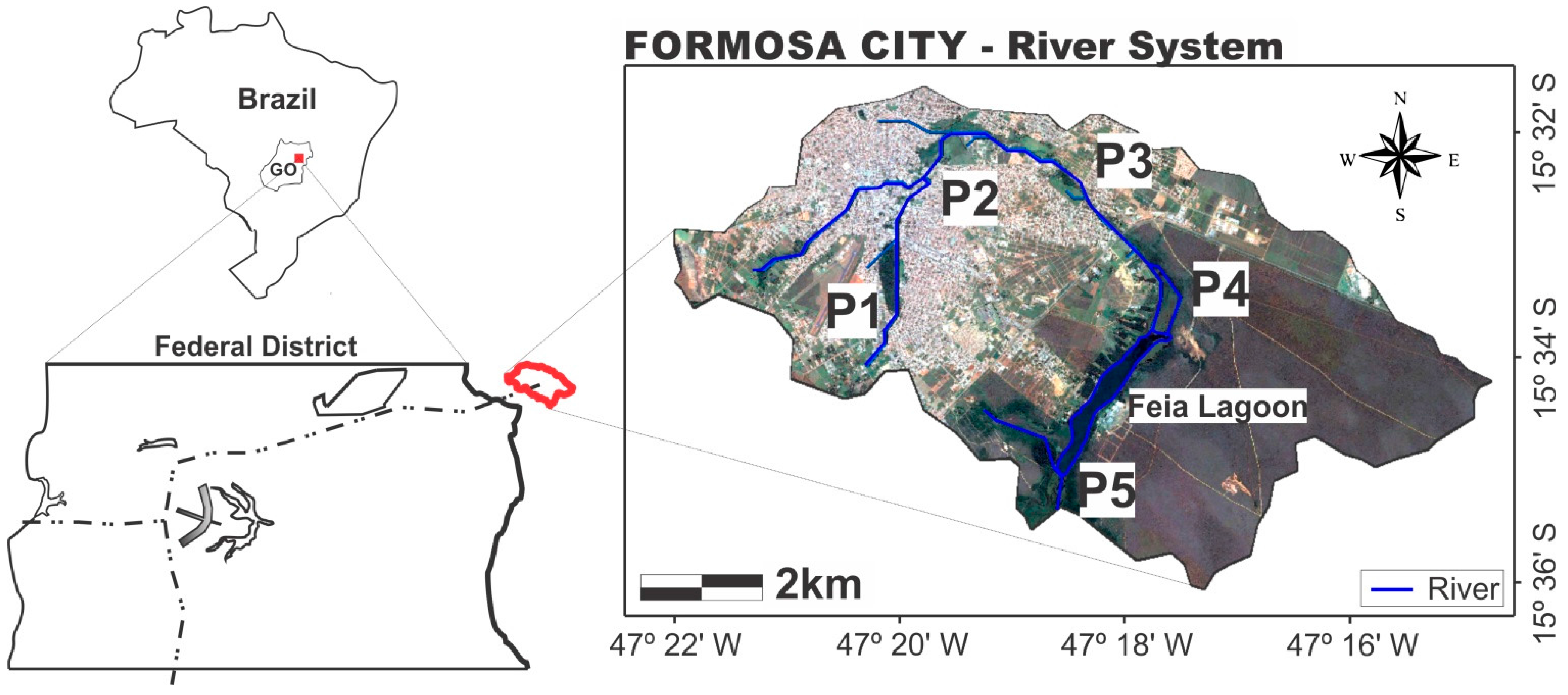
3.1. Physical-Chemical and Biological Analyses
Among the data from physical and chemical analyses, the results of analysis of temperature, EC, TDS, turbidity, pH, DO and water hardness at the sample points are summarized in
Table 1.
Table 1.
Result of the physical and chemical parameters analyzed in the surface water in the headwaters of the Preto River, Formosa, GO, Brazil.
Table 1.
Result of the physical and chemical parameters analyzed in the surface water in the headwaters of the Preto River, Formosa, GO, Brazil.
| Collection Points | Temp. (*) (°C) | EC (µS·cm−1) | TDS (mg·L−1) | Turbidity (NTU) | pH | DO (mg·L−1) | Hardness (mg·L−1 CaCO3) |
|---|
| Collection date: May 07 |
| P1 | 23.5 | 44 | 22 | 0.11 | 5.9 | 2.3 | 9 |
| P2 | 24.7 | 137 | 68 | 0.78 | 7.2 | 5.6 | 34 |
| P3 | 21.9 | 128 | 64 | 1.18 | 7.4 | 6.3 | 45 |
| P4 | (**) | (**) | (**) | (**) | (**) | (**) | (**) |
| P5 | 24.1 | 154 | 77 | 0.02 | 6.9 | 3.3 | 61 |
| Collection date: June 25 |
| P1 | 23.2 | 35 | 18 | 2.29 | 5.8 | 3.2 | 9 |
| P2 | 24.1 | 138 | 69 | 5.29 | 7.4 | 5.6 | 36 |
| P3 | 20.2 | 124 | 62 | 1.86 | 7.7 | 5.3 | 41 |
| P4 | 19.5 | 128 | 64 | 4.94 | 7.7 | 5.1 | 44 |
| P5 | 18.7 | 161 | 81 | 1.43 | 6.7 | 3.4 | 69 |
| Collection date: August 13 |
| P1 | 19.7 | 128 | 64 | 2.52 | 6.3 | 3.5 | 12 |
| P2 | 23.5 | 163 | 82 | 3.98 | 7.2 | 5.1 | 37 |
| P3 | 18.8 | 131 | 65 | 2.12 | 7.2 | 5.3 | 41 |
| P4 | 18.1 | 135 | 68 | 3.08 | 7.6 | 4.7 | 46 |
| P5 | 22.1 | 161 | 81 | 3.72 | 6.4 | 4.4 | 73 |
| Collection date: October 01 |
| P1 | (***) | (***) | (***) | (***) | (***) | (***) | (***) |
| P2 | 27.4 | 165 | 83 | 1.95 | 7.5 | 6.7 | 45 |
| P3 | 23.6 | 163 | 81 | 5.12 | 7.8 | 7.4 | 56 |
| P4 | 22.8 | 168 | 84 | 1.88 | 7.7 | 4.9 | 59 |
| P5 | 25.9 | 159 | 79 | 3.24 | 7.6 | 3.1 | 81 |
After carrying out chromatographic analyses, it was not possible to detect the ions Li
+, Br
- and PO
43− in significant quantities in the collected samples. The ion NH
4+ was detected only in P2, in August 2012, with a concentration of 0.36 mg·L
−1. The results of the concentration of the other ions are presented in
Table 2.
Table 2.
Result of the concentrations of ions analyzed in the surface water at the headwaters of the Preto River, Formosa, GO.
Table 2.
Result of the concentrations of ions analyzed in the surface water at the headwaters of the Preto River, Formosa, GO.
| Points | F− (mg·L−1) | Cl− (mg·L−1) | NO2− (mg·L−1) | NO3− (mg·L−1) | SO42− (mg·L−1) | Na+ (mg·L−1) | K+ (mg·L−1) | Ca+ (mg·L−1) | Mg+ (mg·L−1) |
|---|
| Collection date: May 07 |
| P1 | 0.04 | 2.43 | n.d. | 2.28 | 0.30 | 7.32 | 0.34 | 4.01 | 0.40 |
| P2 | 0.05 | 7.03 | 0.06 | 13.85 | 0.90 | 15.03 | 1.95 | 8.50 | 1.22 |
| P3 | 0.05 | 4.06 | n.d. | 0.17 | 0.48 | 11.76 | 0.57 | 8.46 | 1.38 |
| P4 | (*) | (*) | (*) | (*) | (*) | (*) | (*) | (*) | (*) |
| P5 | 0.03 | 1.58 | n.d. | n.d. | 0.45 | 4.01 | 2.14 | 12.28 | 4.63 |
| Collection date: June 25 |
| P1 | 0.05 | 2.75 | n.d. | 2.53 | 0.57 | 7.26 | 0.41 | 4.59 | 0.45 |
| P2 | 0.09 | 7.59 | 0.10 | 13.81 | 1.13 | 14.38 | 2.19 | 8.51 | 1.40 |
| P3 | 0.07 | 4.95 | n.d. | 0.16 | 0.67 | 11.53 | 0.51 | 9.05 | 1.42 |
| P4 | 0.02 | 4.99 | n.d. | 0.15 | 0.74 | 11.84 | 0.72 | 9.79 | 1.55 |
| P5 | 0.01 | 1.27 | n.d. | n.d. | 0.35 | 3.11 | 1.99 | 10.37 | 5.65 |
| Collection date: August 13 |
| P1 | 0.06 | 5.05 | n.d. | 0.77 | 0.66 | 10.36 | 0.28 | 3.50 | 0.33 |
| P2 | 0.11 | 8.72 | 0.09 | 13.69 | 3.80 | 16.21 | 2.33 | 10.43 | 1.40 |
| P3 | 0.03 | 5.09 | n.d. | 0.19 | 0.56 | 11.99 | 0.55 | 13.26 | 1.35 |
| P4 | 0.08 | 5.49 | n.d. | n.d. | 0.63 | 12.17 | 0.64 | 14.53 | 1.61 |
| P5 | 0.09 | 1.19 | n.d. | n.d. | 0.40 | 2.34 | 2.22 | 15.98 | 6.28 |
| Collection date: October 01 |
| P1 | (**) | (**) | (**) | (**) | (**) | (**) | (**) | (**) | (**) |
| P2 | 0.11 | 8.62 | 0.24 | 12.17 | 2.10 | 16.06 | 2.20 | 14.05 | 1.77 |
| P3 | 0.04 | 5.88 | n.d. | 0.15 | 0.34 | 13.01 | 1.10 | 15.52 | 1.86 |
| P4 | 0.05 | 5.98 | n.d. | 0.08 | 0.46 | 12.92 | 1.43 | 15.71 | 1.98 |
| P5 | 0.02 | 0.65 | n.d. | n.d | 0.07 | 1.89 | 2.11 | 12.79 | 7.10 |
Water temperature, represented in
Figure 3A, presented the highest values at point P2 during the sample collections. An increase in surface water temperature is generally provoked by sewage outflows [
13].
The EC values shown in
Figure 3B were higher than 100 µS·cm
−1 at points P2 to P5. An increase in EC is observed in a water body that receives untreated domestic effluents, and levels of EC above 100 µS·cm
−1 suggest negative environmental impacts [
16].
A reduction in the pH value in a water body functions as an indicator of imbalance in the ecosystem. The values found in P5,
Figure 3C, may be an indication that the ecosystem of this region is environmentally unbalanced [
16].
Points P2, P3 and P4 presented a greater quantity of DO, with values almost always over 5 mg·L
−1, as can be seen in
Figure 3D. These values are due to the incorporation of O2 along the watercourse. The low value of DO at P1 is due to the short contact time with the atmospheric air and with photosynthesizing organisms. At P5 it is due to an environment with little current and probably due to the consumption of oxygen by heterotrophic organisms, because the higher the quantity of organic matter introduced into the water, the greater the quantity of oxygen consumed by it, besides being a lentic environment.
Water hardness is mainly related to the content of Ca
2+ and Mg
2+, a fact that was observed in this work, since the increase in the level of these elements was accompanied by an increase in hardness, shown in
Figure 4A [
9]. The low hardness at P1 (source) is probably related to the low content of calcium and magnesium in the soils of the Cerrado region, as also observed by other studies [
5,
17]. The concentrations of magnesium in Cerrado natural surface waters are generally around or lower than 1 mg/L [
3]. At P5 all the samples showed concentrations above of these baseline values.
Nitrate, as shown in
Figure 4B, was what presented the highest concentrations among the nitrogenated compounds studied. At P2 significant values were detected, indicating contamination by sewage flowing into the Beira Rio Creek, also responsible for the increase in sodium concentrations, shown in
Figure 4C at this point, as well as observed in other studies [
5]. The presence of nitrate in P1, the spring of the river, in May and June, can be due to the runoff of this compound derived from the litter decomposition [
18,
19,
20].
Potassium, shown in
Figure 4D, is essential for the nutrition of plants, and in the form of ions is rapidly assimilated by them. In Josefa Gomes Creek a large number of aquatic macrophytes were found, and these are likely to consume the potassium in this stretch of the river, explaining the low concentrations of potassium at points P3 and P4.
Figure 3.
(A) Temperature; (B) Electrical conductivity; (C) hydrogen ion potential and (D) dissolved oxygen.
Figure 3.
(A) Temperature; (B) Electrical conductivity; (C) hydrogen ion potential and (D) dissolved oxygen.
Toothpaste, medicines, vitamins and chewing gum are also sources of F
− [
9]. The high concentrations found mainly at P2, P4 and P5 may be associated with contamination from domestic sewage, increasing the naturally occurring concentrations of this chemical element.
Table 3 presents the Most Probable Number (MPN) of total and thermotolerant coliforms obtained in 100 mL of water samples. It can be observed that the points P2, P3 and P4 presented a high index of total coliforms during all analyses (>2419.6 MPN/100 mL). At P5 it was only in the last collection (October 2012) that the value was lower than this, totaling 1732.9 MPN/100 mL of total coliforms.
Figure 4.
(A) Total hardness; (B) nitrate (C) sodium and (D) potassium.
Figure 4.
(A) Total hardness; (B) nitrate (C) sodium and (D) potassium.
Table 3.
Results of the biological parameters in the surface water at the headwaters of the Preto River, Formosa, GO.
Table 3.
Results of the biological parameters in the surface water at the headwaters of the Preto River, Formosa, GO.
| Date | P1 | P2 | P3 | P4 | P5 |
|---|
| Total coliforms (NMP/100 mL) |
| 07/05/2012 | 1732.9 | >2419.6 | >2419.6 | (*) | >2419.6 |
| 25/06/2012 | 1299.7 | >2419.6 | >2419.6 | >2419.6 | >2419.6 |
| 13/08/2012 | 920.8 | >2419.6 | >2419.6 | >2419.6 | >2419.6 |
| 01/10/2012 | (**) | >2419.6 | >2419.6 | >2419.6 | 1732.9 |
| Thermotolerant coliforms (NMP/100 mL) |
| 07/05/2012 | 307.6 | >2419.6 | 248.1 | (*) | 33.1 |
| 25/06/2012 | 98.4 | >2419.6 | 648.8 | 727.0 | 18.7 |
| 13/08/2012 | 261.3 | 1986.3 | 365.4 | 770.1 | 290.9 |
| 01/10/2012 | (**) | >2419.6 | 613.1 | 166.4 | 107.1 |
The high density of thermotolerant coliforms in the P2 water samples indicates a high level of contamination by sewage, given that these microrganisms come from the intestine of humans and other warm-blooded animals, and are eliminated in large numbers with feces (106/g–108/g) [
9]. The presence of these bacteria indicates that this water is unsuitable for human consumption [
21].
At P5 large quantities of the macrophyte water hyacinth (
Eichhornia crassipes) were noted. Water hyacinth is recognized in the literature as an indicator of polluted environments, and its presence is associated with eutrophy since it is the species that develops best in such environments [
22].
Another aquatic plant species found in large quantities along Josefa Gomes Creek was Southern Cattail (
Typha domingensis), which is typically present along the banks of drainage channels, lakes and reservoirs, and on várzea floodplains [
23]. In general, the growth of macrophytes is associated with an increase in the availability of nutrients, especially nitrogen and phosphorous, and with a greater amount of light due to the removal of gallery forest [
4].
To relate the obtained results with the season,
Figure 5 shows the rainfall values in the studied area, based on the monitoring of the meteorological stations from Embrapa Cerrados.
Figure 5.
Rainfall in the studied period monitored by the meteorological stations from Embrapa Cerrados. Source: Laboratory of Environmental Biophysics at Embrapa Cerrados.
Figure 5.
Rainfall in the studied period monitored by the meteorological stations from Embrapa Cerrados. Source: Laboratory of Environmental Biophysics at Embrapa Cerrados.
Figure 5 shows that the collection days (7 May, 25 Jun, 13 Aug and 1 Oct) there were not rainfall in the study area and then is possible to concluded that the results were not influenced by the rain presence, but the dry season may have been responsible for the reduction of some values such as nitrate and potassium in P1, suggesting that the observed quantitative may actually be coming from runoff.
In the studied area it can be noted that superficial drainage occasioned by the topography of the city and the constructed micro-drainage encourage the flow of rubbish into the water body. Most of the county of Formosa has no rainwater drainage system. The capture and movement of rainwater directs the flow to Josefa Gomes Creek, altering the water quality, carrying a large amount of solid residues and provoking instability on the banks of the source, compromising aquatic life. Such problems related to the urban drainage system affect various Brazilian cities and occur in function of environmental, political, social and economic aspects [
24]. These characteristics are present in various parts of the town, and the large amount of rubbish thrown into empty plots and on the streets interfere in water quality.
The areas around springs possess a strategic function in the movement of animals, providing ecological corridors [
25] The Municipal Ecological Park of Mata da Bica, where several springs are situated, supplying the Preto River in the town of Formosa, is strategically placed for maintaining fauna since it lies between the Ecological Station of Aguas Emendadas (Planaltina, Federal District) and the Preservation Area of the Army Training Ground of Formosa. This makes a connection for birds that crosses the whole town, helping with the movement of fauna and dispersal of plant species. It is also worth highlighting that the Cerrado biome is on the list of the 25 world hotspots for biodiversity conservation [
26]. Not only the alterations in the quality of the water but also in the structure of the vegetation can provoke significant impacts in the population dynamics of this group, mainly causing alterations in population and the disappearance of species.
3.2. Statistical Analysis
Initially, based on descriptive statistics, it was possible to observe the mean, maximum and minimum values and the standard deviation of the analyzed parameters among all those collected. These are shown in
Table 4.
Table 4.
Descriptive statistics attributed to physical, chemical and biological parameters.
Table 4.
Descriptive statistics attributed to physical, chemical and biological parameters.
| Variables | Mean | Standard Deviation | Minimum | Maximum |
|---|
| Water Temperature | 22.35 | 2.66 | 18.08 | 27.40 |
| pH | 7.15 | 0.64 | 5.81 | 7.85 |
| Conductivity | 134.6 | 37.90 | 35.00 | 168.00 |
| TDS | 67.32 | 18.92 | 18.00 | 84.00 |
| DO | 4.73 | 1.38 | 2.29 | 7.42 |
| Hardness | 44.32 | 20.58 | 9.00 | 81.00 |
| Turbidity | 2.52 | 1.61 | 0.02 | 5.29 |
| Coliforms | 2197.81 | 457.51 | 920.80 | 2419.60 |
| E.coli | 772.28 | 880.66 | 18.70 | 2419.60 |
| Sodium | 10.17 | 4.71 | 1.89 | 16.21 |
| Potassium | 1.31 | 0.28 | 0.34 | 2.33 |
| Calcium | 10.63 | 3.50 | 4.01 | 15.98 |
| Magnesium | 2.32 | 0.33 | 0.40 | 7.10 |
| Fluoride | 0.06 | 0.01 | 0.02 | 0.11 |
| Chloride | 4.63 | 2.53 | 0.66 | 8.72 |
| Nitrite | 0.03 | 0.06 | 0.00 | 0.24 |
| Nitrate | 3.33 | 5.58 | 0.00 | 13.85 |
| Sulfate | 0.82 | 0.85 | 0.30 | 3.80 |
With the exception of the nitrite variable, which had values detected only at point 2, the others were submitted to the normality test. From this, it could be seen that only the data on the variables total coliforms (p = 0.0009), magnesium (p = 0.02), nitrate (p = 0.02) and sulfate (p = 0.04) did not demonstrate normal distribution. Thus, these variables, including nitrite, were not submitted to analyses of variance (ANOVA) and clustering (Cluster).
Considering the variables with parametric data,
i.e., those that presented normal distribution, it was possible to infer from the ANOVA test that only water temperature and turbidity presented significant differences between the months in which sampling was carried out. This demonstrated variation in function of seasonality, as observed in
Table 5. The other variables did not demonstrate significant differences in their means at different sampling periods.
Table 5.
Analysis of variance of means for variables between sampling periods (May, June, August and October).
Table 5.
Analysis of variance of means for variables between sampling periods (May, June, August and October).
| Summary Anova |
|---|
| Variables | F Value | p Value |
|---|
| Water Temperature | 4.116 | 0.0275 * |
| pH | 1.238 | 0.333 |
| Conductivity | 1.779 | 0.197 |
| TDS | 1.766 | 0.2 |
| DO | 0.52 | 0.675 |
| Hardness | 1.075 | 0.391 |
| Turbidity | 4.129 | 0.0272 * |
| E.coli | 0.007 | 0.999 |
| Sodium | 0.084 | 0.968 |
| Potassium | 0.374 | 0.773 |
| Calcium | 3.234 | 0.0547 |
| Fluoride | 1.071 | 0.393 |
| Chloride | 0.287 | 0.834 |
As regards the collection locations, it was also through Analysis of Variance that the lack of significant variation could be observed in the variables water temperature, turbidity and F. Thus, specific environmental characteristics at each point may have influenced the variation in mean values for other variables, such as pH, conductivity, DO and TDS, at least at one of the five points (
Table 6).
Table 6.
Analysis of variance of means for variables between the sampling points (Points 1, 2, 3, 4 and 5).
Table 6.
Analysis of variance of means for variables between the sampling points (Points 1, 2, 3, 4 and 5).
| Summary Anova |
|---|
| Variables | F Value | p Value |
|---|
| Water Temperature | 2.353 | 0.108 |
| pH | 16.39 | 5.37E-05 * |
| Conductivity | 6.831 | 0.00345 * |
| TDS | 6.841 | 0.00342 * |
| DO | 13.41 | 0.000152 * |
| Hardness | 38 | 4.69E-07 * |
| Turbidity | 0.489 | 0.744 |
| E.coli | 74.46 | 7.91E-09 * |
| Sodium | 90.64 | 2.33E-09 * |
| Potassium | 46.31 | 1.44E-07 * |
| Calcium | 6.263 | 0.00491 * |
| Fluoride | 2.214 | 0.124 |
| Chloride | 37.32 | 5.22E-07 * |
The data that were standardized by means of the “standardize” function and transformed into a distance matrix by means of a Euclidean similarity index were submitted to Cluster analysis and resulted in a dendrogram. This, in turn, was analyzed with abline at point 4.5, demonstrating the formation of four groups (A, B, C and D) containing the points in each collection month. M1, M2, M3 and M5 are the points 1, 2, 3 and 5 collected in May; J1, J2, J3, J4 and J5 are the points 1, 2, 3, 4 and 5 collected in June; A1, A2, A3, A4 and A5 = collection of the five points in August; and O2, O3, O4 and O5 are the collection in October at points 2, 3, 4 and 5 (
Figure 6).
As observed in
Figure 6, group A includes point 1 in all its sampling periods, as does group B, formed by the grouping of point 2 in the four monitoring periods. Group C includes points 3 and 4 throughout the collection months. Group D, in turn, is formed by point 5.
It can thus be inferred that the grouping of collection points is due to the standard of the variables analyzed at each locality, with a significant value of cophenetic correlation, and Pearson’s r equal to 0.85. As indicated by Bocard
et al. [
14] a coefficient of cophenetic correlation over 0.7 indicates a good correlation between the variables that characterize the standard grouping obtained.
Analyzing the influence of temporal variation on the configuration of the groups, subgroups can be identified among groups A, B, C and D, conditioned by the factor of collection time. There is a greater similarity between the months of May and June in Group A. The second group, referring to Point 2, presents two more similar periods: a grouping of the months of May and June, and another one with the months of August and October. Group C, in turn, is subdivided into two groups, the first including Points 3 and 4 in the months of May, June and August, and the second with both points in the month of October. Finally, in group D, as with the previous groups, there is a shorter Euclidean distance between the months of May and June, followed by October and August.
As regards collection points, the analysis of variance made it possible to observe greater significant differences between the variables except for water temperature, turbidity and F-, which did not demonstrate a significant difference. The result of ANOVA carried out between the collection points corroborated the result of Cluster analysis, and this in turn allowed us to identify a standard grouping of points in function of the variables.
Thus, the characterization of Point 1 (Group A—
Figure 6), water body spring, shows that it is most different from the rest of the points, possibly because of a configuration that is more favorable to maintaining water quality, such as the existence of riparian forest. Point 2, localized in a channeled region and in contact with untreated effluents (Group B), is also in contrast to the other points, in that it presents compromised water quality that is more evident than at the other points.
Points 3 and 4, grouped together, may present more similar characteristics because of their spatial proximity, which provides similar environmental conditions. Lastly, it was possible to observe grouping at point 5 in the four sampling periods into a single group (Group D), allowing an inference about the influence of the lentic environment upstream from the sampling location.
It can thus be observed that spatial distribution was more significant for the formation of groups than the collection period. The points therefore present greater differentiation among themselves than in function of the temporal variation recorded throughout the study.
Based on the obtained results, it is confirmed that urban occupation near the headwaters of the Preto River watercourse in the town of Formosa (P2) has led to alterations in the natural characteristics of the water.
Josefa Gomes creek underwent several impacts, including elimination of gallery forests, buildings in riparian areas, soil sealing, concentrated release of storm water, untreated wastewater discharge from clandestine inputs, release of solid waste and waste of different nature, erosion formed by leaching, stream bed obstruction by dumping of waste and soil deposit generated by silting processes.
Figure 6.
Dendrogram after Cluster analysis from the collected data.
Figure 6.
Dendrogram after Cluster analysis from the collected data.
The input of waste from the small watershed (storm drainage) located in the urban area and flows into the Feia Lagoon contribute to the silting process. Whereas the urban area of Formosa city is almost entirely located in the study area, water bodies in this catchment zone are subject to the effects of different sources of pollution and intrinsic forms of degradation of a city that had a population growth and expansion of occupied areas without planning.
This scenario reflects the poor planning in use and occupation, and is mainly due to the insufficient infrastructure and environmental sanitation and urban drainage in Formosa city. Throughout the river basin under study there are environmental impacts such as degradation of the surface water quality, aquatic weeds proliferation, losses in aquatic life, landscape degradation and smelly water. Several countries have suffered problems with rapid urbanization of natural areas. Water is a natural resource essential to all living beings, and preserving their quality is to ensure life. This is just one of the great challenges in various cities throughout the world today; to develop sustainably, while preserving the regions of springs and ponds that run through urban centers [
27].
Although in this study the number of collections was small throughout the year, it was possible to observe natural characteristics of the river, based on the P1 data, and the impact of urbanization in these values when looking at the P2 data. This diagnosis is essential to know and seek to minimize the consequences of this impact on the natural resource and subsequently on human health.