Efficiency of a Multi-Soil-Layering System on Wastewater Treatment Using Environment-Friendly Filter Materials
Abstract
:1. Introduction
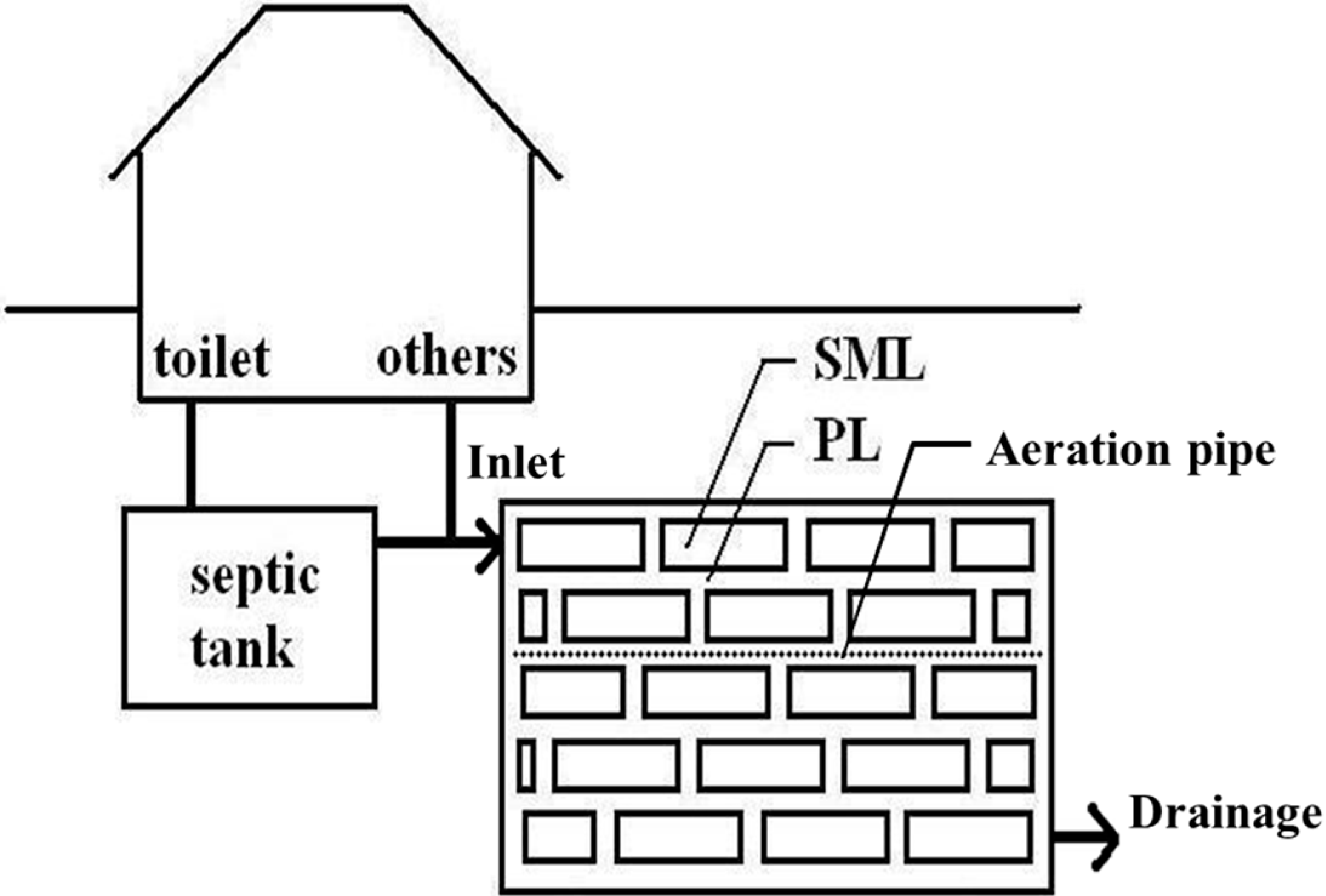
2. Wastewater Treatment Mechanism of MSL
| Pollutant | Primary Reactant | Reaction Conditions | Precautions during Operation |
|---|---|---|---|
| Organic matter | Microorganism | Sufficient organic matter | The accumulated organic matter may cause microbial overgrowth, forming a biofilm that blocks water flow. The accumulation can be reduced by ventilating the system or leaving the system idle. |
| Phosphate | Fe(OH)3 | Dissolution and oxidation of iron | During excessive ventilation, oxidation may cause Fe2O3 low-activation surfaces, which reduces effective surface area and phosphate fixation capacity. |
| NH4+-N NO3-N | Microorganism | Aerobic nitrification Anaerobic Denitrification | Aerobic and anaerobic states determine the removal of TN. These states can be controlled by adjusting the ventilation time and quantity. |
3. Materials and Method
3.1. Materials of the MSL System
3.1.1. Soil Mixture Layers, SMLs
Soil
Powdered Activated Carbon
Organic Matter
Iron Scraps

3.1.2. Permeable Layers, PLs
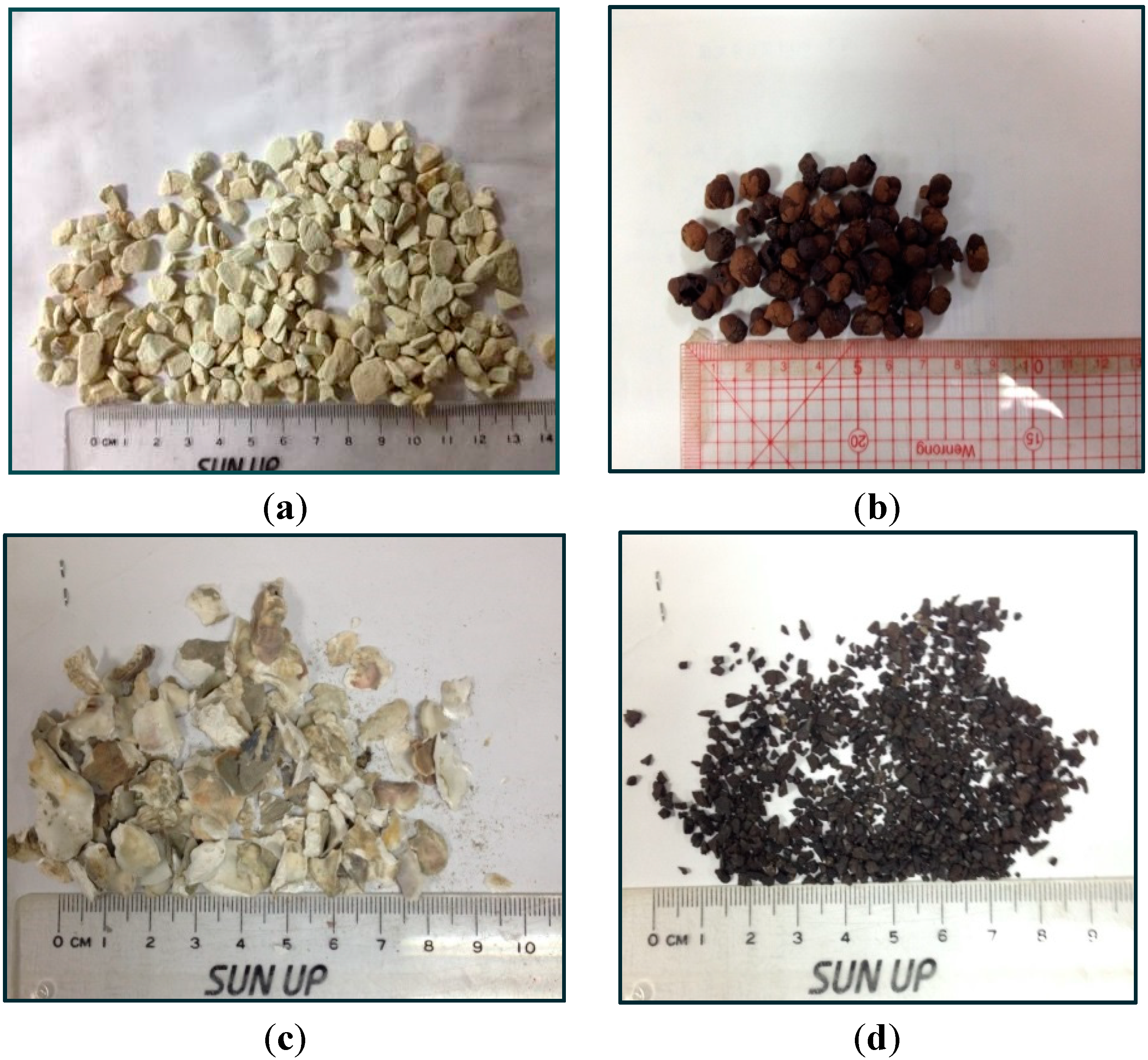
Zeolite
Expanded Clay Aggregate
Oyster Shells
Granular Activated Carbon
3.2. Test Apparatus
3.3. Experiment Process Scheme
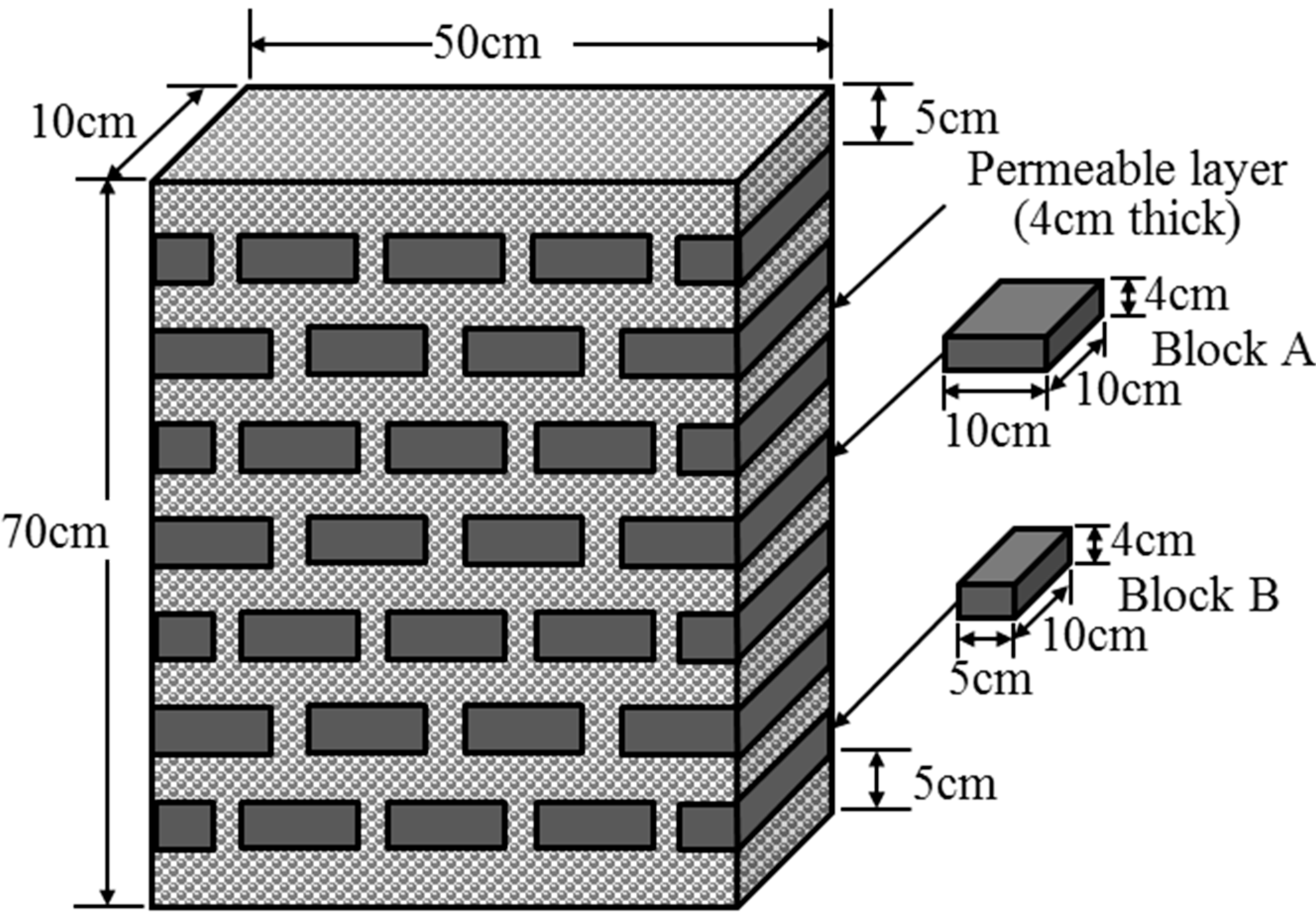
3.3.1. Preparing Test Samples (Sample Chamber)
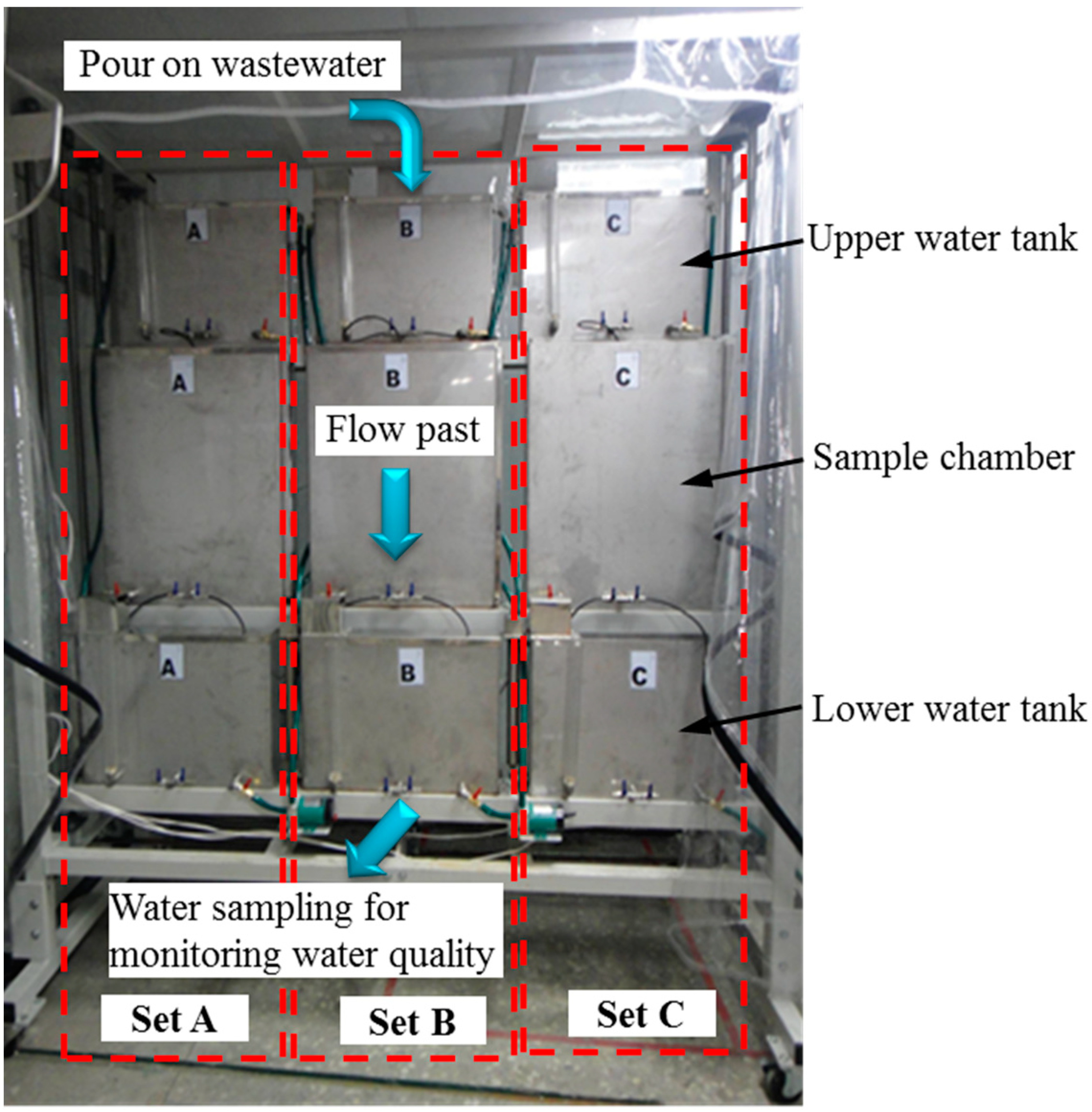
3.3.2. Transferring Wastewater (Upper Water Tank)
3.3.3. Monitoring Water Quality
4. Results and Discussion
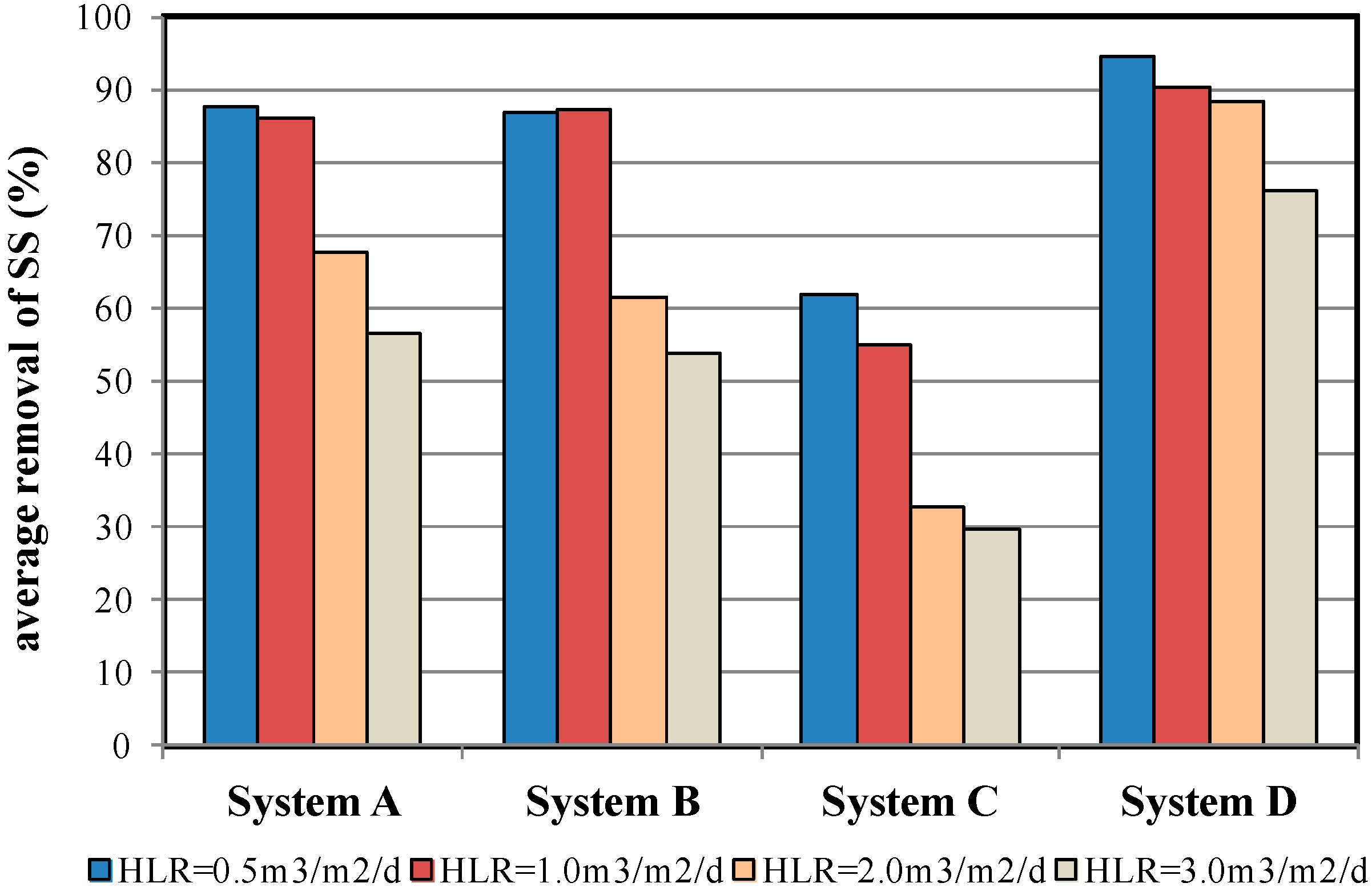
4.1. SS Removal Efficiency
| Test Condition | Filter Medium | ||||
|---|---|---|---|---|---|
| System A | System B | System C | System D | ||
| HLR (m3/m2/d) | Inflow (mg/L) | 11.28 ± 6.90 | 11.28 ±6.90 | 16.77 ± 5.65 | 16.77 ± 5.65 |
| 0.5 | Outflow (mg/L) | 1.80 ± 1.16 | 1.88 ± 1.31 | 6.24 ± 2.09 | 0.88 ± 0.26 |
| % removal | 83.4 ± 10.8 | 82.5 ± 12.1 | 63.2 ± 4.7 | 94.5 ± 1.4 | |
| 1.0 | Outflow (mg/L) | 1.57 ± 1.05 | 1.85 ± 1.19 | 7.56 ± 2.31 | 1.69 ± 0.75 |
| % removal | 84.6 ± 8.9 | 82.8 ± 10.9 | 54.9 ±4.5 | 90.3 ± 2.9 | |
| 2.0 | Outflow (mg/L) | 3.42 ± 1.80 | 4.16 ± 2.14 | 11.57 ± 4.62 | 2.12 ± 0.83 |
| % removal | 65.4 ± 6.9 | 62.4 ± 11.4 | 33.5 ± 6.6 | 88.9 ± 1.8 | |
| 3.0 | Outflow (mg/L) | 4.00 ± 1.86 | 4.38 ± 1.87 | 12.24 ± 4.67 | 3.97 ± 1.10 |
| % removal | 54.6 ± 5.0 | 54.2 ± 11.5 | 32.4 ± 8.8 | 74.2 ± 4.9 | |
4.2. COD Removal Efficiency
| Test Condition | Filter Medium | ||||
|---|---|---|---|---|---|
| System A | System B | System C | System D | ||
| HLR (m3/m2/d) | Inflow (mg/L) | 170.7 ± 61.4 | 170.7 ± 61.4 | 203.2 ± 51.5 | 203.2 ± 51.5 |
| 0.5 | Outflow (mg/L) | 36.5 ± 9.1 | 51.1 ± 17.0 | 94.6 ± 25.1 | 52.8 ± 13.6 |
| % removal | 76.9 ± 7.3 | 68.8 ± 7.5 | 54.6 ± 2.7 | 73.9 ± 4.1 | |
| 1.0 | Outflow (mg/L) | 68.3 ± 29.1 | 78.3 ± 24.5 | 130.1 ± 32.8 | 85.4 ± 17.3 |
| % removal | 65.2 ± 11.9 | 51.8 ± 4.1 | 33.9 ± 3.7 | 57.0 ± 2.7 | |
| 2.0 | Outflow (mg/L) | 78.8 ± 15.6 | 110.6 ± 33.5 | 152.7 ± 35.4 | 111.6 ± 24.5 |
| % removal | 49.4 ± 14.7 | 33.7 ± 4.2 | 22.9 ± 3.3 | 40.6 ± 6.0 | |
| 3.0 | Outflow (mg/L) | 113.3 ± 41.0 | 114.8 ± 35.6 | 143.9 ± 29.8 | 119.5 ± 29.7 |
| % removal | 30.5 ± 18.2 | 33.0 ± 8.6 | 26.8 ± 5.0 | 42.7 ± 3.1 | |

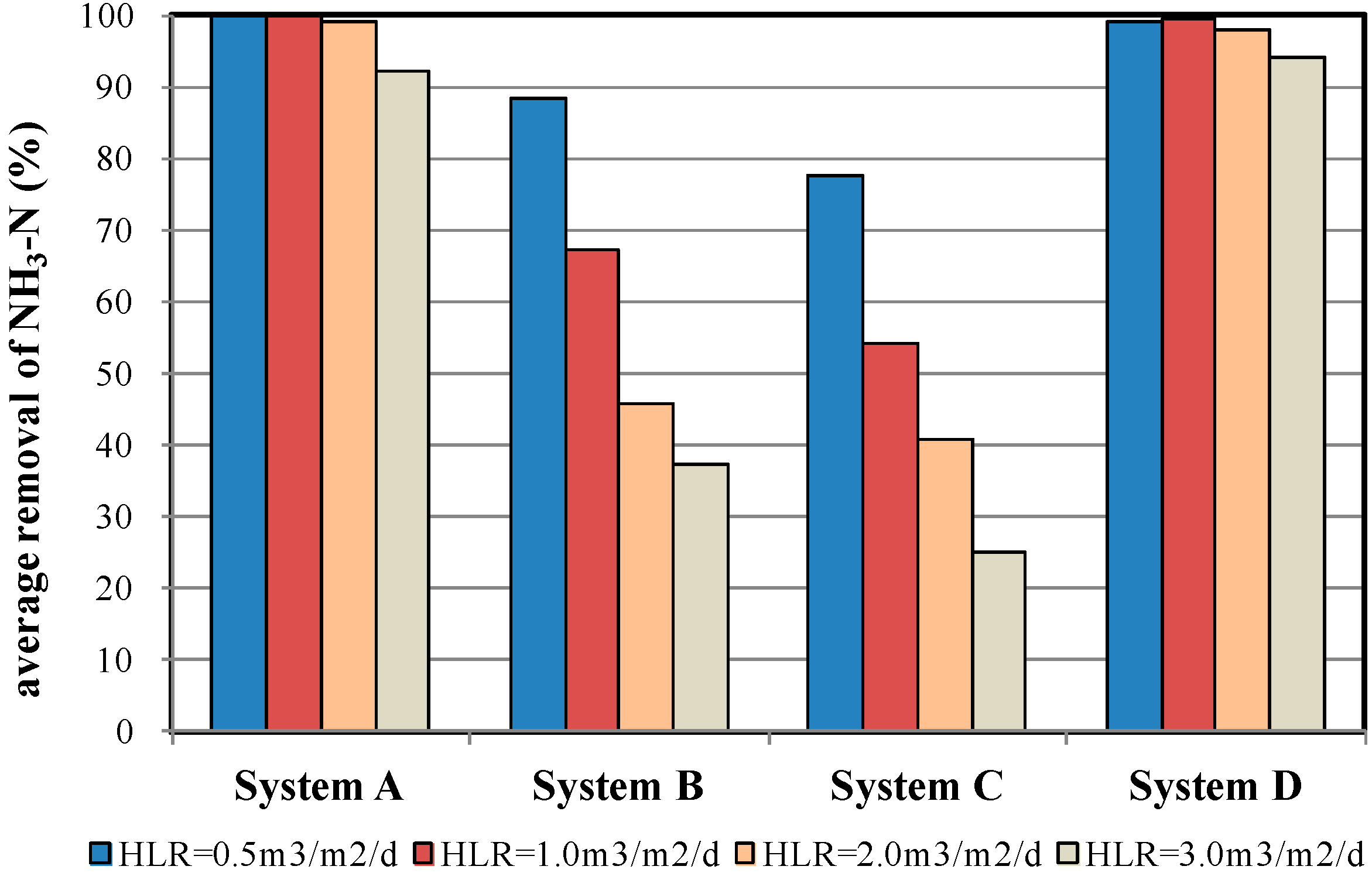
4.3. NH3-N Removal Efficiency
| Test Condition | Filter Medium | ||||
|---|---|---|---|---|---|
| System A | System B | System C | System D | ||
| HLR (m3/m2/d) | Inflow (mg/L) | 24.6 ± 3.7 | 24.6 ± 3.7 | 27.5 ± 6.9 | 27.5 ± 6.9 |
| 0.5 | Outflow (mg/L) | 0.07 ± 0.05 | 2.82 ± 0.79 | 6.26 ± 1.59 | 0.27 ± 0.14 |
| % removal | 99.7 ± 0.2 | 87.2 ± 4.5 | 77.9 ± 1.6 | 99.2 ± 0.4 | |
| 1.0 | Outflow (mg/L) | 0.08 ± 0.06 | 7.73 ± 0.89 | 12.91 ± 2.62 | 0.22 ± 0.12 |
| % removal | 99.7 ± 0.2 | 67.0 ± 5.2 | 53.8 ± 3.9 | 99.3 ± 0.4 | |
| 2.0 | Outflow (mg/L) | 0.37 ± 0.19 | 14.15 ± 2.37 | 16.54 ± 4.44 | 0.57 ± 0.18 |
| % removal | 98.6 ± 0.7 | 45.6 ± 4.0 | 41.3 ± 1.9 | 97.6 ± 1.3 | |
| 3.0 | Outflow (mg/L) | 2.96 ± 1.00 | 14.63 ± 2.74 | 20.19 ± 4.82 | 1.72 ± 0.41 |
| % removal | 87.5 ± 4.5 | 37.2 ± 5.9 | 24.8 ± 2.5 | 93.4 ± 2.0 | |
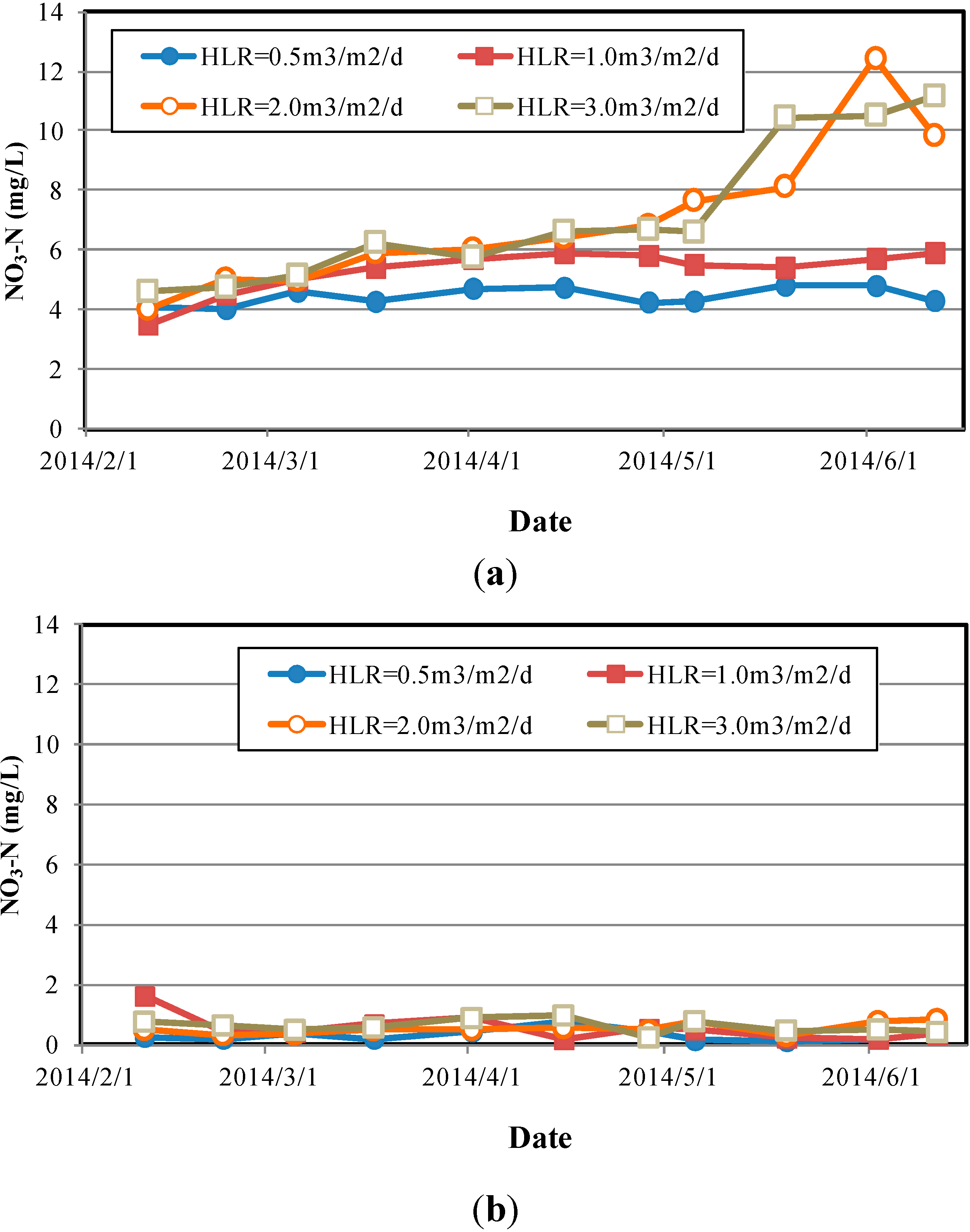
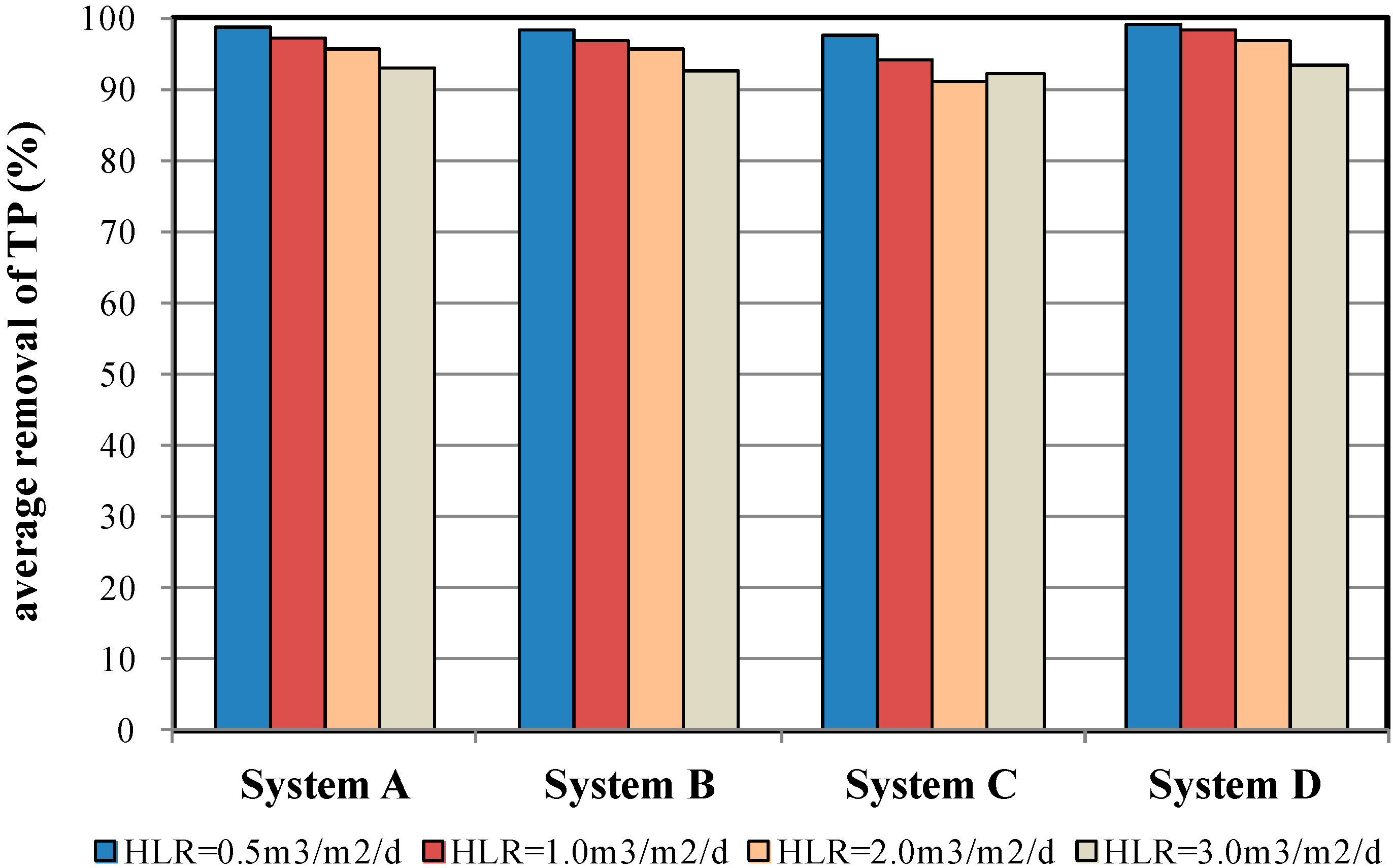
4.4. TP Removal Efficiency
| Test Condition | Filter Medium | ||||
|---|---|---|---|---|---|
| System A | System B | System C | System D | ||
| HLR (m3/m2/d) | Inflow (mg/L) | 7.9 ± 3.8 | 7.9 ± 3.8 | 10.7 ± 5.0 | 10.7 ± 5.0 |
| 0.5 | Outflow (mg/L) | 0.15 ± 0.12 | 0.14 ± 0.06 | 0.28 ± 0.19 | 0.08 ± 0.04 |
| % removal | 98.5 ± 0.8 | 98.4 ± 0.4 | 97.8 ± 0.7 | 99.1 ± 0.1 | |
| 1.0 | Outflow (mg/L) | 0.25 ± 0.12 | 0.27 ± 0.16 | 0.57 ± 0.36 | 0.12 ± 0.03 |
| % removal | 96.9 ± 1.2 | 96.4 ± 0.5 | 94.4 ± 2.3 | 98.3 ± 0.6 | |
| 2.0 | Outflow (mg/L) | 0.35 ± 0.16 | 0.37 ± 0.14 | 0.90 ± 0.37 | 0.49 ± 0.35 |
| % removal | 95.8 ± 1.7 | 95.6 ± 0.6 | 90.5 ± 1.4 | 96.3 ± 1.5 | |
| 3.0 | Outflow (mg/L) | 0.45 ± 0.24 | 0.63 ± 0.30 | 0.80 ± 0.31 | 0.58 ± 0.29 |
| % removal | 92.5 ± 3.1 | 93.2 ± 1.2 | 91.4 ± 2.3 | 93.3 ± 1.6 | |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, C.F.; Lin, J.Y.; Huang, C.H.; Chen, W.L.; Chueh, N.L. Performance evaluation of a full-scale natural treatment system for nonpoint source and point source pollution removal. Environ. Monit. Assess. 2009, 157, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Wang, J.Y.; Lee, H.Y.; Wen, C.K. Application of a constructed wetland for non-point source pollution control. Water Sci. Technol. 2001, 44, 585–590. [Google Scholar] [PubMed]
- Masunaga, T.; Sato, K.; Shirahama, M.; Kudo, H.; Wakatsuki, T. Characteristics of wastewater treatment using a multi-soil-layering system in relation to wastewater contamination levels and hydraulic loading rates. Soil Sci. Plant Nutr. 2007, 53, 215–223. [Google Scholar] [CrossRef]
- Chen, X.; Luo, A.C.; Sato, K.; Wakasuki, T.; Masunaga, T. An introduction of a multi-soil-layering system: A novel green technology for wastewater treatment in rural areas. Water Environ. J. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Luanmanee, S.; Boonsook, P.; Attanandana, T.; Saitthiti, B.; Panichajakul, C.; Wakatsuki, T. Effect of intermittent aeration regulation of a multi-soil-layering system on domestic wastewater treatment in Thailand. Ecol. Eng. 2002, 18, 415–428. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N&P removable on-site domestic waste water treatment system by Multi-Soil-Layering method. Water Sci. Technol. 1993, 27, 31–40. [Google Scholar]
- Wakatsuki, T.; Luanmanee, S.; Masunaga, T.; Attanandana, T. High rade on-site treatment of domestic wastewater and polluted river water by Multi-Soil-Layering method. In Proceedings of the IWA (International Water Association) Conference, Johannesburg, South Africa, 23–26 May 2000.
- Wen, D.; Ho, Y.S.; Tang, X. Comparative sorption kinetic studies of ammonium onto zeolite. J. Hazard. Mater. 2006, 133, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Saltali, K.; Sari, A.; Aydin, M. Removal of ammonium ion from aqueous solution by natural Turkish (Yildizeli) zeolite for environmental quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J. Hazard. Mater. 2007, 170, 1186–1191. [Google Scholar] [CrossRef]
- Cintoli, R.; Sabatino, B.D.; Galeotti, L.; Bruno, G. Ammonium uptake by zeolite and treatment in UASB reactor of piggery wastewater. Water Sci. Technol. 1995, 32, 73–81. [Google Scholar] [CrossRef]
- Sato, K.; Masunaga, T.; Wakatsuki, T. Water movement characteristics in a Multi-Soil-Layering system. Soil Sci. Plant Nutr. 2005, 51, 75–82. [Google Scholar] [CrossRef]
- Sato, K.; Masunaga, T.; Wakatsuki, T. Characterization of treatment processes and mechanisms of COD, phosphorus and nitrogen removal in a multi-soil-layering system. Soil Sci. Plant Nutr. 2005, 51, 213–221. [Google Scholar] [CrossRef]
- Boonsook, P.; Luanmanee, S.; Attanandana, T.; Kamidouzono, A.; Masunaga, T.; Wakatsuki, T. A comparative study of permeable layer materials and aeration regime on efficiency of multi-soil-layering system for domestic wastewater treatment in Thailand. Soil Sci. Plant Nutr. 2003, 49, 873–882. [Google Scholar] [CrossRef]
- Pattnaik, R.; Yost, R.S.; Porter, G.; Masunaga, T.; Attanandana, T. Improving Multi-Soil-Layering (MSL) system remediation of dairy effluent. Ecol. Eng. 2007, 32, 1–10. [Google Scholar] [CrossRef]
- Attanandana, T.; Saitthiti, B.; Thongpae, S.; Kritapirom, S.; Luanmanee, S.; Wakatsuki, T. Multi-media-layering system for food service wastewater treatment. Ecol. Eng. 2000, 15, 133–138. [Google Scholar] [CrossRef]
- Masunaga, T.; Wakatsuki, T. High quality water remediation by the Multi Soil Layering Method. In Proceedings of the 12th International Conference on Chemistry for Protection of the Environment, Nanjing, China, 19 September 1999; pp. 303–309.
- Harada, K.; Wakatsuki, T. Advanced treatment for livestock wastewater by MSL system. In Proceedings of the 31st Annual Conference of Japanese Society of Water Environment Tokyo, Japanese Society of Water Environment, Tokyo, Japan, 17 March 1997; p. 140. (In Japanese)
- Masunaga, T.; Sato, K.; Wakatsuki, T. Environmental remediation using purification function of soil by Multi-Soil-Layering system. In Proceedings of the 17th World Congress of Soil Science, Symposium 55, Bangkok, Thailand, 14–21 August 2002; p. 1108.
- Wakatsuki, T.; Omura, S.; Abe, Y.; Izumi, K. Treatment of domestic waste water by Multi-Soil-Layering method. Jpn. J. Soil Sci. Plant. Nutr. 1989, 60, 335–351. (In Japanese) [Google Scholar]
- Wakatsuki, T.; Omura, S.; Abe, Y.; Izumi, K. Role and life of purification abilities of soil materials in the MSL system. Jpn. J. Soil Sci. Plant. Nutr. 1990, 61, 74–84. (In Japanese) [Google Scholar]
- Rhodes, C.J. Properties and applications of zeolites. Sci. Prog. 2010, 93, 223–284. [Google Scholar] [CrossRef] [PubMed]
- Rinzema, A.; van Lier, J.; Lettinga, G. Sodium inhibition of acetoclastic methanogens in granular sludge from a UASB reactor. Enzyme Microb. Technol. 1988, 10, 24–32. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-C.; Wang, P.-H. Efficiency of a Multi-Soil-Layering System on Wastewater Treatment Using Environment-Friendly Filter Materials. Int. J. Environ. Res. Public Health 2015, 12, 3362-3380. https://doi.org/10.3390/ijerph120303362
Ho C-C, Wang P-H. Efficiency of a Multi-Soil-Layering System on Wastewater Treatment Using Environment-Friendly Filter Materials. International Journal of Environmental Research and Public Health. 2015; 12(3):3362-3380. https://doi.org/10.3390/ijerph120303362
Chicago/Turabian StyleHo, Chia-Chun, and Pei-Hao Wang. 2015. "Efficiency of a Multi-Soil-Layering System on Wastewater Treatment Using Environment-Friendly Filter Materials" International Journal of Environmental Research and Public Health 12, no. 3: 3362-3380. https://doi.org/10.3390/ijerph120303362





