Serum Adiponectin and Leptin Concentrations in Relation to Body Fat Distribution, Hematological Indices and Lipid Profile in Humans
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Measures
2.2.1. Anthropometric Measurements and Bioelectrical Impedance Analysis (BIA)
2.2.2. Biochemical Parameters of Venous Blood
2.2.3. Statistical Analysis
3. Results
3.1. Anthropomorphic Parameters in the Study Group
| Parameter | Female and Male n = 95 | Female n = 55 | Male n = 40 |
|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | |
| Age [years] | 37 (23–58) | 38 (23–54) | 32 (24–58) |
| Height [cm] | 170 (153–193) | 168 (153–178) | 178.0 ***F (158–193) |
| Weight [kg] | 95.25 (61.0–139) | 81.90 (61–139) | 97.60 *F (66.9–135.1) |
| BMI [kg/m2] | 30.35 (23.3–53) | 30.20 (23.3–53) | 31.9 (24.6–41.2) |
| WHR | 0.85 (0.82–1.09) | 0.89 (0.82–1.03) | 0.91 (0.84–1.09) |
| SBP[mm Hg] | 130 (105–160) | 125 (105–140) | 140.0 ***F (125–160) |
| DBP[mm Hg] | 80 (70–100) | 75 (70–85) | 80.0 *F (70–100) |
| LBM [kg] [%] | 58.30 (40.6–96.5) 64.82 (51.58–77.36) | 51.30 (40.6–71.7) 61.17 (51.58–68.87) | 70.9 ***F (43.20–96.5) 68.78 ***F (62.80–77.36) |
| SMM [kg] [%] | 25.75 (13.3–50.0) 27.487 (19.11–41.51) | 23.20 (13.9–30.9) 25.10 (19.11–37.2) | 33.40 ***F (13.3–50.0) 30.70 **F (19.88–41.51) |
| VFM [kg] [%] | 4.45 (2.4–12.4) 5.26 (3.18–8.92) | 4.60 (2.4–12.4) 5.46 (3.52–8.92) | 4.30 (3.1–9.6) 4.63 (3.18–7.19) |
| SFM [kg] [%] | 25.85 (18.0–54.9) 30.67 (19.47–39.49) | 27.20 (18.0–54.9) 33.21 (27.61–39.5) | 22.3 (18.5–40.1) 25.84 ***F (19.47–30.79) |
| SFM:VFM | 5.91 (3.91–7.83) | 6.15 (4.43–7.83) | 5.44 **F (3.99–6.65) |
| MBF:LBM | 0.54 (0.29–0.94) | 0.63 (0.45–0.94) | 0.45 ***F (0.29–0.59) |
| SLMT [kg] [%] | 26.26 (18.94–42.73) 29.43 (22.12–35.66) | 23.37 (18.94–31.26) 27.85 (22.12–31.98) | 31.68 ***F (20.30–42.73) 31.63 ***F (27.86–35.66) |
| MBFT [kg] [%] | 15.74 (10.49–34.52) 18.09 (11.62–24.83) | 16.31 (10.49–34.52) 19.915 (16.04–24.83) | 13.67 (11.25–25.56) 16.05 ***K (11.62–19.13) |
| SFMT [kg] [%] | 11.08 (7.85–22.12) 13.64 (8.44–15.91) | 11.71 (8.09–22.12) 14.37 (12.51–15.91) | 9.57 (7.85–15.96) 10.61 ***F (8.44–13.57) |
| SFMT:VFMT | 2.55 (1.57–3.55) | 2.68 (1.78–3.55) | 2.31**F (1.57–2.93) |
| MBFRL [kg] [%] | 5.57 (3.70-11.94) 6.36 (4.10–8.59) | 5.73 (3.70–11.94) 6.99 (5.64–8.59) | 4.81 **MLL MBF (3.99–8.99) 5.64 ***F, ** MLL MBF(4.1–6.73) |
| MBFLL [kg] [%] | 5.58 (3.70–11.93) 6.42 (4.08–8.58) | 5.68 (3.70–11.93) 6.97 (5.64–8.58) | 4.86 (3.98–9.04) 5.64 ***F (4.08–6.77) |
3.2. Clinical and Biochemical Characteristics of the Study Population
| Parameter | Female and Male n = 95 | Female n = 55 | Male n = 40 |
|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | |
| Adiponectin[ng/mL] | 41.37 (6.01–145.40) | 59.40 (16.37–145.40) | 34.98 **F (6.01–85.53) |
| Leptin [pg/mL] | 221.85 (45.68–894.50) | 255.30 (98.83–894.50) | 102.50 ***F (45.68–430.70) |
| ADPN/LEP ratio | 0.2 (0.03–1.28) | 0.2 (0.03–0.86) | 0.2 (0.11–1.27) |
| RBC [1012/L] | 4.92 (3.85–5.89) | 4.76 (3.85–5.46) | 5.20 **F (4.49–5.89) |
| HGB [mmol/L] | 7.65 (5.00–9.00) | 7.50 (5.00–8.00) | 8.40 ***F (7.20–9.00) |
| HCT [L/L] | 0.41 (0.29–0.51) | 0.40 (0.29–0.43) | 0.45 ***F (0.39–0.51) |
| MCV [fL] | 85.00 (70.00–93.00) | 84.00 (70.00–92.00) | 86.00 (82.00–93.00) |
| MCH [fmol] | 1.57 (1.19–1.77) | 1.55 (1.19–1.73) | 1.59 (1.49–1.77) |
| MCHC [mmol/L] | 18.60 (17.10–19.60) | 18.60 (17.10–19.10) | 18.70 (17.20–19.60) |
| RDW [%] | 12.70 (10.90–16.30) | 12.80 (10.90–16.30) | 12.60 (11.50–13.50) |
| WBC [109/L] | 7.25 (5.00–10.20) | 7.80 (5.00–10.20) | 6.70 (5.70–8.50) |
| LYM [109/L] | 2.60 (1.80–4.20) | 2.60 (1.80–4.20) | 2.60 (1.90–3.20) |
| MON [109/L] | 0.50 (0.30–0.80) | 0.50 (0.30–0.80) | 0.50 (0.40–0.80) |
| GRA [109/L] | 4.15 (2.50–6.20) | 4.30 (2.50–6.20) | 3.800 (2.70–5.30) |
| Extracellular hemoglobin [g/dL] | 16.19 (8.76–110.48) | 13.33 (8.76–110.48) | 17.14 **F (14.10–36.19) |
| Total bilirubin [mg/dL] | 2.36 (1.24–7.20) | 2.25 (1.35–4.16) | 3.60 *F (1.24–7.20) |
| Total protein [g/L] | 52.88 (21.24–67.35) | 53.333 (21.24–67.35) | 51.98 (33.45–57.85) |
| Albumin [g/L] | 38.87 (19.72–59.95) | 36.53 (19.72–59.95) | 40.53 (26.15–55.89) |
| Cholesterol [mg/dL] | 206.50 (123.00–314.00) | 205.00 (142.00–287.00) | 222.00 (123.00–314.00) |
| HDL [mg/dL] | 28.32 (7.08–41.59) | 30.09 (16.37–41.59) | 25.66 *F (7.08–34.07) |
| LDL [mg/dL] | 147.43 (39.58–238.80) | 148.74 (102.16–238.80) | 133.97 (39.58–228.05) |
| TG [mg/dL] | 132.61 (37.68–372.46) | 118.84 (37.68–297.10) | 171.01 **F (89.86–372.46) |
| TCh:HDL | 7.12 (4.40–34.32) | 6.66 (4.40–14.90) | 8.16 (5.17–34.32) |
| LDL:HDL | 4.99 (2.24–26.61) | 4.96 (2.85–11.37) | 5.13 (2.24–26.61) |
| TG:TCh | 0.64 (0.24–2.87) | 0.58 (0.24–1.21) | 0.96 *F (0.41–2.87) |
3.3. Relationships between the Analyzed Anthropomorphic Parameters and Serum Adiponectin and Leptin Concentrations
| Parameter | Female and Male n = 95 | Female n = 55 | Male n = 40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ADPN [ng/mL] | LEP [pg/mL] | ADPN/LEP Ratio | ADPN [ng/mL] | LEP [pg/mL] | ADPN/LEP Ratio | ADPN [ng/mL] | LEP [pg/mL] | ADPN/LEP Ratio | |
| Height [cm] | –0.33 | - | - | - | - | - | –0.59 | - | - |
| Weight [kg] | –0.42 | - | –0.33 | - | 0.59 | - | - | - | –0.52 |
| BMI [kg/m2] | - | - | –0.42 | - | 0.59 | –0.52 | - | - | - |
| WHR | - | - | - | - | 0.57 | –0.42 | - | - | - |
| SBP [mmHg] | –0.49 | - | - | - | - | - | - | - | - |
| LBM [kg]/[%] | –0.50/- | -/–0.81 | -/- | -/- | 0.55/–0.64 | -/- | –0.62/- | -/–0.71 | –0.54/- |
| MBF [kg]/[%] | -/- | 0.54/- | –0.47/- | -/- | 0.62/0.65 | –0.42/- | -/- | 0.54/0.71 | -/- |
| SMM [kg]/[%] | –0.49/- | –0.37/–0.62 | -/- | -/- | -/- | -/- | -/- | -/–0.59 | -/- |
| VFM [kg]/[%] | - | 0.34/0.56 | –0.41/- | -/- | 0.61/0.63 | –0.47/- | -/- | -/0.55 | -/- |
| SFM [kg]/[%] | -/- | 0.58/0.81 | –0.49/- | -/- | 0.62/0.64 | –0.42/- | -/- | 0.60/0.69 | –0.55/- |
| SFM:VFM | - | - | - | - | –0.57 | - | - | - | - |
| MBF:LBM | - | 0.8 | - | - | 0.64 | - | - | 0.71 | - |
| SLMT [kg]/[%] | –0.48/- | -/–0.77 | -/0.43 | -/- | 0.54/–0.63 | -/0.49 | –0.59/- | -/–0.69 | –0.52/- |
| MBFT[kg]/[%] | -/- | 0.54/0.80 | –0.47/–0.42 | -/- | 0.62/0.64 | –0.42/–0.48 | -/- | 0.53/0.71 | -/–0.53 |
| SFMT [kg]/[%] | -/- | 0.61/0.81 | –0.51/–0.39 | -/- | 0.63/0.66 | –0.42/–0.49 | -/- | 0.65/0.73 | –0.63/–0.58 |
| SFMT:VFMT | - | - | - | - | –0.58 | 0.40 | - | - | - |
| MBFRL [kg]/[%] | -/- | 0.53/0.8 | -/- | -/- | 0.60/0.64 | -/- | -/- | 0.56/0.68 | -/- |
| MBFLL [kg]/[%] | -/- | 0.53/0.81 | -/- | -/- | 0.62/0.68 | -/- | -/- | 0.55/0.68 | -/- |
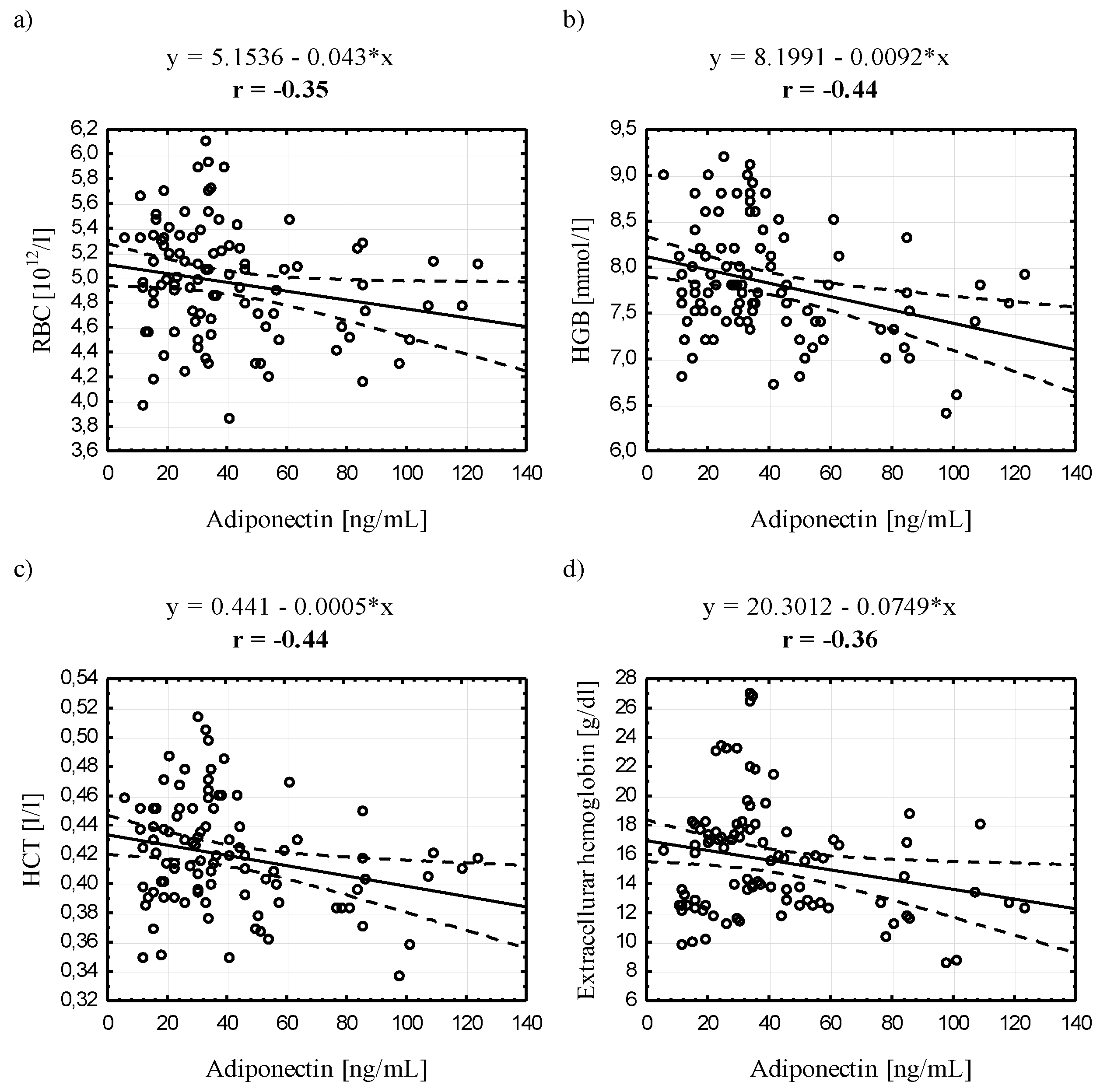
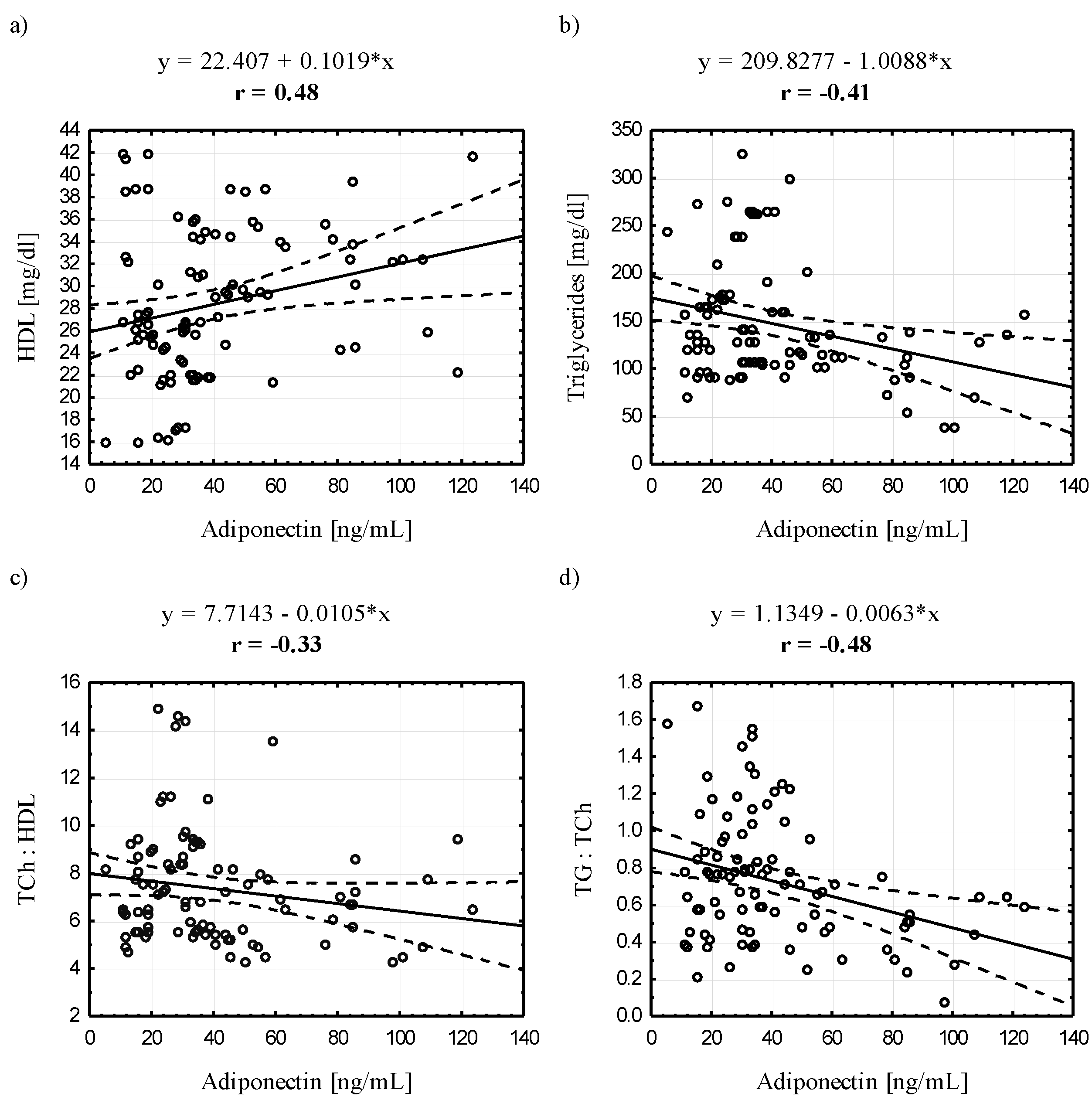
3.4. Relationships between Serum Adiponectin and Leptin Concentrations and Selected Blood Biochemical Indicators

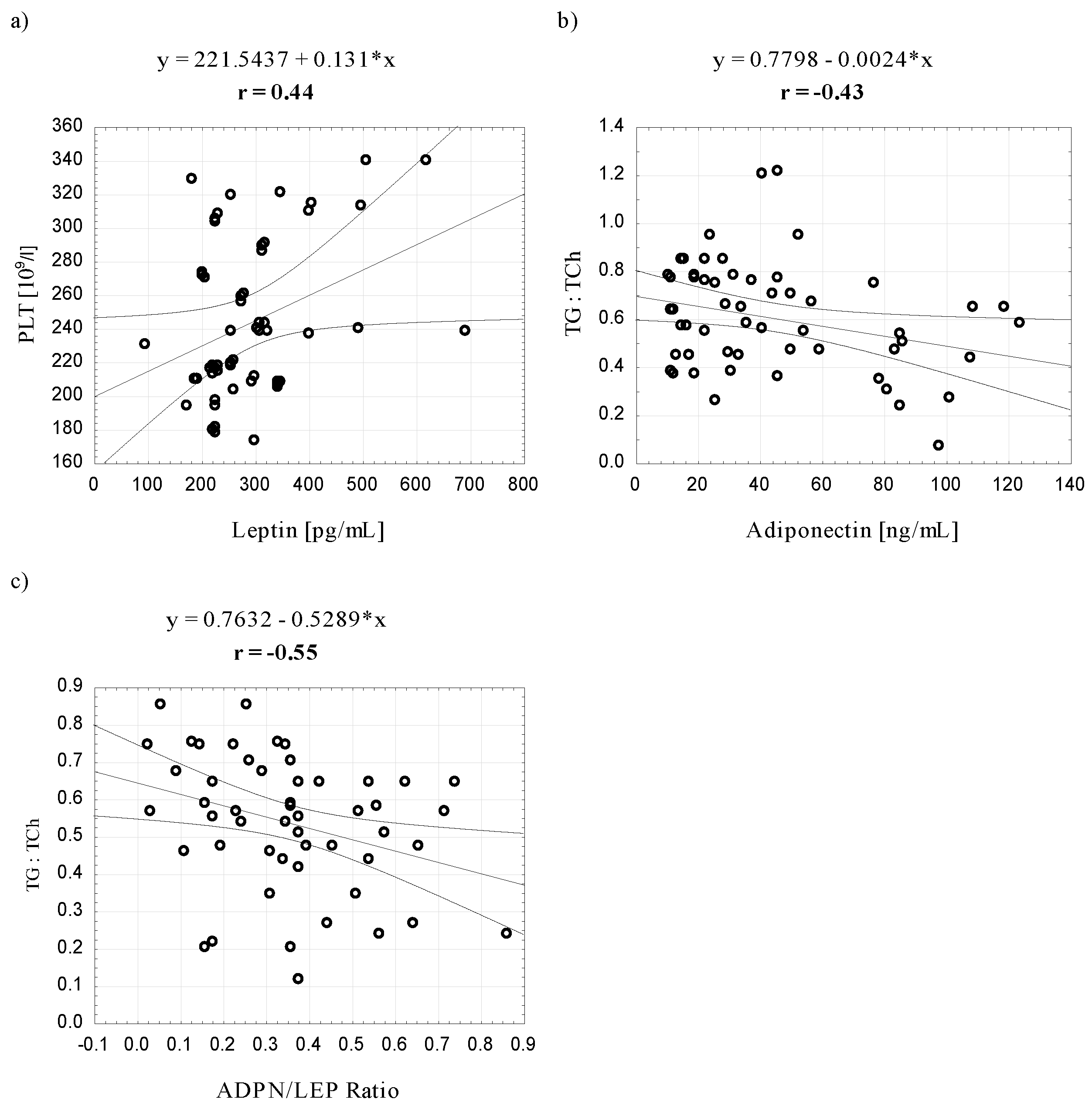
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Donnell, C.J.; Elosua, R. Cardiovascular risk factors. Insights from Framingham. Rev. Esp. Cardiol. 2008, 61, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, F.; Laviola, L.; Eriksson, J.W. Regional differences of insulin action in adipose tissue: Insights from in vivo and in vitro studies. Acta Physiol. Scand. 2005, 183, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Waki, H.; Kamon, J.; Murakami, K.; Motojima, K.; Komeda, K.; Miki, H.; Kubota, N.; Terauchi, Y.; Tsuchida, A.; et al. Inhibition of RXR and PPAR gamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 2001, 108, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.W.; Scherer, P.E. Minireview: The adipocyte—At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Lyon, C.J.; Law, R.E.; Hsueh, W.A. Minireview: Adiposity, inflammation, and atherogenesis. Endocrinology 2003, 144, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, L.; Bengtsson, C.; Larsson, B.; Pennert, K.; Rybo, E.; Sjöström, L. Distribution of adipose tissue and risks of cardiovascular disease and death: A 12-year follow up of participants in the population study of women in Gothenburg, Sweden. Br. Med. J. 1984, 289, 1257–1261. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.; Riederer, M.; Shah, P.; Peters, P.; Quehenberger, F.; Wood, A.; Scharnagl, H.; März, W.; Kostner, K.M.; Kostner, G. Expression of fat mobilizing genes in human epicardial adipose tissue. Atherosclerosis 2012, 220, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, R.; Iglesias, M.J.; Gallego, R.; Raghay, K.; Eiras, S.; Rubio, J.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; Lago, F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005, 579, 5163–5169. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Tsao, T.S.; Saha, A.K.; Murrey, H.E.; Zhang, C.C.; Itani, S.I.; Lodish, H.F.; Ruderman, N.B. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 2002, 99, 16309–16313. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Oh-I, S.; Okada, S.; Mori, M. Leptin resistance and obesity. Endocr. J. 2007, 54, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lam, Q.L.K.; Lu, L. Role of leptin in immunity. Cell. Mol. Immunol. 2007, 4, 1–13. [Google Scholar] [PubMed]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Canavan, B.; Salem, R.O.; Schurgin, S.; Koutkia, P.; Lipinska, I.; Laposata, M.; Grinspoon, S. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J. Clin. Endocrinol. Metab. 2005, 90, 5779–5785. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Invest. 2000, 106, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ono, K.; Kawamura, T.; Wada, H.; Kita, T.; Shimatsu, A.; Hasegawa, K. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am. J. Physiol. Heart. Circ. Physiol. 2007, 292, H2387–H2396. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.L.; Morgan, D.A.; Sivitz, W.I.; Mark, A.L.; Haynes, W.G. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension 2001, 37, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.U.; Melikian, N.; Shah, A.M.; Grieve, D.J.; Wheatcroft, S.B.; John, L.; Gamel, A.E.; Desai, J.B.; Nelson, B.; Driver, C.; et al. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: Evidence for tissue specificity of leptin resistance. Eur. Heart. J. 2006, 27, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Verovská, R.; Lacnák, Z.; Haluzíková, D.; Fábin, P.; Hájek, P.; Horák, L.; Haluzík, M.; Svacina, S.; Matoulek, M. Comparison of various methods of body fat analysis in overweight and obese women. Vnitr. Lek. 2009, 55, 455–461. [Google Scholar] [PubMed]
- Fürstenberg, A.; Davenport, A. Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient hemodialysis patients. Am. J. Kidney. Dis. 2011, 57, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Al Mutairi, S.; Mojiminiyi, O.A.; Al Alawi, A.; Al Rammah, T.; Abdella, N. Study of leptin and adiponectin as disease markers in subjects with obstructive sleep apnea. Dis. Markers 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity distribution influences circulating adiponectin levels. Transl. Res. 2014, 164, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Wang, P.W.; Chen, T.Y. The relationship between regional abdominal fat distribution and both insulin resistance and subclinical chronic inflammation in non-diabetic adults. Diabetol. Metab. Syndr. 2014, 6, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.L.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J. Leptin and atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Horlick, M.B.; Rosenbaum, M.; Nicolson, M.; Levine, L.S.; Fedun, B.; Wang, J.; Pierson, R.N., Jr.; Leibel, R.L. Effect of puberty on the relationship between circulating leptin and body composition. J. Clin. Endocrinol. Metab. 2000, 85, 2509–2518. [Google Scholar] [PubMed]
- Rosenbaum, M.; Nicolson, M.; Hirsch, J.; Heymsfield, S.B.; Gallagher, D.; Chu, F.; Leibel, R.L. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 1996, 81, 3424–3427. [Google Scholar]
- Zhao, Y.N.; Li, Q.; Li, Y.C. Effects of body mass index and body fat percentage on gestational complications and outcomes. J. Obstet. Gynaecol. Res. 2014, 40, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Suyila, Q.; Cui, H.; Yang, L.; Zhao, L.; Zhang, R.; Su, X. Serum leptin concentrations in Mongolian women. Obes. Res. Clin. Pract. 2013, 7, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, M.; Korzeniewska, A.; Jankowska, R. The evaluation of body composition, adiponectin, C-reactive protein and cholesterol levels in patients with obstructive sleep apnea syndrome. Adv. Clin. Exp. Med. 2013, 22, 817–824. [Google Scholar] [PubMed]
- Goropashnaya, A.V.; Herron, J.; Sexton, M.; Havel, P.J.; Stanhope, K.L.; Plaetke, R.; Mohatt, G.V.; Boyer, B.B. Relationships between plasma adiponectin and body fat distribution, insulin sensitivity, and plasma lipoproteins in Alaskan Yup’ik Eskimos: The Center for Alaska Native Health Research study. Metabolism 2009, 58, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L.; Grundy, S.M. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J. Obes. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, T.; Sudi, K.; Jürimäe, J.; Payerl, D.; Rüütel, K. Relationships between plasma leptin levels and body composition parameters measured by different methods in postmenopausal women. Am. J. Hum. Biol. 2003, 15, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Markocka-Mączka, K.; Szelachowski, P.; Grabowski, K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis. Markers 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Salvador, J.; Páramo, J.A.; Orbe, J.; de Irala, J.; Diez-Caballero, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin. Biochem. 2002, 35, 315–320. [Google Scholar] [CrossRef]
- Karbowska, J.; Kochan, Z. Leptin as a mediator between obesity and cardiac dysfunction. Postepy Hig. Med. Dosw. 2012, 66, 267–274. [Google Scholar] [CrossRef]
- Akasaka, Y.; Tsunoda, M.; Ogata, T.; Ide, T.; Murakami, K. Direct evidence for leptin-induced lipid oxidation independent of long-form leptin receptor. Biochim. Biophys. Acta. 2010, 1801, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Park, C.G.; Seo, H.S.; Oh, D.J.; Ro, Y.M. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004, 13, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Shek, E.W.; Brands, M.W.; Hall, J.E. Chronic leptin infusion increases arterial pressure. Hypertension 1998, 31, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 1999, 282, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.M.; McMahon, A.D.; Packard, C.J.; Kelly, A.; Shepherd, J.; Gaw, A.; Sattar, N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001, 104, 3052–3056. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, S.; Colquhoun, D.; Keech, A.; Yallop, J.; Barnes, E.H.; Pollicino, C.; Simes, J.; Tonkin, A.M.; Nestel, P. Leptin, but not adiponectin, is a predictor of recurrent cardiovascular events in men: Results from the LIPID study. Int. J. Obes. 2009, 33, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Park, K.G.; Park, K.S.; Kim, M.J.; Kim, H.S.; Suh, Y.S.; Ahn, J.D.; Park, K.K.; Chang, Y.C.; Lee, I.K. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res. Clin. Pract. Suppl. 2004, 63, 135–142. [Google Scholar] [CrossRef]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Cheung, B.M.Y.; Tso, A.W.K.; Xu, A.; Wat, N.M.S.; Fong, C.H.Y.; Ong, L.H.Y.; Tam, S.; Tan, K.C.B.; Janus, E.D.; et al. Hypoadiponectinemia as a predictor for the development of hypertension: A 5-year prospective study. Hypertension 2007, 49, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Ouchi, N.; Ito, M.; Kihara, S.; Shiojima, I.; Pimentel, D.R.; Kumada, M.; Sato, K.; Schiekofer, S.; Ohashi, K.; et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004, 10, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.M.; Winnicki, M.; Wolk, R.; Svatikova, A.; Phillips, B.G.; Davison, D.E.; Berger, P.B.; Somers, V.K. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation 2004, 109, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Kazumi, T.; Kawaguchi, A.; Hirano, T.; Yoshino, G. C-reactive protein in young, apparently healthy men: Associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism 2003, 52, 1113–1116. [Google Scholar] [CrossRef]
- Haffner, S.M.; Mykkänen, L.; Festa, A.; Burke, J.P.; Stern, M.P. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: Implications for preventing coronary heart disease during the prediabetic state. Circulation 2000, 101, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M.; Matsuyama, A.; Okamoto, Y.; Ishigami, M.; Kuriyama, H.; Kishida, K.; Nishizawa, H.; et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kieć-Klimczak, M.; Malczewska-Malec, M.; Huszno, B. Leptin to adiponectin ratio, as an index of insulin resistance and atherosclerosis development. Przegl. Lek. 2008, 65, 844–849. [Google Scholar] [PubMed]
- Inoue, M.; Yano, M.; Yamakado, M.; Maehata, E.; Suzuki, S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism 2006, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.; Amato, S.; Degano, C.; Farina, M.G.; Patanè, G.; Vigneri, R.; Frittitta, L. Adiponectin relationship with lipid metabolism is independent of body fat mass: Evidence from both cross-sectional and intervention studies. J. Clin. Endocrinol. Metab. 2004, 89, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, E.M.; Shalan, M. Plasma adiponectin levels for prediction of cardiovascular risk among hemodialysis patients. Ther. Apher. Dial. 2014, 18, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Benedetto, F.A.; Cutrupi, S.; Parlongo, S.; Malatino, L.S.; Bonanno, G.; Seminara, G.; Rapisarda, F.; et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end—Stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 134–141. [Google Scholar] [PubMed]
- Gray, B.; Steyn, F.; Davies, P.S.; Vitetta, L. Liver function parameters, cholesterol, and phospholipid α-linoleic acid are associated with adipokine levels in overweight and obese adults. Nutr. Res. 2014, 34, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirrakhimov, E.; Kerimkulova, A.; Lunegova, O.; Mirrakhimov, A.; Alibaeva, N.; Nabiev, M. Lipids and leptin level in natives of Kyrgyzstan. Turk. Kardiyol. Dern. Ars. 2014, 42, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Tchernova, J.; Whincup, P.; Lowe, G.D.; Kelly, A.; Rumley, A.; Wallace, A.M.; Sattar, N. Plasma leptin: Associations with metabolic, inflammatory and haemostatic risk factors for cardiovascular disease. Atherosclerosis 2007, 191, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Qu, H.Q.; Li, Q.; Martinez, P.J.; Rentfro, A.R.; McCormick, J.B.; Fisher-Hoch, S.P. Adiponectin/leptin ratio and metabolic syndrome in a Mexican American population. Clin. Invest. Med. 2011, 34, E290. [Google Scholar] [PubMed]
- Yoon, J.H.; Park, J.K.; Oh, S.S.; Lee, K.H.; Kim, S.K.; Cho, I.J.; Kim, J.K.; Kang, H.T.; Ahn, S.G.; Lee, J.W.; et al. The ratio of serum leptin to adiponectin provides adjunctive information to the risk of metabolic syndrome beyond the homeostasis model assessment insulin resistance: The Korean Genomic Rural Cohort Study. Clin. Chim. Acta 2011, 412, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Gannagé-Yared, M.H.; Khalife, S.; Semaan, M.; Fares, F.; Jambart, S.; Halaby, G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur. J. Endocrinol. 2006, 155, 167–176. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubkowska, A.; Radecka, A.; Bryczkowska, I.; Rotter, I.; Laszczyńska, M.; Dudzińska, W. Serum Adiponectin and Leptin Concentrations in Relation to Body Fat Distribution, Hematological Indices and Lipid Profile in Humans. Int. J. Environ. Res. Public Health 2015, 12, 11528-11548. https://doi.org/10.3390/ijerph120911528
Lubkowska A, Radecka A, Bryczkowska I, Rotter I, Laszczyńska M, Dudzińska W. Serum Adiponectin and Leptin Concentrations in Relation to Body Fat Distribution, Hematological Indices and Lipid Profile in Humans. International Journal of Environmental Research and Public Health. 2015; 12(9):11528-11548. https://doi.org/10.3390/ijerph120911528
Chicago/Turabian StyleLubkowska, Anna, Aleksandra Radecka, Iwona Bryczkowska, Iwona Rotter, Maria Laszczyńska, and Wioleta Dudzińska. 2015. "Serum Adiponectin and Leptin Concentrations in Relation to Body Fat Distribution, Hematological Indices and Lipid Profile in Humans" International Journal of Environmental Research and Public Health 12, no. 9: 11528-11548. https://doi.org/10.3390/ijerph120911528






