.P. Genetic multiplicity of the human UDP glucuronosyltransferases and regulation in the gastrointestinal tract. Mol. Pharmacol. 2001, 59, 405–414. [Google Scholar] [PubMed]
- Zheng, Z.; Fang, J.L.; Lazarus, P. Glucuronidation: An important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab. Dispos. 2002, 30, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Beland, F.A.; Doerge, D.R.; Wiener, D.; Guillemette, C.; Marques, M.M.; Lazarus, P. Characterization of benzo(a) pyrenetrans-7,8-dihydrodiol glucuronidation by human tissue microsomes and overexpressed UDP-glucuronosyltransferase enzymes. Cancer Res. 2002, 62, 1978–1986. [Google Scholar] [PubMed]
- Lacko, M.; Roelofs, H.M.; TeMorsche, R.H.; Voogd, A.C.; Ophuis, M.B.; Peters, W.H.; Manni, J.J. Genetic polymorphism in the conjugating enzyme UGT1A1 and the risk of head and neck cancer. Int. J. Cancer 2010, 127, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Szanyi, I.; Ráth, G.; Móricz, P.; Somogyvári, K.; Révész, P.; Gerlinger, I.; Orsós, Z.; Ember, I.; Kiss, I. Effects of cytochrome P450 1A1 and uridine-diphosphate-glucuronosyltransferase 1A1 allelic polymorphisms on the risk of development and the prognosis of head and neck cancers. Eur. J. Cancer Prev. 2012, 21, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Baranano, D.E.; Rao, M.; Ferris, C.D.; Snyder, S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA 2002, 99, 16093–16098. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.D.; Horn, P.S.; Sherman, K.E. Serum bilirubin levels in the U.S. population: Gender effect and inverse correlation with colorectal cancer. Hepatology 2004, 40, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Temme, E.H.; Zhang, J.; Schouten, E.G.; Kesteloot, H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control 2001, 12, 887–894. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. “Iatrogenic Gilbert syndrome”—A strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med. Hypotheses 2007, 69, 974–994. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Gelbart, T.; Demina, A. Racial variability in the UDP glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl. Acad. Sci. USA 1998, 95, 8170–8174. [Google Scholar] [CrossRef] [PubMed]
- Bosma, P.J.; Chowdhury, J.R.; Bakker, C.; Gantla, S.; de Boer, A.; Oostra, B.A.; Lindhout, D.; Tytgat, G.N.; Jansen, P.L.; Oude Elferink, R.P.; et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N. Engl. J. Med. 1995, 333, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, G.; Ryan, M.; Seddon, R.; Hume, R.; Burchell, B. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 1996, 347, 578–581. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Jansen, P.L.; Steegers, E.A.; Peters, W.H. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J. Hepatol. 2000, 33, 348–351. [Google Scholar] [CrossRef]
- Mercke Odeberg, J.; Andrade, J.; Holmberg, K.; Hoglund, P.; Malmqvist, U.; Odeberg, J. UGT1A polymorphisms in a Swedish cohort and a human diversity panel, and the relation to bilirubin plasma levels in males and females. Eur. J. Clin. Pharmacol. 2006, 62, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, X.; Wang, Y.; Zhang, F.; Wang, Y.; Fu, W.; Yu, T.; Li, S.; Xiong, M.; Huang, W.; et al. Association of polymorphisms in four bilirubin metabolism genes with serum bilirubin in three Asian populations. Hum. Mutat. 2009, 30, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Aono, S.; Yamada, Y.; Keino, H.; Hanada, N.; Nakagawa, T.; Sasaoka, Y.; Yazawa, T.; Sato, H.; Koiwai, O. Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem. Biophys. Res. Commun. 1993, 197, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Kowalski, L.P.; Oliveira, B.V.; Curado, M.P.; Pereira, R.N.; Silva, M.E.; Fava, A.S.; Torloni, H. Risk factors for oral cancer in Brazil: A case-control study. Int. J. Cancer 1989, 43, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Xiang, Y.B.; Yang, G.; Cai, Q.; Fazio, S.; Linton, M.F.; Elasy, T.; Xu, W.H.; Li, H.; Cai, H.; et al. Prevalence and determinants of metabolic syndrome according to three definitions in middle-aged Chinese men. Metab. Syndr. Relat. Disord. 2009, 7, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cheong, H.S.; Park, B.L.; Kim, L.H.; Namgoong, S.; Kim, J.O.; Kim, H.D.; Kim, Y.H.; Chung, M.W.; Han, S.Y.; et al. Comprehensive variant screening of the UGT gene family. Yonsei Med. J. 2014, 55, 232–239. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, R.F.; Bergmann, A.; Thuler, L.C. Alcohol consumption and risk of cancer: A systematic literature review. Asian Pac. J. Cancer Prev. 2013, 14, 4965–4972. [Google Scholar] [CrossRef] [PubMed]
- Szyfter, K.; Szmeja, Z.; Szyfter, W.; Hemminki, K.; Banaszewski, J.; Jaskuła-Sztul, R.; Louhelainen, J. Molecular and cellular alterations in tobacco smoke-associated larynx cancer. Mutat. Res. 1999, 445, 259–274. [Google Scholar] [CrossRef]
- McDonald, S.; Haie, C.; Rubin, P.; Nelson, D.; Divers, L.D. Second malignant tumors in patients with laryngeal carcinoma: Diagnosis, treatment, and prevention. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 457–465. [Google Scholar] [CrossRef]
- Albino, A.P.; Huang, X.; Jorgensen, E.; Yang, J.; Gietl, D.; Traganos, F.; Darzynkiewicz, Z. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: A new assay for carcinogens. Cell Cycle 2004, 3, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Jeggo, P.A. The role of double-strand break repair-insights from human genetics. Nat. Rev. Genet. 2006, 7, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and sites of distant metastases from head and neck cancer. ORL J. Otorhinolaryngol. Relat. Spec. 2001, 63, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Dobrossy, L. Epidemiology of head and neck cancer: Magnitude of the problem. Cancer Metastasis Rev. 2005, 24, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, J.; Gao, Q.; Yu, W.; Yang, Q.; Li, X. Laryngeal cancer risk and common single nucleotide polymorphisms in nucleotide excision repair pathway genes ERCC1, ERCC2, ERCC3, ERCC4, ERCC5 and XPA. Gene 2014, 542, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Starska, K.; Krześlak, A.; Forma, E.; Olszewski, J.; Lewy-Trenda, I.; Osuch-Wójcikiewicz, E.; Bryś, M. Genetic polymorphism of metallothionein 2A and risk of laryngeal cancer in a Polish population. Med. Oncol. 2014, 31, 75. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lin, F.; Liao, S.; Bao, Q.; Ni, L. Effects of SNPs (CYP1B1*2 G355T, CYP1B1*3 C4326G, and CYP2E1*5 G-1293C), smoking, and drinking on susceptibility to laryngeal cancer among Han Chinese. PLoS ONE 2014, 9, e106580. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zeng, Y.; Xu, W.D.; Liu, C.; Wang, Y.J.; Wang, Y.D.; Wang, Y.D. Genetic Association between the XPG Asp1104His Polymorphism and Head and Neck Cancer Susceptibility: Evidence Based on a Meta-Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.H.; Min, J.W.; Jeon, H.B.; Cho, D.H.; Oh, J.S.; Lee, H.G.; Hwang, S.G.; An, S.; Han, Y.H.; Kim, J.S. ERp57 modulates STAT3 activity in radioresistant laryngeal cancer cells and serves as a prognostic marker forlaryngeal cancer. Oncotarget 2015, 6, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huangfu, H.; Pan, H.; Wang, B.; Wen, S.; Han, R.; Li, L. Association between UGT1A1 Polymorphism and Risk of Laryngeal Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2016, 13, 112. https://doi.org/10.3390/ijerph13010112
Huangfu H, Pan H, Wang B, Wen S, Han R, Li L. Association between UGT1A1 Polymorphism and Risk of Laryngeal Squamous Cell Carcinoma. International Journal of Environmental Research and Public Health. 2016; 13(1):112. https://doi.org/10.3390/ijerph13010112
Chicago/Turabian StyleHuangfu, Hui, Hong Pan, Binquan Wang, Shuxin Wen, Rui Han, and Li Li. 2016. "Association between UGT1A1 Polymorphism and Risk of Laryngeal Squamous Cell Carcinoma" International Journal of Environmental Research and Public Health 13, no. 1: 112. https://doi.org/10.3390/ijerph13010112





