Diuron in Water: Functional Toxicity and Intracellular Detoxification Patterns of Active Concentrations Assayed in Tandem by a Yeast-Based Probe
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials, Apparatus, Samples
2.2. Methods
- 50 mg ± 1.0 mg dried S. cerevisiae cells were rehydrated in test tubes always 12 h prior to the experiment with 10 mL of Milli-Q water without any agitation; no nutrient was added to prevent proliferation of cells.
- 0.5 M of glucose solution with Milli-Q water was prepared at weekly basis for replications of experiments eliminating extra variations of test.
- diuron stock solution, 10−2 M, was prepared by adding 60.3 mg of herbicide in 25 mL of methanol: dilutions with water Milli-Q were carried out to obtain 10−4 M, 10−5 M, 10−6 M and 10−8 M of diuron respectively.
- two-point calibration of the Clark-type oximeter was completed using: (a) atmospheric oxygen and (b) a sodium sulfite solution (10 g/L). Calibration with atmospheric oxygen was done before every single test because atmospheric oxygen measurements depend on both temperature and barometric pressure [24]. The calibration point with sodium sulfite gives the electrode a zero reference point since this compound binds with the dissolved O2.
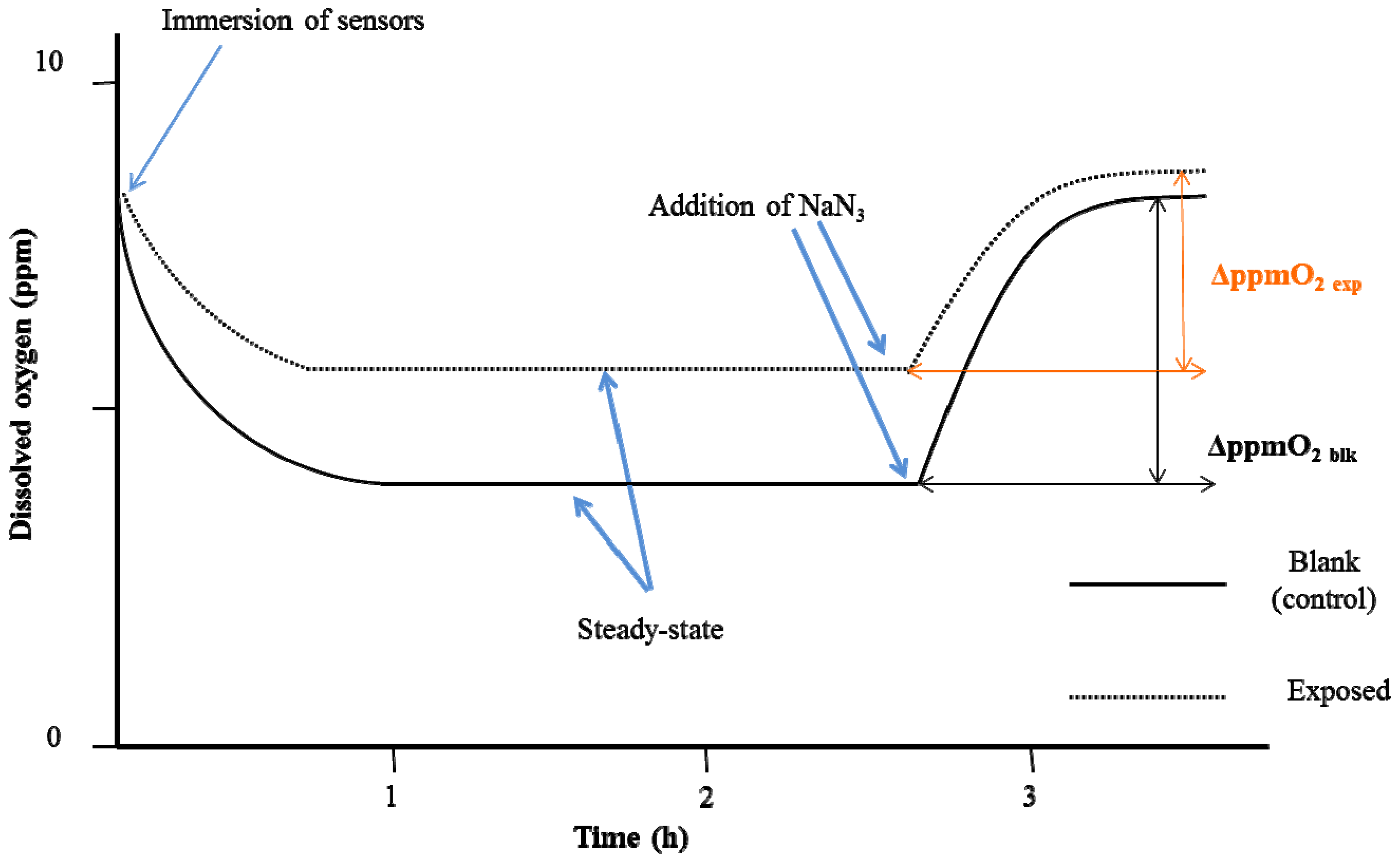
3. Results and Discussion
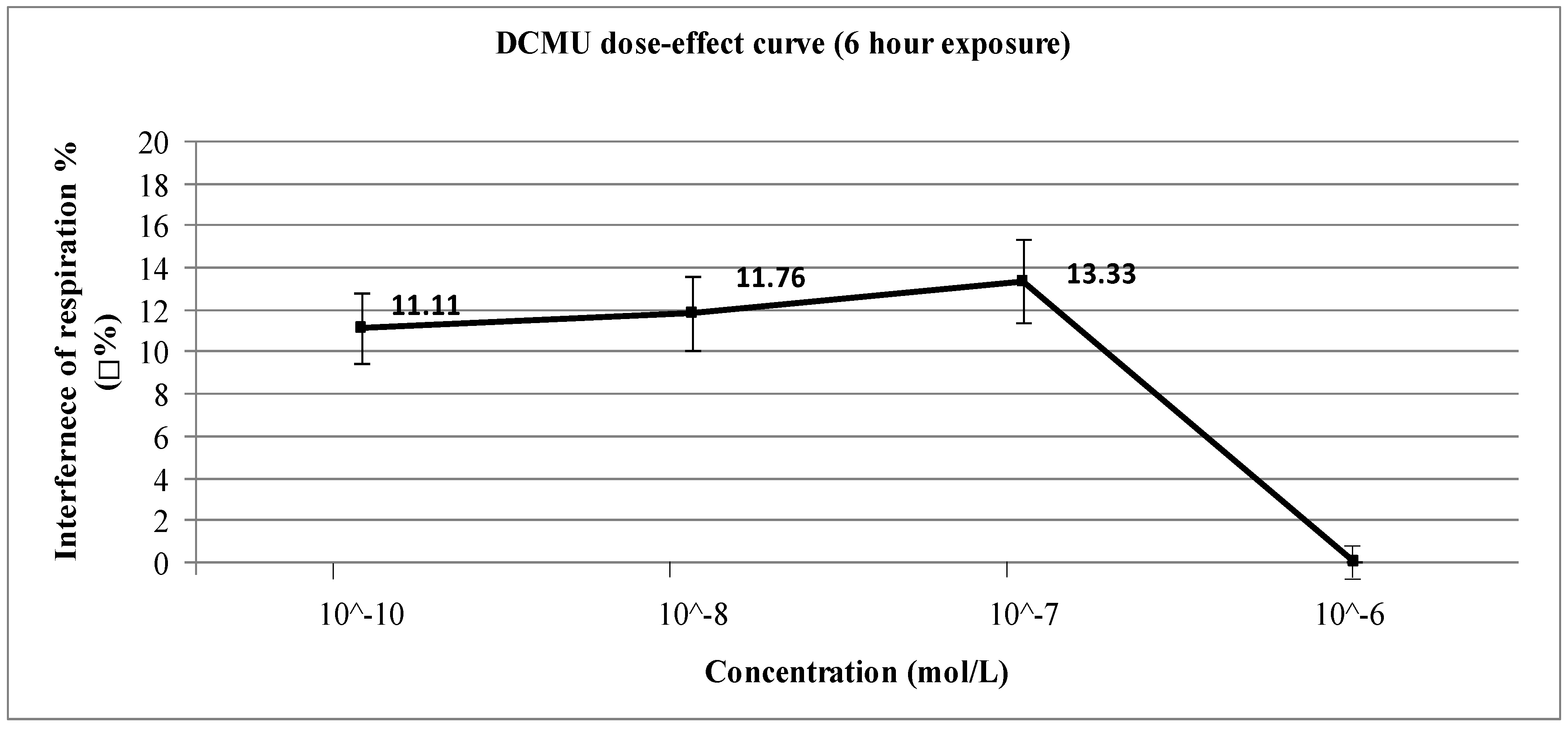
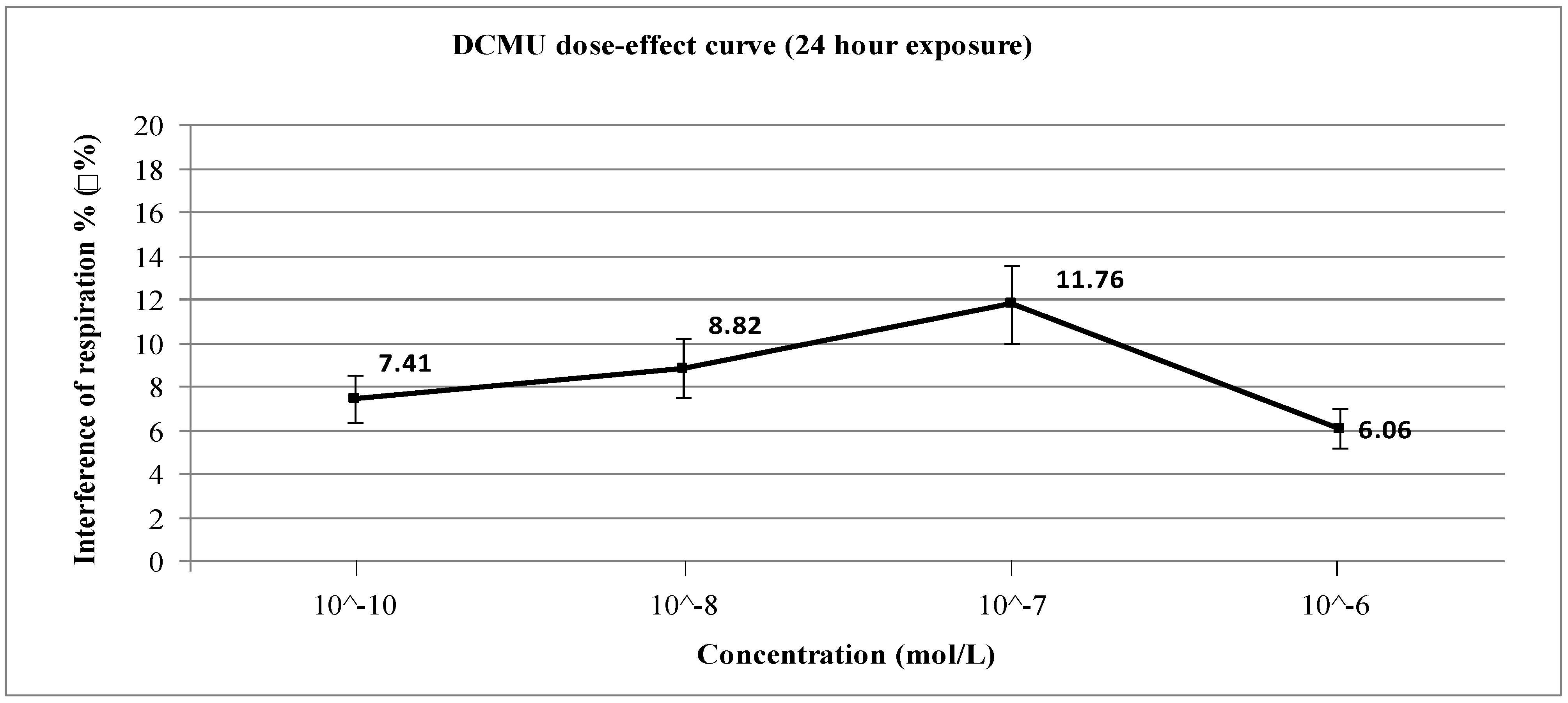
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Worldwide pesticide use. In Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2014; pp. 5–6. [Google Scholar]
- Estève, K.; Poupot, C.; Dabert, P.; Mietton-Peuchot, M.; Milisic, V. A Saccharomyces cerevisiae-based bioassay for assessing pesticide toxicity. J. Ind. Microbiol. Biotechnol. 2009, 36, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Karalliedde, L.; Buckley, N.; Fernando, R.; Hutchinson, G.; Isbister, G.; Konradsen, F.; Murray, D.; Piola, J.C.; Senanayake, N.; Sheriff, R.; Singh, S.; Siwach, S.B.; Smit, L. Pesticide poisoning in the developing world—A minimum pesticides list. Lancet 2002, 360, 1163–1167. [Google Scholar] [CrossRef]
- Sass, J.B.; Needleman, H.L. Industry testing of toxic pesticides on human subjects concluded “no effect” despite the evidence. Environ. Health Perspect. 2004, 112, 150–151. [Google Scholar]
- Barra Caracciolo, A.; Giuliano, G.; Grenni, P.; Guzzella, L.; Pozzoni, F.; Bottoni, P.; Fava, L.; Crobe, A.; Orrù, M.; Funari, E. Degradation and leaching of the herbicides metolachlor and diuron: A case study in an area of northern Italy. Environ. Pollut. 2005, 134, 525–534. [Google Scholar]
- Italian Ministry of Health. Ministero Del Lavoro, Della Salute E Delle Politiche Sociali. Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=29100 (accessed on 26 November 2014).
- European Food Safety Authority. Reasoned opinion of EFSA: Review of the Existing Maximum Residue Levels (MRLs) for diuron according to article 12 of regulation (EC) No. 396/2005. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Convent, B.; Briquet, M.; Goffeau, A. Kinetic evidence for two sites in the inhibition by diuron of the electron transport in the bc1 segment of the respiratory chain in Saccharomyces cerevisiae. Eur. J. Biochem. 1978, 92, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Convent, B.; Briquet, M. Properties of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea and other inhibitors of the cytochrome bc1 segment of the mitochondrial respiratory chain in Saccharomyces cerevisiae. Eur. J. Biochem. 1978, 82, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Giessler, A.; Geier, B.M.; Rago, J.P.; Slonimski, P.P.; Jagow, G. Analysis of cytochrome-b amino acid residues forming the contact face with the iron-sulfur subunit of ubiquinol: Cytochrome-c reductase in Saccharomyces cerevisiae. Eur. J. Biochem. 1994, 222, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B.; Colson, A.M. Two nuclearly inherited loci conferring increased diuron resistance to NADH oxidase in Saccharomyces cerevisiae. Curr. Genetics 1989, 15, 31–38. [Google Scholar] [CrossRef]
- Dragone, R.; Frazzoli, C.; Grappelli, C.; Campanella, L. A new respirometric endpoint-based biosensor to assess the relative toxicity of chemicals on immobilized human cells. Ecotoxicol. Environ. Safety 2009, 72, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G.; Koopman, B.; Wang, H.D. Baker’s yeast assay procedure for testing heavy metal toxicity. Bull. Environ. Contam. Toxicol. 1984, 32, 80–84. [Google Scholar] [CrossRef]
- Altschuh, D. Immunochemistry and biosensors. Current Opin. Chem. Biol. 1999, 3, 106–111. [Google Scholar] [CrossRef]
- Altschuh, D. Esoon’04, Grenoble-France. 2004. Available online: http://esonn.fr/0oldweb/ESONN04/Lectures/esonn_2004_Altschuh.pdf (accessed on 28 November 2014).
- Pérez-García, A.; Codina, J.C.; Cazorla, F.M.; Vicente, A. Rapid respirometric toxicity test: Sensitivity to metals. Bull. Environ. Contam. Toxicol. 1993, 50, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, R.M.; Keenan, P. The eukaryote alternative: Advantages of using yeasts in place of bacteria in microbial biosensor development. Biotechnol. Bioprocess Eng. 2000, 5, 387–394. [Google Scholar] [CrossRef]
- Dragone, R.; Frazzoli, C.; Grasso, G.; Rossi, G. Sensor with intact or modified yeast cells as rapid device for toxicological test of chemicals. J. Agr. Chem. Environ. 2014, 3, 35–40. [Google Scholar]
- Ribeiro, I.C.; Verı́ssimo, I.; Moniz, L.; Cardoso, H.; Sousa, M.J.; Soares, A.M.V.M.; Leão, C. Yeasts as a model for assessing the toxicity of the fungicides Penconazol, Cymoxanil and Dichlofluanid. Chemosphere 2000, 41, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Favero, G.; Mastrofini, D.; Tomassetti, M. Further developments in toxicity cell biosensors. Sens. Actuat. B-Chem. 1997, 44, 279–285. [Google Scholar] [CrossRef]
- Campanella, L.; Favero, G.; Tomassetti, M. Immobilised yeast cells biosensor for total toxicity testing. Sci. Total Environ. 1995, 171, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Favero, G.; Mastrofini, D.; Tomassetti, M. Respirometric biomonitor for the control of industrial effluent toxicity. In European Symposium on Optics for Environmental and Public Safety; International Society for Optics and Photonics: Munich, Germany, 1995; pp. 221–230. [Google Scholar]
- Frazzoli, C.; Dragone, R.; Mantovani, A.; Massimi, C.; Campanella, L. Functional toxicity and tolerance patterns of bioavailable Pd (II), Pt (II), and Rh (III) on suspended Saccharomyces cerevisiae cells assayed in tandem by a respirometric biosensor. Anal. Bioanal. Chem. 2007, 389, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.P.; Killham, K.; Glover, L.A. Design and application of a biosensor for monitoring toxicity of compounds to eukaryotes. Appl. Environ. Microbiol. 2000, 66, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1405609372533&uri=CELEX:31998L0083 (accessed on 2 December 2014).
- Fisher, N.; Meunier, B. Molecular basis of resistance to cytochrome bc1 inhibitors. FEMS Yeast Res. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Rieske, J.S. Inhibitors of respiration at energy-coupling site 2 of the respiratory chain. Pharmacol. Therapeut. 1980, 11, 415–450. [Google Scholar] [CrossRef]
- Jungwirth, H.; Kuchler, K. Yeast ABC transporters—A tale of sex, stress, drugs and aging. FEBS Lett. 2006, 580, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.; Decottignies, A.; Kolaczkowski, M.; Carvajal, E.; Balzi, E.; Goffeau, A. The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 2001, 3, 207–214. [Google Scholar] [PubMed]
- Moradas-Ferreira, P.; Costa, V. Adaptive response of the yeast Saccharomyces cerevisiae to reactive oxygen species: Defences, damage and death. Redox Rep. 2000, 5, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Bba-Gen. Subjects 2008, 1780, 1217–1235. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Telo, J.P.; Duarte, N.F.; Sá-Correia, I. The herbicide 2,4-dichlorophenoxyacetic acid induces the generation of free-radicals and associated oxidative stress responses in yeast. Biochem. Biophys. Res. Commun. 2004, 324, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.F.W.; Koorengevel, M.C.; de Kruijff, B.; de Kroon, A.I.P.M. Transbilayer movement of phosphatidylcholine in the mitochondrial outer membrane of Saccharomyces cerevisiae is rapid and bidirectional. Bba-Biomembranes 1999, 1421, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Recio, E.; Maqueda, C.; Sánchez-Verdejo, T.; Balek, V. Slow diuron release formulations based on clay-phosphatidylcholine complexes. Appl. Clay Sci. 2012, 55, 53–61. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragone, R.; Cheng, R.; Grasso, G.; Frazzoli, C. Diuron in Water: Functional Toxicity and Intracellular Detoxification Patterns of Active Concentrations Assayed in Tandem by a Yeast-Based Probe. Int. J. Environ. Res. Public Health 2015, 12, 3731-3740. https://doi.org/10.3390/ijerph120403731
Dragone R, Cheng R, Grasso G, Frazzoli C. Diuron in Water: Functional Toxicity and Intracellular Detoxification Patterns of Active Concentrations Assayed in Tandem by a Yeast-Based Probe. International Journal of Environmental Research and Public Health. 2015; 12(4):3731-3740. https://doi.org/10.3390/ijerph120403731
Chicago/Turabian StyleDragone, Roberto, Rachel Cheng, Gerardo Grasso, and Chiara Frazzoli. 2015. "Diuron in Water: Functional Toxicity and Intracellular Detoxification Patterns of Active Concentrations Assayed in Tandem by a Yeast-Based Probe" International Journal of Environmental Research and Public Health 12, no. 4: 3731-3740. https://doi.org/10.3390/ijerph120403731
APA StyleDragone, R., Cheng, R., Grasso, G., & Frazzoli, C. (2015). Diuron in Water: Functional Toxicity and Intracellular Detoxification Patterns of Active Concentrations Assayed in Tandem by a Yeast-Based Probe. International Journal of Environmental Research and Public Health, 12(4), 3731-3740. https://doi.org/10.3390/ijerph120403731







