Investigation of the Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) on Apoptosis and Cell Cycle in a Zebrafish (Danio rerio) Liver Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZFL Culture
2.2. Cellular Modeling and MTT Assay
2.3. Quantitative Real Time-Polymerase Chain Reaction (qPCR)
2.4. Flow Cytometry of Cell Apoptosis Detection
| Primer | Sequences (5′ to 3′) | PCR Fragments (bp) |
|---|---|---|
| P53 forward primer | CCACCATGAGAGAACACCTGAT | 144 |
| P53 reverse primer | GCTGAGGAGCTTCATAGAGAACC | |
| Bcl-2 forward primer | TGGGCTCATCTCCTTCTCC | 178 |
| Bcl-2 reverse primer | TCTCCTCACAGCTCCACATC | |
| Bax forward primer | GGAGGCGATACGGGCAGT | 151 |
| Bax reverse primer | TTGCGAATCACCAATGCTGTG | |
| Caspase-3 forward primer | ACTGATGGTTCTGTGGAGC | 200 |
| Caspase-3 reverse primer | CGCATAGAGGAAGTCTGCTT | |
| NFKBNF-κB p65 forward primer | AAGATGAGAACGGAGACACGC | 404 |
| NFKBNF-κB p65 reverse primer | TACCAGCAATCGCAAACAACG | |
| B-actin forward primer | CAGGGCGTGATGGTGGGGAT | 226 |
| β-actin reverse primer | GGTTGGCTTTGGGGTTGAG | |
| GAPDH forward primer | AGTTGTAAGCAATGCCTCCTG | 191 |
| GAPDH reverse primer | CTGGGATGATGTTCTGACTGG | |
| B2M forward primer | GCACTCATCACTTTTGCACTTC | 135 |
| B2M reverse primer | GCTCACATAGCAGATCAGGGT |
2.5. Cell Cycle Analysis
2.6. Western Blot Assay
2.7. Statistical Analysis
3. Results
3.1. Detection of IC50 and IC80 Concentrations
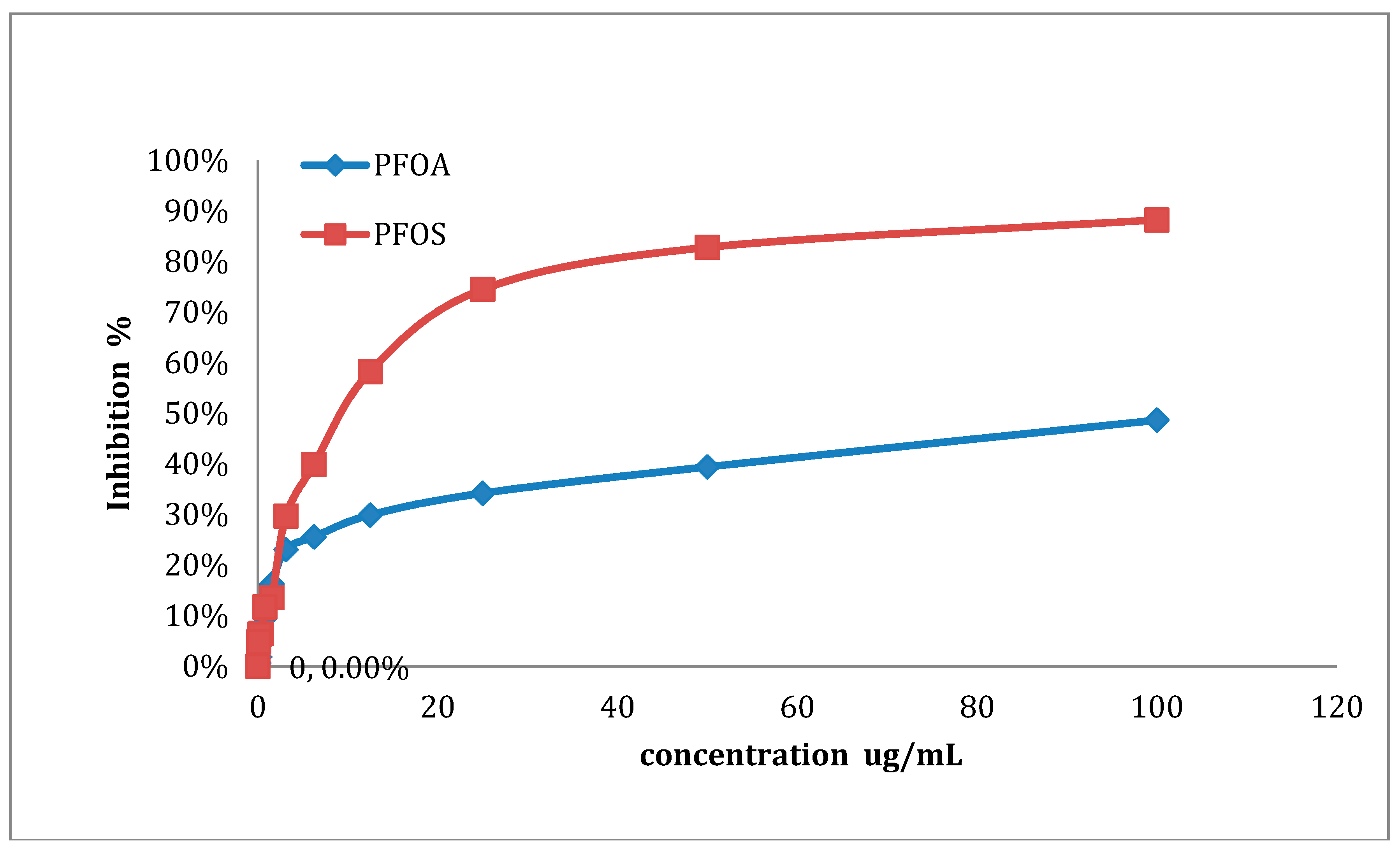
| Substances | IC50 | IC80 |
|---|---|---|
| PFOA (μg/mL) | 84.76 | 150.97 |
| PFOS (μg/mL) | 27.92 | 56.77 |
3.2. Relative Expression of p53, Bcl-2, Bax, Caspase-3 and NFκB p65
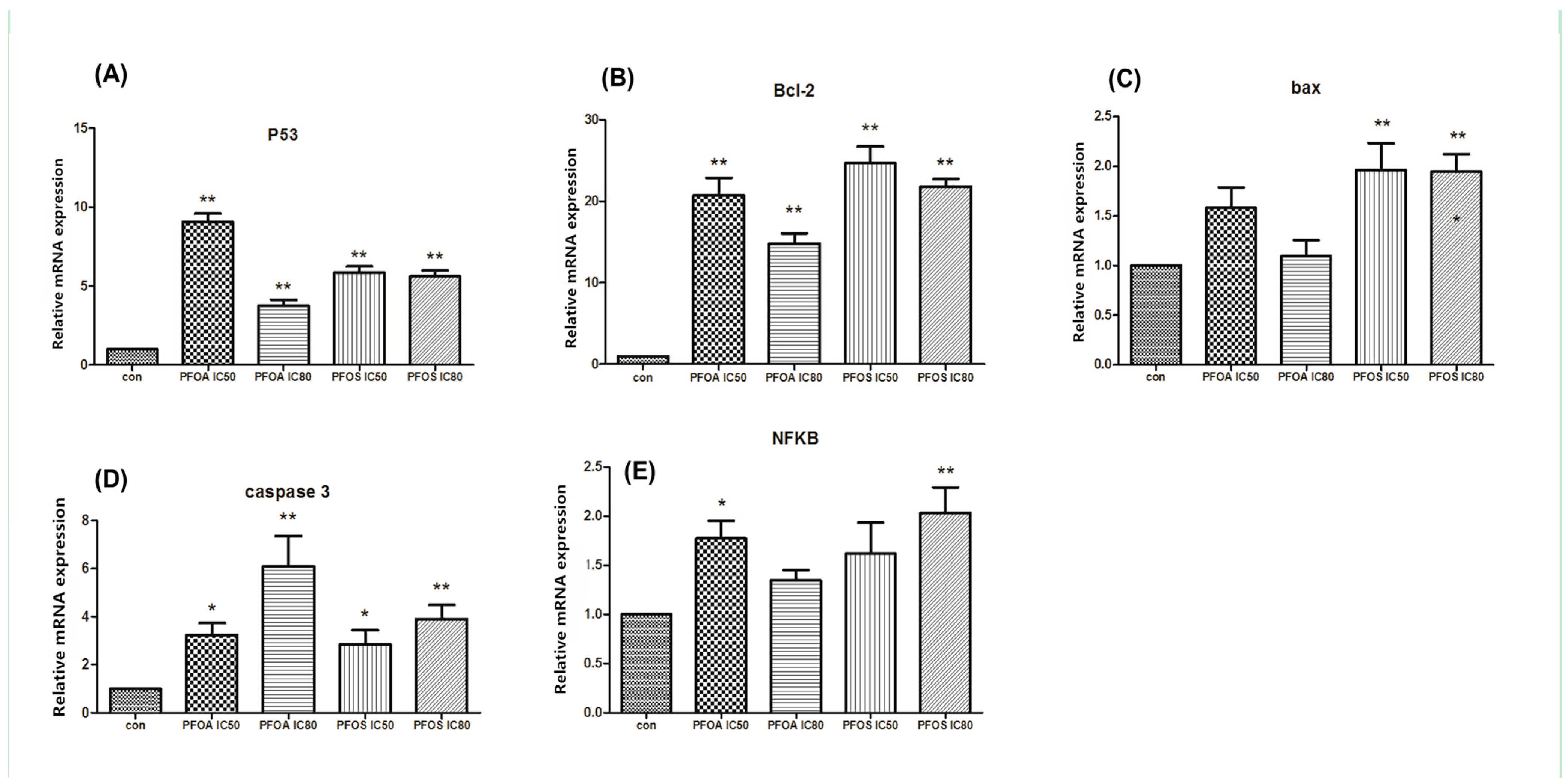
3.3. Cell Apoptosis and Cycle in ZFL Cells
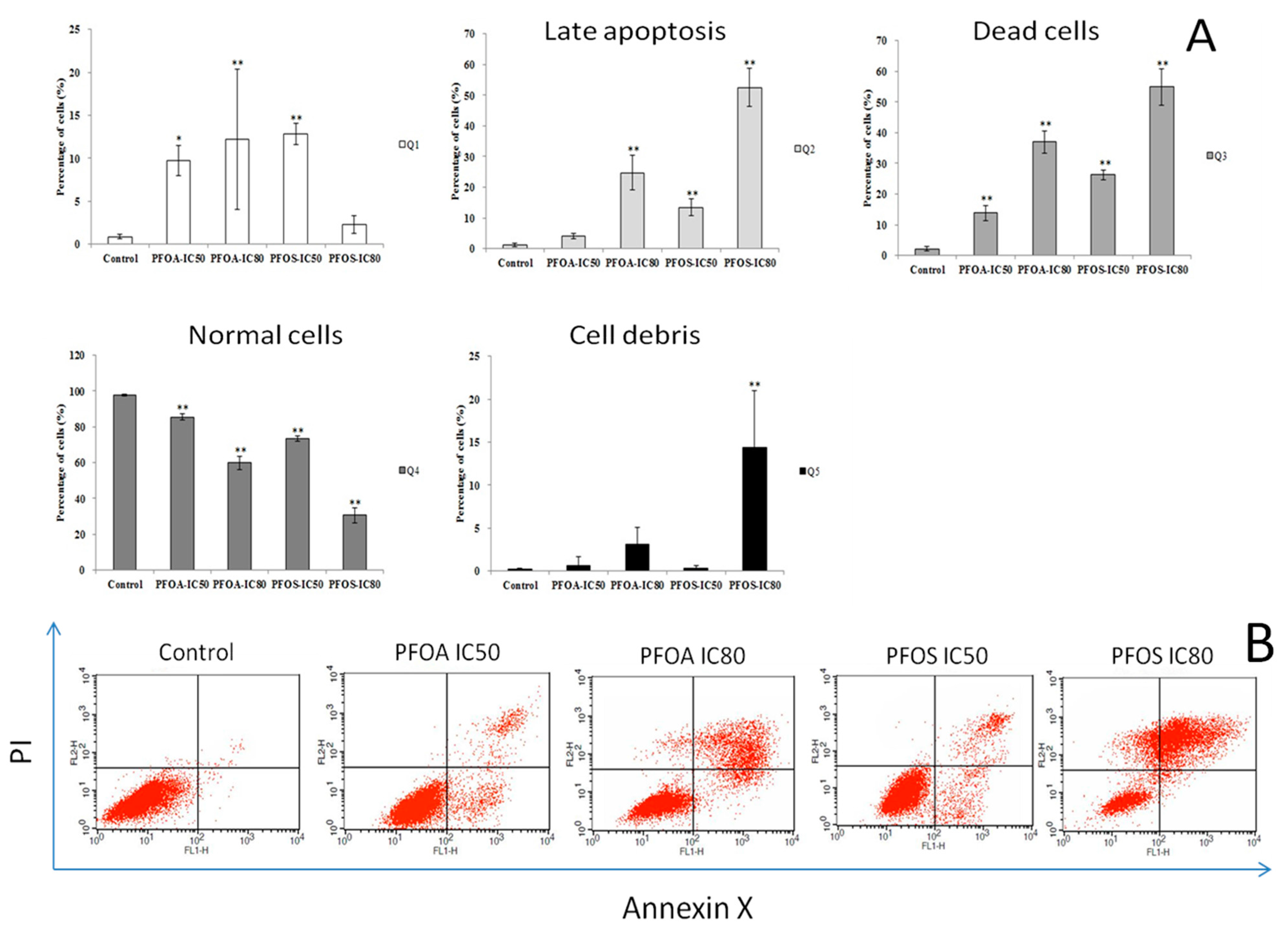

3.4. Protein Expression Levels of p53, Bcl-2, and Active-Caspase 3 in ZFL Cells Induced with PFOA and PFOS
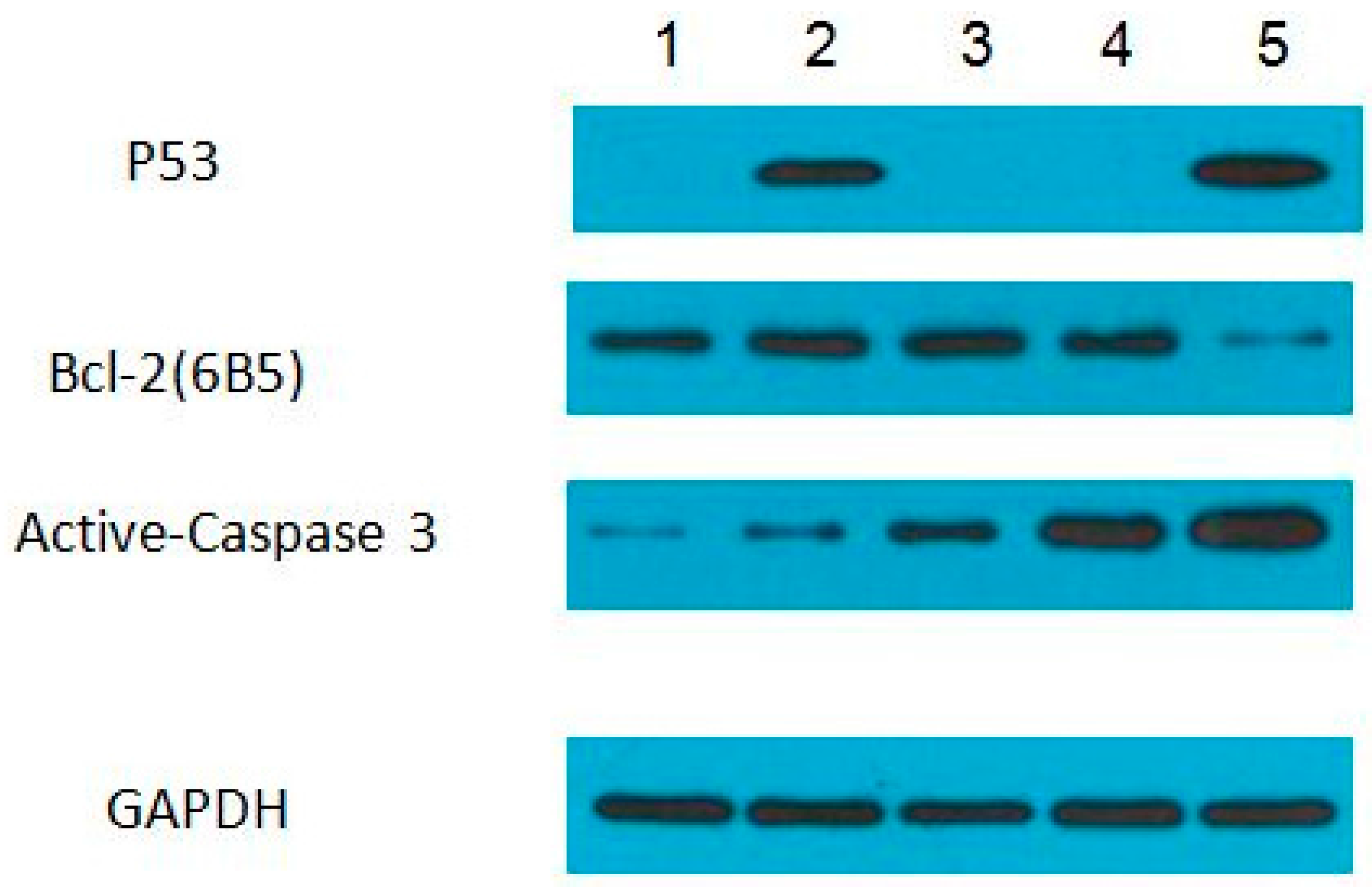
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Ham, T.J.; Mapes, J.; Kokel, D.; Peterson, R.T. Live imaging of apoptotic cells in zebrafish. FASEB J. 2010, 24, 4336–4342. [Google Scholar] [CrossRef] [PubMed]
- Hilger-Eversheim, K.; Moser, M.; Schorle, H.; Buettner, R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 2000, 260, 1–12. [Google Scholar] [CrossRef]
- Bonneau, B.; Nougarède, A.; Prudent, J.; Popgeorgiev, N.; Peyriéras, N.; Rimokh, R.; Gillet, G. The Bcl-2 homolog Nrz inhibits binding of IP3 to its receptor to control calcium signaling during zebrafish epiboly. Sci. Signal. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Coffin, A.B.; Rubel, E.W.; Raible, D.W. Bax, Bcl2, and p53 differentially regulate neomycin-and gentamicin-induced hair cell death in the zebrafish lateral line. J. Assoc. Res. Otolaryngol. 2013, 14, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Liew, H.; Guo, L.; Lane, D. Tumor-specific signaling to p53 is mimicked by Mdm2 inactivation in zebrafish: Insights from mdm2 and mdm4 mutant zebrafish. Oncogene 2015, 34, 5933–5941. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, H.; Ge, S.; Wang, M.; Zhao, H.; Wang, L.; An, L.; Zhang, J.; Zhang, H.; Hu, B. Controlled intracellular self-assembly and disassembly of 19F nanoparticles for MR imaging of caspase 3/7 in zebrafish. ACS Nano 2014, 9, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- De Felip, E.; Abballe, A.; Albano, F.L.; Battista, T.; Carraro, V.; Conversano, M.; Franchini, S.; Giambanco, L.; Iacovella, N.; Ingelido, A.M. Current exposure of Italian women of reproductive age to PFOS and PFOA: A human biomonitoring study. Chemosphere 2015, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van de Vijver, K.I.; Hoff, P.; Das, K.; Brasseur, S.; Van Dongen, W.; Esmans, E.; Reijnders, P.; Blust, R.; De Coen, W. Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea. Environ. Sci. Technol. 2005, 39, 6978–6984. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.W.; So, M.; Jiang, G.; Taniyasu, S.; Yamashita, N.; Song, M.; Wu, Y.; Li, J.; Giesy, J.; Guruge, K. Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ. Sci. Technol. 2006, 40, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Loccisano, A.E.; Longnecker, M.P.; Campbell, J.L., Jr.; Andersen, M.E.; Clewell, H.J., III. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J. Toxicol. Environ. Health Part A 2013, 76, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.; Al-Tonsy, A.H.; Awad, M.E.; Roberfroid, M.; Khan, M. Peroxisomal enzymes and 8-hydroxydeoxyguanosine in rat liver treated with perfluorooctanoic acid. Dis. Mark. 2003, 19, 19–25. [Google Scholar] [CrossRef]
- Kennedy, G.L.; Butenhoff, J.L.; Olsen, G.W.; O’Connor, J.C.; Seacat, A.M.; Perkins, R.G.; Biegel, L.B.; Murphy, S.R.; Farrar, D.G. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 2004, 34, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Directorate, O.E. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts: ENV/JM/RD (2002) 17/FINAL; Organisation for Economic Co-operation and Development: Paris, France, 2002. [Google Scholar]
- Seacat, A.M.; Thomford, P.J.; Hansen, K.J.; Olsen, G.W.; Case, M.T.; Butenhoff, J.L. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 2002, 68, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Guruge, K.S.; Yeung, L.W.; Yamanaka, N.; Miyazaki, S.; Lam, P.K.; Giesy, J.P.; Jones, P.D.; Yamashita, N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol. Sci. 2006, 89, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-Y.; Kim, S.-H.; Shin, H.-I.; Bae, H.I.; Yang, J.-H. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch. Toxicol. 2008, 82, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.G.; Panaretakis, T.; Bergstrand, A.; Depierre, J.W. Effects of the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid, on apoptosis in human hepatoma HepG2 cells. Carcinogenesis 1999, 20, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Panaretakis, T.; Shabalina, I.G.; Grandér, D.; Shoshan, M.C.; DePierre, J.W. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol. Appl. Pharmacol. 2001, 173, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhong, L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat. Res./Gen. Toxicol. Environ. Mutagen. 2005, 587, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, K.; Shi, X.; Wang, J.; Lam, P.K.; Wu, R.S.; Zhou, B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat. Toxicol. 2007, 82, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Kastan, M.B. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes Dev. 1998, 12, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Prives, C. Signaling to p53: Breaking the MDM2-p53 circuit. Cell 1998, 95, 5–8. [Google Scholar] [CrossRef]
- Vousden, K.H. p53: Death star. Cell 2000, 103, 691–694. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Lien, J.-C.; Liu, H.-C.; Hsu, S.-C.; Lin, H.-Y.; Chueh, F.-S.; Ji, B.-C.; Yang, M.-D.; Hsu, W.-H.; Chung, J.-G. Demethoxycurcumin-induced DNA damage decreases DNA repair-associated protein expression levels in NCI-H460 human lung cancer cells. Anticancer Res. 2015, 35, 2691–2698. [Google Scholar] [PubMed]
- Carrillo, A.M.; Hicks, M.; Khabele, D.; Eischen, C.M. Pharmacologically increasing Mdm2 inhibits DNA repair and cooperates with genotoxic agents to kill p53 inactivated ovarian cancer cells. Mol. Cancer Res. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Pieroni, L.; Monteleone, V.; Lucà, R.; Fici, L.; Luca, E.; Urbani, A.; Xiong, S.; Soddu, S.; Masetti, R. MDM4/HIPK2/p53 cytoplasmic assembly uncovers coordinated repression of molecules with anti-apoptotic activity during early DNA damage response. Oncogene 2015. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, B.; Hermant, M.; Castrogiovanni, C.; Staudt, C.; Dumont, P. Resveratrol induces DNA damage in colon cancer cells by poisoning topoisomerase II and activates the ATM kinase to trigger p53-dependent apoptosis. Toxicol. Vitr. 2015, 29, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Prykhozhij, S.V. In the absence of Sonic hedgehog, p53 induces apoptosis and inhibits retinal cell proliferation, cell-cycle exit and differentiation in zebrafish. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zeng, G.; Li, X.; Wu, Z.; Wang, L. Cantharidin inhibits cell proliferation and promotes apoptosis in tongue squamous cell carcinoma through suppression of miR-214 and regulation of p53 and Bcl-2/Bax. Oncol. Rep. 2015, 33, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jiang, Z.; Bi, L.; Yang, Y.; Chen, W. Salvianolic acid A attenuates TNF-α-and d-GalN-induced ER stress-mediated and mitochondrial-dependent apoptosis by modulating Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, S.; Li, N.; Chen, L.; Su, J.; Niu, G.; Zhu, S.; Liang, Y. Sasanquasaponin from Camellia oleifera Abel. induces apoptosis via Bcl-2, Bax and caspase-3 activation in HepG2 cells. Mol. Med. Rep. 2015, 12, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Espino, J.; Bejarano, I.; López, J.; Rodriguez, A.; Pariente, J. Caspase-3 and-9 are activated in human myeloid HL-60 cells by calcium signal. Mol. Cell. Biochem. 2010, 333, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kuranaga, E. Caspase signaling in animal development. Dev. Growth Differ. 2011, 53, 137–148. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Liu, W.; Xie, W.; Yu, W.; Wang, C.; Chen, H. Investigation of the Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) on Apoptosis and Cell Cycle in a Zebrafish (Danio rerio) Liver Cell Line. Int. J. Environ. Res. Public Health 2015, 12, 15673-15682. https://doi.org/10.3390/ijerph121215012
Cui Y, Liu W, Xie W, Yu W, Wang C, Chen H. Investigation of the Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) on Apoptosis and Cell Cycle in a Zebrafish (Danio rerio) Liver Cell Line. International Journal of Environmental Research and Public Health. 2015; 12(12):15673-15682. https://doi.org/10.3390/ijerph121215012
Chicago/Turabian StyleCui, Yuan, Wei Liu, Wenping Xie, Wenlian Yu, Cheng Wang, and Huiming Chen. 2015. "Investigation of the Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) on Apoptosis and Cell Cycle in a Zebrafish (Danio rerio) Liver Cell Line" International Journal of Environmental Research and Public Health 12, no. 12: 15673-15682. https://doi.org/10.3390/ijerph121215012





