An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
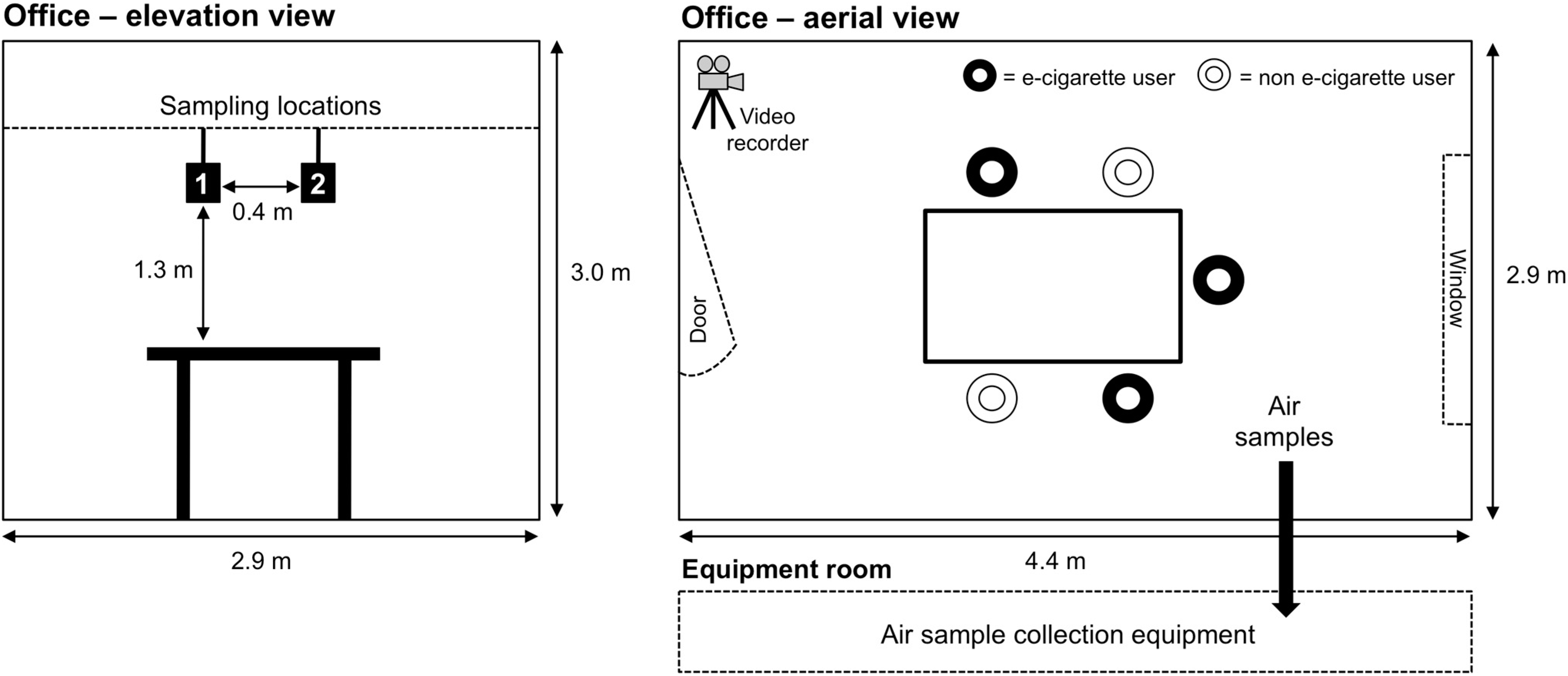
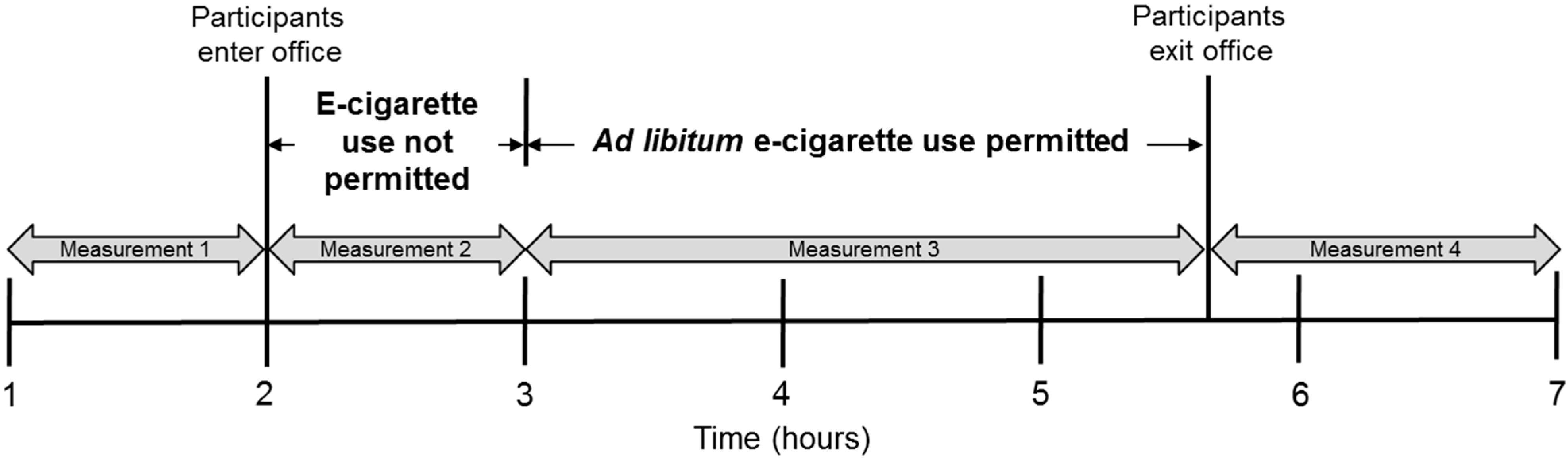
2.2. Analysis of Indoor Air Parameters
2.2.1. Indoor Climate
2.2.2. Nicotine
2.2.3. Volatile Organic Compounds (VOCs)
2.2.4. Glycerol
2.2.5. Low Molecular Weight Carbonyls
2.2.6. Polycyclic Aromatic Hydrocarbons (PAHs)
2.2.7. Trace Metals
2.2.8. Tobacco-Specific Nitrosamines (TSNAs)
2.3. Analysis of Outdoor Air Parameters
3. Results and Discussion
3.1. Product Use: Puff Rate
3.2. Indoor Climate Parameters
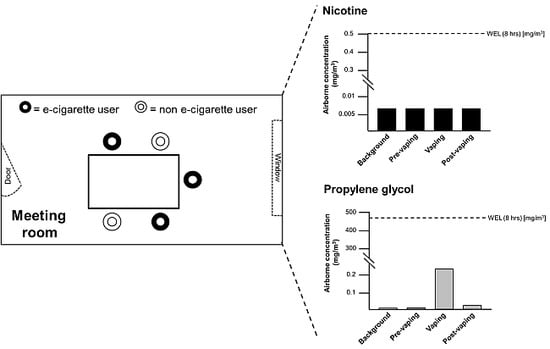
3.3. Volatile Organic Compounds (VOCs; Including Nicotine, Propylene Glycol and Glycerol) and Low Molecular Weight Carbonyls
| Chemical Compound | Background (before Participants Enter Room) | Room Occupied (No Vaping) | Room Occupied (Vaping Permitted) | Room Unoccupied (after Participants Leave Room) | Air Quality Guidelines or UK Workplace Exposure Limit as Published (WEL; 8 h Average) (mg/m3) | Air Quality Guidelines or UK Workplace Exposure Limit * (WEL; 8 h Average) (µg/m3) |
|---|---|---|---|---|---|---|
| Measurement 1 (µg/m3) | Measurement 2 (µg/m3) | Measurement 3 (µg/m3) | Measurement 4 (µg/m3) | |||
| Propylene glycol | <0.5 | <0.5 | 203.6 | 10.2 | UK WEL: 474 | 474,000 |
| Glycerol | <150 | <225 | <250 | <200 | UK WEL: 10 | 10,000 |
| Nicotine | <7.0 | <7.0 | <7.0 | <7.0 | UK WEL: 0.5 | 500 |
| Isoprene | <0.5 | 6.2 | 9.5 | <0.5 | Not established | Not established |
| Acetone | 1.3 | 9.2 | 10.7 | 1.2 | UK WEL: 1210 | 1,210,000 |
| Propan-2-ol | 55.3 | 13.6 | 8.0 | 29.2 | UK WEL: 999 | 999,000 |
| Hexamethylenecyclotri-siloxane | 5.3 | 29.1 | 13.3 | 4.4 | Not established | Not established |
| Octamethylcyclotetra-siloxane | <0.5 | 14.2 | 3.6 | 0.9 | Not established | Not established |
| Limonene | 2.2 | 2.1 | 2.9 | 1.5 | Not established | Not established |
| Octanal | 2.1 | 3.5 | 5.4 | 4.6 | Not established | Not established |
| Decamethylcyclo-pentanesiloxane | 6.3 | 307 | 460.8 | 107.5 | Not established | Not established |
| Nonanal | 6.3 | 7.9 | 10.6 | 11.0 | Not established | Not established |
| Decanal | 2.8 | 5.7 | 9.5 | 11.6 | Not established | Not established |
| 2,2,4-Trimethyl-1,3-pentanediol monoisobutyrate | 7.7 | 16.1 | 17.3 | 18.0 | Not established | Not established |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | <0.5 | <0.5 | 1.5 | 2.2 | Not established | Not established |
| Di-isobutyl phthalate | 3.5 | 4.4 | 2.3 | 2.8 | UK WEL: 5 | 5000 |
| Formaldehyde | 32.0 | 31.0 | 37.6 | 21.0 | WHO: 0.1 | 100 |
| Acetaldehyde | 9.0 | 6.5 | 12.4 | 6.0 | EU Indoor Air Quality: 0.2 | 200 |
| Acrolein | <2.0 | <2.0 | <2.0 | <2.0 | UK WEL: 0.23 | 230 |
| Total VOC | 65.0 | 237.0 | 379.8 | 129.0 | UK Building Regulations: 0.3 (8 h average) | 300 |
3.4. Polycyclic Aromatic Hydrocarbons (PAHs)
| Chemical Compound | Background (before Participants Enter Room) | Room Occupied (No Vaping) | Room Occupied (Vaping Permitted) | Room Unoccupied (after Participants Leave Room) |
|---|---|---|---|---|
| Measurement 1 (µg/m3) | Measurement 2 (µg/m3) | Measurement 3 (µg/m3) | Measurement 4 (µg/m3) | |
| Acenaphthene | <1.25 | <1.25 | <1.25 | <1.25 |
| Acenaphthylene | <1.25 | <1.25 | <1.25 | <1.25 |
| Anthracene | <1.25 | <1.25 | <1.25 | <1.25 |
| Benz[a]anthracene | <1.25 | <1.25 | <1.25 | <1.25 |
| Benzo[b]fluoranthene | <1.25 | <1.25 | <1.25 | <1.25 |
| Benzo[k]fluoranthene | <1.25 | <1.25 | <1.25 | <1.25 |
| Benzo[ghi]perylene | <1.25 | <1.25 | <1.25 | <1.25 |
| Benzo[a]pyrene | <1.25 | <1.25 | <1.25 | <1.25 |
| Chrysene | <1.25 | <1.25 | <1.25 | <1.25 |
| Dibenz[ah]anthracene | <1.25 | <1.25 | <1.25 | <1.25 |
| Fluoranthene | <1.25 | <1.25 | <1.25 | <1.25 |
| Fluorene | <1.25 | <1.25 | <1.25 | <1.25 |
| Indeno[1,2,3-cd]pyrene | <1.25 | <1.25 | <1.25 | <1.25 |
| Naphthalene | <1.25 | <1.25 | <1.25 | <1.25 |
| Phenanthrene | <1.25 | <1.25 | <1.25 | <1.25 |
| Pyrene | <1.25 | <1.25 | <1.25 | <1.25 |
3.5. Trace Metals
| Chemical Compound | Background (before Participants Enter Room) | Room Occupied (No Vaping) | Room occupied (Vaping Permitted) | Room unoccupied (after Participants Leave Room) | UK Workplace Exposure Limit as Published (WEL; 8 h Average) (mg/m3) | UK Workplace Exposure Limit * (WEL; 8 h Average) (µg/m3) |
|---|---|---|---|---|---|---|
| Measurement 1 (µg/m3) | Measurement 2 (µg/m3) | Measurement 3 (µg/m3) | Measurement 4 (µg/m3) | |||
| Aluminium | <2.0 | <2.0 | <2.0 | <2.0 | 10 | 10,000 |
| Antimony | <1.0 | <1.0 | <1.0 | <1.0 | 0.5 | 500 |
| Arsenic | <1.0 | <1.0 | <1.0 | <1.0 | 0.1 | 100 |
| Barium | <1.0 | <1.0 | <1.0 | <1.0 | 0.5 | 500 |
| Beryllium | <2.0 | <2.0 | <2.0 | <2.0 | 0.002 | 2.0 |
| Cadmium | <1.0 | <1.0 | <1.0 | <1.0 | 0.025 | 25 |
| Chromium | <1.0 | <1.0 | <1.0 | <1.0 | 0.5 | 500 |
| Cobalt | <1.0 | <1.0 | <1.0 | <1.0 | 0.1 | 100 |
| Copper | <1.0 | <1.0 | <1.0 | <1.0 | 1 | 1000 |
| Lead | <1.0 | <1.0 | <1.0 | <1.0 | Not established | Not established |
| Manganese | <1.0 | <1.0 | <1.0 | <1.0 | 0.5 | 500 |
| Mercury | <1.0 | <1.0 | <1.0 | <1.0 | 0.02 | 20 |
| Nickel | <1.0 | <1.0 | <1.0 | <1.0 | 0.1 | 100 |
| Phosphorus | <10.0 | <10.0 | <10.0 | <10.0 | Not established | Not established |
| Selenium | <1.0 | <1.0 | <1.0 | <1.0 | 0.1 | 100 |
| Silver | <2.0 | <2.0 | <2.0 | <2.0 | 0.1 | 100 |
| Thallium | <2.0 | <2.0 | <2.0 | <2.0 | 0.1 | 100 |
| Zinc | <1.0 | <1.0 | <1.0 | <1.0 | Not established | Not established |
3.6. Tobacco-Specific Nitrosamines (TSNAs)
| Chemical Compound | Background (before Participants Enter Room) | Room Occupied (No Vaping) | Room Occupied (Vaping Permitted) | Room Unoccupied (after Participants Leave Room) |
|---|---|---|---|---|
| Measurement 1 (µg/m3) | Measurement 2 (µg/m3) | Measurement 3 (µg/m3) | Measurement 4 (µg/m3) | |
| N’-Nitrosonornicotine (NNN) | <0.5 | <0.5 | <0.5 | <0.5 |
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) | <0.5 | <0.5 | <0.5 | <0.5 |
| N’-Nitrosoanatabine (NAT) | <0.5 | <0.5 | <0.5 | <0.5 |
| N’-Nitrosoanabasine (NAB) | <0.5 | <0.5 | <0.5 | <0.5 |
3.7. Study Limitations and Strengths
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ASH. Use of electronic cigarettes in Great Britain. Available online: http://www.ash.org.uk/files/documents/ASH_891.pdf (accessed on 17 August 2014).
- Farsalinos, K.E.; Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 2014, 5, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Riker, C.A.; Lee, K.; Darville, A.; Hahn, E.J. E-cigarettes: Promise or peril? Nurs. Clin. N. Am. 2012, 47, 159–171. [Google Scholar] [CrossRef]
- WHO. World Health Organisation Framework Convention on Tobacco Control. Available online: http://apps.who.int/gb/fctc/PDF/cop6/FCTC_COP6_10-en.pdf?ua=1 (accessed on 17 August 2014).
- Burstyn, I. Peering through the mist: Systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- BMA. BMA Calls for Stronger Regulation of E-Cigarettes. Available online: file:///C:/Users/ukbhgco/Downloads/tobaccoecigarettespublicplaces_jan2013.pdf (accessed on 20 November 2014).
- ASH. E-Cigarettes. Available online: http://www.scottish.parliament.uk/S4_HealthandSport Committee/Meeting%20Papers/Papers_for_Meeting-18_November_2014.pdf (accessed on 20 November 2014).
- Petry, T.; Vitale, D.; Joachim, F.J.; Smith, B.; Cruse, L.; Mascarenhas, R.; Schneider, S.; Singal, M. Human health risk evaluation of selected VOC, SVOC and particulate emissions from scented candles. Regul. Toxicol. Pharmacol. 2014, 69, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Schripp, T.; Markewitz, D.; Uhde, E.; Salthammer, T. Does e-cigarette consumption cause passive vaping? Indoor Air 2013, 23, 25–31. [Google Scholar] [CrossRef] [PubMed]
- McAuley, T.R.; Hopke, P.K.; Zhao, J.; Babaian, S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 2012, 24, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Czogala, J.; Goniewicz, M.L.; Fidelus, B.; Zielinska-Danch, W.; Travers, M.J.; Sobczak, A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2014, 16, 655–662. [Google Scholar] [CrossRef]
- Schober, W.; Szendrei, K.; Matzen, W.; Osiander-Fuchs, H.; Heitmann, D.; Schettgen, T.; Jorres, R.A.; Fromme, H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases feno levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 2014, 217, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Long, G.A. Comparison of select analytes in exhaled aerosol from e-cigarettes with exhaled smoke from a conventional cigarette and exhaled breaths. Int. J. Environ. Res. Public Health 2014, 11, 11177–11191. [Google Scholar] [CrossRef] [PubMed]
- Romagna, G.; Zabarini, L.; Barbiero, L.; Bocchietto, E.; Todeschi, S.; Caravati, E.; Voster, D.; Farsalinos, K. Characterization of Chemicals Released to the Environment by Electronic Cigarettes Use (Clearstream-Air Project): Is Passive Vaping a Reality. Available online: http://clearstream.flavourart.it/site/wp-content/uploads/2012/09/CSA_ItaEng.pdf (accessed on 18 March 2015).
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chang, H. Research gaps related to the environmental impacts of electronic cigarettes. Tob. Control 2014, 23, ii54–ii58. [Google Scholar] [CrossRef] [PubMed]
- Upton, S.; Kukadia, V. Ventilation Rate Measurement: New Tracer Gases and Techniques for Healthy Indoor Environments; BRE: Bracknell, UK, 2011. [Google Scholar]
- ISO. ISO 16000–6:2011 Indoor Air—Part 6: Determination of Volatile Organic Compounds in Indoor and Test Chamber Air by Active Sampling on Tenax ta Sorbent, Thermal Desorption and Gas Chromatography Using MS or MS-FID; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ISO. ISO 16000–3:2011 Indoor Air—Part 3: Determination of Formaldehyde and Other Carbonyl Compounds in Indoor Air and Test Chamber Air—Active Sampling Method; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- USEPA. Office of the Federal Registration (OFR) Appendix A: Priorty Pollutants. Available online: law.cornell.edu/cfr/text/40/part-423/appendix-A (accessed 18 March 2015).
- USEPA. Method 29-Metals Emissions from Stationary Sources. Available online: http://www.epa.gov/ttn/emc/promgate/m-29.pdf (accessed on 4 June 2014).
- CIBSE. Guide B: Heating, Ventilating, Air Conditioning and Refrigeration; Chartered Institute of Building Services Engineers: London, UK, 2005. [Google Scholar]
- General Fact Sheet: Limiting Conditions and Reliability, Ventilation, Room Size, Body Surface Area. Available online: http://www.rivm.nl/en/Documents_and_publications/Scientific/Reports/2006/augustus/General_fact_sheet_Limiting_conditions_and_reliability_ventilation_room_size_body_surface_area_Updated_version_for_ConsExpo_4 (accessed on 18 March 2015).
- HSE. The Six Basic Factors. Available online: http://www.hse.gov.uk/temperature/thermal/factors.htm (accessed on 6 November 2014).
- McGrath-Morrow, S.A.; Hayashi, M.; Aherrera, A.; Lopez, A.; Malinina, A.; Collaco, J.M.; Neptune, E.; Klein, J.D.; Winickoff, J.P.; Breysse, P.; et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Colard, S.; O’Connell, G.; Verron, T.; Cahours, X.; Pritchard, J.D. Electronic cigarettes and indoor air quality: A simple approach to modeling potential bystander exposures to nicotine. Int. J. Environ. Res. Public Health 2015, 12, 282–299. [Google Scholar] [CrossRef]
- HSE. Environmental Hygiene Guidance Note eh40/2005 Workplace Exposure Limits: Containing the List of Workplace Exposure Limits for Use with the Control of Substances Hazardous to Health Regulations (as Amended), 2nd ed.; HSE: Liverpool, UK, 2011. [Google Scholar]
- Armitage, A.K.; Dixon, M.; Frost, B.E.; Mariner, D.C.; Sinclair, N.M. The effect of tobacco blend additives on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Chem. Res. Toxicol. 2004, 17, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Lee, L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2014, 8. [Google Scholar] [CrossRef]
- Tran, T.M.; Kannan, K. Occurrence of cyclic and linear siloxanes in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Sci. Total Environ. 2015, 511, 138–144. [Google Scholar] [CrossRef] [PubMed]
- HM Government. The Building Regulations 2010: Ventilation Document F; HM Government: London, UK, 2010.
- Bekki, K.; Uchiyama, S.; Ohta, K.; Inaba, Y.; Nakagome, H.; Kunugita, N. Carbonyl compounds generated from electronic cigarettes. Int. J. Environ. Res. Public Health 2014, 11, 11192–11200. [Google Scholar] [CrossRef] [PubMed]
- Riess, U.; Tegtbur, U.; Fauck, C.; Fuhrmann, F.; Markewitz, D.; Salthammer, T. Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOx in exhaled human breath. Anal. Chim. Acta 2010, 669, 53–62. [Google Scholar] [CrossRef] [PubMed]
- CPSC. The U.S. Consumer Product Safety Commission: An Update on Formaldehyde. Available online: http://www.cpsc.gov/PageFiles/121919/AN%20UPDATE%20ON%20FORMALDEHYDE%20final%200113.pdf (accessed on 18 March 2015).
- WHO. Who Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Copenhagen, Denmark, 2010. [Google Scholar]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- EU. The Index Project, Critical Appraisal of the Setting and Implementation of Indoor Exposure Limits in the EU. Available online: http://ec.europa.eu/health/ph_projects/2002/pollution/ fp_pollution_2002_frep_02.pdf (accessed on 11 November 2014).
- EPA. Candles and Incense as Potential Sources of Indoor Air Pollution: Market Analysis and Literature Review. Available online: http://nepis.epa.gov/Adobe/PDF/P1009BZL.pdf (accessed on 13 November 2014).
- King, J.; Kupferthaler, A.; Frauscher, B.; Hackner, H.; Unterkofler, K.; Teschl, G.; Hinterhuber, H.; Amann, A.; Hogl, B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012, 33, 413–428. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicity of Polycyclic Aromatic Hydrocarbons (PAHs). Available online: http://www.atsdr.cdc.gov/csem/pah/docs/pah.pdf (accessed on 9 November 2014).
- Farsalinos, K.E.; Voudris, V. E-cigarette use and indoor air quality: Methodological limitations: Response to W. Schober et al.’s “use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases feno levels of e-cigarette consumers”. Int. J. Hyg. Environ. Health 2014, 217, 705–706. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- IARC. Air pollution, part 1, some non-heterocyclic polycyclic aromatic hydrocarbons and some related industrial exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 82, 1–556. [Google Scholar]
- Lerner, C.A.; Sundar, I.K.; Watson, R.M.; Elder, A.; Jones, R.; Done, D.; Kurtzman, R.; Ossip, D.J.; Robinson, R.; McIntosh, S.; et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ. Pollut. 2015, 198, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T. Chemical evaluation of electronic cigarettes. Tob. Control 2014, 23, ii11–ii17. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Etter, J.F.; Farsalinos, K.; Hajek, P.; le Houezec, J.; McRobbie, H. A critique of a WHO-commissioned report and associated article on electronic cigarettes. Addiction 2014, 12, 2128–2134. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O'Connell, G.; Colard, S.; Cahours, X.; Pritchard, J.D. An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room. Int. J. Environ. Res. Public Health 2015, 12, 4889-4907. https://doi.org/10.3390/ijerph120504889
O'Connell G, Colard S, Cahours X, Pritchard JD. An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room. International Journal of Environmental Research and Public Health. 2015; 12(5):4889-4907. https://doi.org/10.3390/ijerph120504889
Chicago/Turabian StyleO'Connell, Grant, Stéphane Colard, Xavier Cahours, and John D. Pritchard. 2015. "An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room" International Journal of Environmental Research and Public Health 12, no. 5: 4889-4907. https://doi.org/10.3390/ijerph120504889
APA StyleO'Connell, G., Colard, S., Cahours, X., & Pritchard, J. D. (2015). An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room. International Journal of Environmental Research and Public Health, 12(5), 4889-4907. https://doi.org/10.3390/ijerph120504889