Assessment of Malawian Mothers’ Malaria Knowledge, Healthcare Preferences and Timeliness of Seeking Fever Treatments for Children Under Five
Abstract
:1. Introduction
2. Materials and Methods
The Data and Sampling Methods
3. Analytical Models
3.1. Multinomial Logit Modeling of Healthcare Preferences
3.2. Timeliness of Treatment Modeling With Poisson Regression
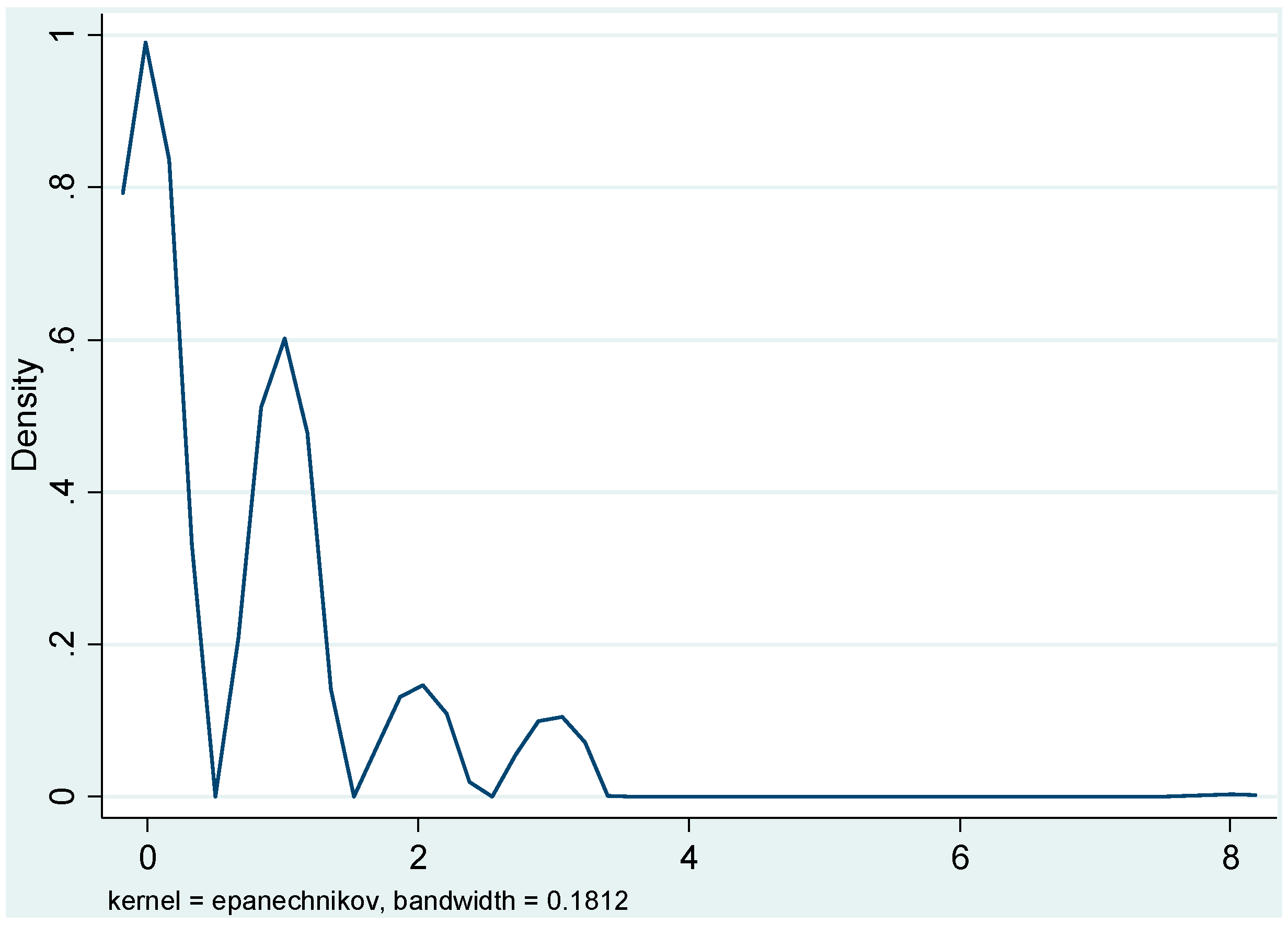
4. Results
4.1. Socio-Economic Profiles, Malaria Knowledge and Fever Treatment Preferences
| Variables | Mean | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Number of under five children | 1.63 | 0.7063 | 0 | 4 |
| Sex of child | 0.49 | 0.4998 | 0 | 1 |
| Child's age in months | 28.15 | 15.5719 | 0 | 59 |
| Age of household head | 36.26 | 15.0545 | 16 | 98 |
| Urban residence | 0.11 | 0.3074 | 0 | 1 |
| North Region | 0.11 | 0.3098 | 0 | 1 |
| Central Region | 0.44 | 0.4968 | 0 | 1 |
| South Region | 0.45 | 0.4974 | 0 | 1 |
| Highest mothers’ years education | 3.52 | 2.7047 | 0 | 8 |
| Mothers’ age | 27.97 | 6.5819 | 15 | 48 |
| Male household headship | 0.79 | 0.4071 | 0 | 1 |
| Variables | Percentage |
|---|---|
| Knowledge of Malaria Danger Signals | |
| Danger signs of malaria: Seizure/convulsions | 44.52 |
| Danger signs of malaria: Fainting | 17.37 |
| Danger signs of malaria: Any fever | 7.41 |
| Danger signs of malaria: High fever | 22.44 |
| Danger signs of malaria: Stiff neck | 3.63 |
| Danger signs of malaria: Feeling weak | 11.38 |
| Danger signs of malaria: Not active | 0.78 |
| Danger signs of malaria: Chills/shivering | 7.90 |
| Danger signs of malaria: Unable to eat | 2.39 |
| Danger signs of malaria: Vomiting | 9.94 |
| Danger signs of malaria: Crying all the time | 1.40 |
| Danger signs of malaria: Restless | 2.36 |
| Danger signs of malaria: Diarrhea | 4.29 |
| Danger signs of malaria: Other | 4.92 |
| Danger signs of malaria: Don’t know | 11.79 |
| Knowledge of Malaria Symptoms | |
| Knows main malaria symptoms: fever | 84.98 |
| Knows main malaria symptoms: chills | 44.17 |
| Knows main malaria symptoms: headache | 17.22 |
| Knows main malaria symptoms: Nausea/vomiting | 40.73 |
| Knows main malaria symptoms: Diarrhea | 13.29 |
| Knows main malaria symptoms: Dizziness | 1.85 |
| Knows main malaria symptoms: Loss of appetite | 3.90 |
| Knows main malaria symptoms: Body ache or joint pain | 23.78 |
| Knows main malaria symptoms: Pale eyes | 2.80 |
| Knows main malaria symptoms: Feeling weak | 6.35 |
| Knows main malaria symptoms: other | 2.42 |
| Variables | Frequency | Percentage |
|---|---|---|
| Days ago had malaria | ||
| 0 | 9 | 1.41 |
| 1 | 33 | 4.64 |
| 2 | 105 | 14.02 |
| 3 | 143 | 20.20 |
| 4 | 52 | 7.66 |
| 5 | 48 | 8.02 |
| 6 | 26 | 3.98 |
| 7 | 152 | 23.31 |
| 8 | 25 | 3.85 |
| 9 | 13 | 2.25 |
| 10 | 32 | 5.30 |
| 11 | 1 | 0.23 |
| 12 | 9 | 1.37 |
| 13 | 6 | 0.68 |
| 14 | 22 | 3.07 |
| Days after fever sought treatments | ||
| 0 | 353 | 54.42 |
| 1 | 220 | 32.43 |
| 2 | 54 | 6.81 |
| 3 | 40 | 6.16 |
| 8 | 1 | 0.18 |
| Healthcare Preferences | ||
| Public hospital/health centers | 317 | 46.89 |
| Private hospital/health centers | 31 | 4.59 |
| Other sources | 328 | 48.52 |
| Variables | Percentage |
|---|---|
| Sources of Malaria Treatment Fees | |
| Regular Income | 9.70 |
| Occasional income | 4.79 |
| Borrowed income | 1.04 |
| Sale of assets | 4.21 |
| Other incomes | 1.40 |
4.2. Factors Influencing Health Care Preferences
| Variables | Public Health Centers | Private Health Centers | Tolerance | ||||
|---|---|---|---|---|---|---|---|
| Coefficient | RRR | z-stat | Coefficient | RRR | z-stat | ||
| Demographic variables | |||||||
| Age of household head | 0.0160 | 1.0161 | 2.35 | 0.0573 | 1.0589 | 2.97 | 0.9708 |
| Type of place of residence | 0.6532 | 1.9217 | 2.33 | 2.2204 | 9.2108 | 3.75 | 0.8444 |
| Northern region | −0.7258 | 0.4839 | −2.35 | −29.3764 | 0.0000 | −24.12 | 0.7659 |
| Central region | −0.5102 | 0.6004 | −2.27 | −0.5992 | 0.5492 | −0.81 | 0.7014 |
| Highest year of education | 0.0633 | 1.0654 | 1.78 | 0.2400 | 1.2712 | 2.02 | 0.9252 |
| Number of days taken off | 0.3841 | 1.4683 | 2.42 | 0.3112 | 1.3651 | 0.86 | 0.8089 |
| Danger signals of malaria | |||||||
| Danger signs of malaria: High fever | 0.2237 | 1.2507 | 0.95 | −0.3675 | 0.6925 | −0.66 | 0.8811 |
| Danger signs of malaria: Restless | −1.0087 | 0.3647 | −1.58 | 0.6106 | 1.8416 | 0.58 | 0.9211 |
| Danger signs of malaria: Fainting | 0.2090 | 1.2324 | 0.80 | 0.3693 | 1.4468 | 0.53 | 0.7909 |
| Danger signs of malaria: Any fever | −0.0457 | 0.9553 | −0.14 | −0.4657 | 0.6277 | −0.53 | 0.9111 |
| Danger signs of malaria: Stiff neck | −1.1541 | 0.3153 | −2.08 | −1.0175 | 0.3615 | −0.93 | 0.9471 |
| Danger signs of malaria: Chills/shivering | 0.4260 | 1.5312 | 1.02 | 1.5705 | 4.8091 | 1.87 | 0.9033 |
| Danger signs of malaria: Unable to eat | 0.4013 | 1.4938 | 0.76 | −1.7407 | 0.1754 | −1.60 | 0.9062 |
| Danger signs of malaria: Diarrhea | 0.3035 | 1.3546 | 0.62 | 0.1678 | 1.1827 | 0.22 | 0.9165 |
| Knows main malaria symptoms: Diarrhea | 0.0836 | 1.0872 | 0.30 | 2.0943 | 8.1200 | 2.66 | 0.9002 |
| Knows main malaria symptoms: chills | −0.3434 | 0.7094 | −1.67 | −0.5909 | 0.5538 | −0.93 | 0.8442 |
| Knows main malaria symptoms: headache | 0.6356 | 1.8881 | 2.43 | −0.3394 | 0.7122 | −0.46 | 0.8563 |
| Knows main malaria symptoms: Dizziness | 0.7073 | 2.0286 | 1.02 | −26.2484 | 0.0000 | −23.10 | 0.9235 |
| Knows main malaria symptoms: Body ache or joint pain | 0.3103 | 1.3638 | 1.31 | −0.0231 | 0.9772 | −0.03 | 0.9067 |
| Source of payment for treatment (Income) | 1.3009 | 3.6726 | 3.56 | 4.1525 | 63.5925 | 5.65 | 0.8444 |
| Source of payment for treatment (Occasional income) | 0.0797 | 1.0830 | 0.17 | 3.7961 | 44.5260 | 4.11 | 0.9165 |
| Source of payment for treatment (Borrowed) | 2.5953 | 13.4006 | 2.24 | 2.8936 | 18.0573 | 1.80 | 0.9408 |
| Source of payment for treatment (Sale of assets) | −1.7047 | 0.1818 | −1.60 | 1.8172 | 6.1543 | 1.35 | 0.8561 |
| Source of payment for treatment (Other) | −1.8972 | 0.1500 | −2.76 | 2.2981 | 9.9551 | 1.91 | 0.9708 |
| Constant | −0.8924 | 0.3346 | −2.67 | −7.9550 | 0.0004 | −5.54 | |
| Log likelihood | −239.8497 | ||||||
| Number of observations | 667 | ||||||
| Wald Chi Square (50) | 4969.90 *** | ||||||
| Pseudo R square | 0.1719 | ||||||
| Knows main malaria symptoms: Diarrhea | 0.0836 | 1.0872 | 0.30 | 2.0943 | 8.1200 | 2.66 | 0.9002 |
| Knows main malaria symptoms: chills | −0.3434 | 0.7094 | −1.67 | −0.5909 | 0.5538 | −0.93 | 0.8442 |
| Knows main malaria symptoms: headache | 0.6356 | 1.8881 | 2.43 | −0.3394 | 0.7122 | −0.46 | 0.8563 |
4.3. Factors Influencing Timeliness of Fever Treatment
| Variables | Coefficients | Robust Std. Error | Z Statistics | Tolerance |
|---|---|---|---|---|
| Demographic factors | ||||
| Age of household head | 0.0118 | 0.0029 | 4.04 | 0.9708 |
| Type of place of residence | 0.1334 | 0.1215 | 1.10 | 0.8444 |
| Northern region | −0.3975 | 0.1689 | −2.35 | 0.7659 |
| Central region | −0.3378 | 0.1198 | −2.82 | 0.7014 |
| Highest year of education | 0.0304 | 0.0199 | 1.53 | 0.9252 |
| Number of days taken off | 0.1358 | 0.0610 | 2.23 | 0.8089 |
| Danger signals of malaria | ||||
| Danger signs of malaria: High fever | 0.1359 | 0.1267 | 1.07 | 0.8811 |
| Danger signs of malaria: Restless | −0.6570 | 0.4253 | −1.54 | 0.9211 |
| Danger signs of malaria: Fainting | −0.2343 | 0.1469 | −1.59 | 0.7909 |
| Danger signs of malaria: Any fever | −0.7472 | 0.2084 | −3.59 | 0.9111 |
| Danger signs of malaria: Stiff neck | −0.4624 | 0.4182 | −1.11 | 0.9471 |
| Danger signs of malaria: Chills/shivering | 0.2162 | 0.1853 | 1.17 | 0.9033 |
| Danger signs of malaria: Unable to eat | −0.3452 | 0.3243 | −1.06 | 0.9062 |
| Danger signs of malaria: Diarrhea | −0.3547 | 0.2391 | −1.48 | 0.9165 |
| Knowledge of malaria symptoms | ||||
| Knows main malaria symptoms: Refuse to eat or drink | −0.9128 | 0.6461 | −1.41 | 0.8902 |
| Knows main malaria symptoms: Diarrhea | 0.1395 | 0.1478 | 0.94 | 0.9002 |
| Knows main malaria symptoms: chills | −0.1443 | 0.1113 | −1.30 | 0.8442 |
| Knows main malaria symptoms: headache | 0.1969 | 0.1380 | 1.43 | 0.8563 |
| Knows main malaria symptoms: Dizziness | 0.4323 | 0.2609 | 1.66 | 0.9235 |
| Knows main malaria symptoms: Body ache or joint pain | 0.1287 | 0.1248 | 1.03 | 0.9067 |
| Source of payment for treatment (Income) | 0.8694 | 0.1336 | 6.51 | 0.8444 |
| Source of payment for treatment (Occasional income) | 0.8170 | 0.2494 | 3.28 | 0.9165 |
| Source of payment for treatment (Borrowed) | 0.3054 | 0.4395 | 0.69 | 0.9408 |
| Source of payment for treatment (Sale of assets) | 0.9908 | 0.2335 | 4.24 | 0.8561 |
| Source of payment for treatment (Other) | 0.9650 | 0.3176 | 3.04 | 0.9708 |
| Constant | −1.0611 | 0.1694 | −6.26 | |
| lnalpha | −3.4010 | 2.7538 | ||
| Alpha | 0.0333 | 0.0918 | ||
| Number of obs = 667 | ||||
| Log pseudo likelihood = −7.488e + 08 | ||||
| Wald Chi Square (25) = 190.96 *** | ||||
5. Discussions
6. Conclusions
Acknowledgments
Conflicts of interest
References
- World Health Organization. World Malaria Report 2013; World Health Organization (WHO): Geneva, Switzerland, 2013. [Google Scholar]
- Kassile, T. Prevention and management of malaria in under-five children in Tanzania: A review. Tanzania J. Health Res. 2012, 14. [Google Scholar] [CrossRef]
- Bryce, J.; Boschi-Pinto, C.; Shibuya, K.; Black, R.E. WHO estimates of the causes of death in children. Lancet 2005, 365, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Cairns, M.; Roca-Feltrer, A.; Garske, T.; Wilson, A.L.; Diallo, D.; Milligan, P.J.; Ghani, A.C.; Greenwood, B.M. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nature Communication 2012, 3. [Google Scholar] [CrossRef]
- Depetris-Chauvin, E.; Weil, D.N. Malaria and Early African Development: Evidence from the Sickle cell Trait; Working Paper No. 19603; The National Bureau of Economic Research (NBER): Cambridge, MA, USA, 2013. [Google Scholar]
- Rowe, A.K.; Steketee, R.W. Predictions of the impact of malaria control efforts on all-cause child mortality in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2007, 77, 48–55. [Google Scholar] [PubMed]
- Okiro, E.A.; Kazembe, L.N.; Kabaria, C.W.; Ligomeka, J.; Noor, A.M.; Ali, D.; Snow, R.W. Childhood malaria admission rates to four hospitals in malawi between 2000 and 2010. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Kazembe, L.; Mathanga, D.P.; Kinyoki, D.; Ali, D.; Snow, R.W.; Noor, A.M. Mapping malaria transmission intensity in Malawi, 2000–2010. Am. J. Trop. Med. Hyg. 2013, 89, 840–849. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2010. Available online: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf (accessed on 29 December 2014).
- Centers for Disease Control and Prevention, Impact of Malaria. Available online: http://www.cdc.gov/malaria/malaria_worldwide/impact.html (accessed on 2 May 2014).
- Bloland, P.B. Drug Resistance in Malaria; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Nosten, F.; Brasseur, P. Combination therapy for malaria: the way forward. Drugs 2002, 62, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.I.; Watkins, W.M.; White, N.J. Antimalarial dosing regimens and drug resistance. Trends Parasitol. 2008, 24, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mace, K.E.; Mwandama, D.; Jafali, J.; Luka, M.; Filler, S.J.; Sande, J.; Ali, D.; Kachur, S.P.; Mathanga, D.P.; Skarbinski, J. Adherence to treatment with artemether-lumefantrine for uncomplicated malaria in rural Malawi. Clin. Infect. Dis. 2011, 53, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Malenga, G.; Wirima, J.; Kazembe, P.; Nyasulu, Y.; Mbvundula, M.; Nyirenda, C.; Sungani, F.; Campbell, C.; Molyneux, M.; Bronzan, R.; et al. Developing national treatment policy for falciparum malaria in Africa: Malawi experience. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Bradley, A.K.; Greenwood, A.M.; Snow, R.W.; Byass, P.; Hayes, R.J.; N’Jie, A.B. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia. Lancet 1988, 1, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.C.; Kaseje, D.C.O.; Mosley, W.H.; Sempebwa, E.K.N.; Huong, A.Y.; Roberts, J.M. Impact on mortality and fertility of a community-based malaria control programmes in Saradidi. Kenya. Ann. Trop. Med. Parasitol. 1987, 81, 36–45. [Google Scholar] [PubMed]
- Plowe, C.V.; Cortese, J.F.; Djimde, A.; Nwanyanwu, O.C.; Watkins, W.M.; Winstanley, P.A.; Estrade-Franco, J.G.; Mollinedo, R.E.; Avila, J.C.; Cespedes, J.L.; et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 1997, 176, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, A. Patients and Healers in the Context of Culture; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
- Cunningham-Burley, S. Mothers’ beliefs about and perceptions of their children’s illness. In Readings in Medical Sociology; Cunningham-Burley, S., McKeganey, N., Eds.; Routledge: London, UK, 1990. [Google Scholar]
- Lars, O.; Beth, E. Malaria in the United Republic of Tanzania: Cultural considerations and health seeking behaviour. Bull. World Health Organ. 2000, 78, 1352–1357. [Google Scholar] [PubMed]
- Pagnoni, F.; Convelbo, N.; Tiendrebeogo, J.; Cousens, S.; Esposito, F. A community-based programme to provide prompt and adequate treatment of presumptive malaria in children. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Fosu, G. Childhood morbidity and health services utilization: Cross-national comparisons of user-related factors from DHS data. Soc. Sci. Med. 1994, 38, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, C.P. Human ecology and behaviour in malaria control in tropical Africa. Bull. World Health Organ. 1984, 62, 81–87. [Google Scholar] [PubMed]
- Kazembe, L.N.; Appleton, C.C.; Kleinschmidt, I. Choice of treatment for fever at household level in Malawi: Examining spatial patterns. Malar. J. 2007, 6. [Google Scholar] [CrossRef]
- Wirima, J.J. A nation-wide malaria knowledge, attitudes and practices survey in Malawi. Trop. Med. Parasitol. 1996, 45, 52–53. [Google Scholar]
- Government of Malawi: Malaria Strategic Plan 2005–2010 Scaling up Malaria Control Intervention Government of Malawi, 2010. Available online: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Malawi/malawi_malaria_strategic_plan_2005_-_2010_.pdf (assessed on 25 November 2014).
- Holtz, T.H.; Kachur, S.P.; Marum, L.H.; Mkandala, C.; Chizani, N.; Roberts, J.M.; Macheso, A.; Parise, M.E. Care seeking behaviour and treatment of febrile illness in children aged less than five years: A household survey in Blantyre District, Malawi. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Oreagba, A.I.; Onajole, A.T.; Olayemi, S.O.; Mabadeje, A.F.B. Knowledge of malaria amongst caregivers of young children in rural and urban communities in Southwest Nigeria. Trop. J. Pharm. Res. 2004, 3, 299–304. [Google Scholar]
- Nsimba, S.E.; Warsame, M.; Thompson, G.; Masselle, A.; Mbatiga, Z.A. A household’s survey of source, availability and use of antimalarials in a rural area in Tanzania. Drug Int. J. 1999, 33, 1025–1032. [Google Scholar]
- Deming, M.S.; Gayibor, A.; Murphy, K.; Jones, T.S.; Karsa, T. Home treatment of febrile children with antimalarial drugs in Togo. Bull. World Health Organ. 1989, 67, 695–700. [Google Scholar] [PubMed]
- Hamel, M.J.; Odhacha, A.; Roberts, J.M.; Deming, M.S. Malaria control in Bungoma district, Kenya: A survey of home treatment of children with fever, bednet use and attendance at antenatal clinics. Bull. World Health Organ. 2001, 79, 1005–1092. [Google Scholar] [PubMed]
- Fawole, O.I.; Onadeko, M.O. Knowledge and home management of malaria by mothers and caregivers of under five children. West Afr. J. Med. 2001, 20, 152–157. [Google Scholar] [PubMed]
- Massele, Y.A.; Sayi, J.; Nsimba, D.E.S.; Ofori-Adjei, D.; Laing, O.R. Knowledge and management of malaria in Dar es Salaam, Tanzania. East Afr. Med. J. 1993, 70, 639–641. [Google Scholar] [PubMed]
- Ajibade, B.L.; Alao, M.T. Mothers’ action and preferences of treatment of febrile illnesses among under-five-year-old children in Osun State. Biol. Agric. Healthc. 2013, 3, 148–155. [Google Scholar]
- Malawi Malaria Indicator Survey (MIS) 2012. Available online: http://dhsprogram.com/pubs/pdf/MIS13/MIS13.pdf (assessed on 24 November 2014).
- Greene, W.H. Econometric Analysis, 5th ed.; Prentice Hall Press: New Jersey USA, 2002; p. 802. [Google Scholar]
- Ali, S.; Tahir, C.; Qurat-ul-ain, N. Effect of maternal literacy on child health: Myth or reality. Ann. PIMS-Pak. Inst. Med. Sci. 2011, 7, 100–103. [Google Scholar]
- Kabubo-Mariara, J.; Ndenge, G.K.; Mwabu, D.K. Determinants of children’s nutritional status in Kenya: Evidence from demographic and health surveys. J. Afr. Econ. 2009, 18, 363–387. [Google Scholar] [CrossRef]
- Makoka, D. The Impact of Maternal Education on Child Nutrition: Evidence from Malawi, Tanzania, and Zimbabwe; DHS Working Paper No 84; ICF Macro: Calverton, MD, USA, 2013. [Google Scholar]
- Mosley, W.H.; Chen, L.C. An analytical framework for the study of child survival in developing countries. Popul. Dev. Rev. 1984, 10, 25–45. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Media Centre. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 15 December 2014).
- Sharma, A.K.; Bhasin, S.; Chaturvedi, S. Predictors of knowledge about malaria in India. J. VectorBorne Dis. 2007, 44, 189–197. [Google Scholar]
- Osero, J.S.; Otieno, M.F.; Orago, A.S. Mothers’ knowledge on malaria and vector management strategies in Nyamira District, Kenya. East Afr. Med. J. 2006, 83, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Fatungase, K.O.; Amoran, O.E.; Alausa, K.O. The effect of health education intervention on the home management of malaria amongthe caregivers of children aged under 5 years in Ogun State, Nigeria. Eur. J. Med. Res. 2012, 17. [Google Scholar] [CrossRef]
- Ettling, M.; Steketee, R.W.; Macheso, A.; Schultz, L.J.; Nyasulu, Y.; Chitsulo, L. Malaria knowledge, attitudes and practices in Malawi: Survey population characteristics. Trop. Med. Parasitol. 1994, 45, 57–60. [Google Scholar] [PubMed]
- Townes, L.R.; Mwandama, D.; Mathanga, D.P.; Wilson, M.L. Elevated dry-season malaria prevalence associated with fine-scale spatial patterns of environmental risk: A case–control study of children in rural Malawi. Malar. J. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Ustrup, M.; Ngira, B.; Stockman, L.J.; Deming, M.; Nyasulu, P.; Bowie, C.; Msyamboza, K.; Meyrowitsch, D.W.; Cunliffe, N.A.; Bresee, J.; et al. Potential barriers to healthcare in Malawi for under-five children with cough and fever: A national household survey. J. Health Popul. Nutr. Malar. 2014, 32, 68–78. [Google Scholar]
- Presumptive Malaria Treatment in Schools: Successes and Lessons Learned from Mangochi District, Malawi, September 2008. Available online: www.schoolsandhealth.org/Shared%20Documents/Downloads/Presumptive%20Malaria%20Treatment%20in%20Schools-Lessons%20learned%20from%20Malawi.pdf (accessed on 30 December 2014).
- Abdulraheem, I.S. Health needs assessment and determinants of health-seeking behaviour among elderly Nigerians: A house-hold survey. Ann. Afr. Med. 2007, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Iloh, G.U.P.; Amadi, A.N.; Nwankwo, B.O.; Ugwu, V.C. Common under-five morbidity in South-Eastern Nigeria: A study of its pattern in a rural mission general hospital in Imo state. Niger J. Med. 2011, 20, 99–104. [Google Scholar] [PubMed]
- Salako, I.A. National malaria day, malaria awareness walk. Text of an address given on the occasion of the malaria awareness walk held in Abuja. J. Malar. Afr. Trop. 2002, 1, 6–7. [Google Scholar] [CrossRef]
- Fotso, J.C.; Kuate-Defo, B. Measuring socioeconomic status in health research indeveloping countries: Should we be focusing on household, communities or both? Soc. Indic. Res. 2005, 72, 189–237. [Google Scholar] [CrossRef]
- Fotso, J.C.; Kuate-Defo, B. Socioeconomic inequalities in early childhood malnutritionand morbidity: Modification of the household-level effects by the community socioeconomicstatus. Health Place 2005, 11, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Ruel, M.T.; Ndiaye, A. Why is child malnutrition lower in urban than rural areas? Evidence from 36 developing countries. World Dev. 2005, 33, 1285–1305. [Google Scholar] [CrossRef]
- Garrett, J.L.; Ruel, M.T. Are determinants of rural and urban food security and nutritionalstatus different? Some insights from Mozambique. World Dev. 1999, 27, 1955–1975. [Google Scholar] [CrossRef]
- Sastry, N. What explains rural-urban differentials in child mortality in Brazil? Sci. Med. 1997, 44, 989–1002. [Google Scholar] [CrossRef]
- Lalou, R.; Legrand, T.K. Child mortality in the urban and rural Sahel. Population 1997, 9, 147–168. [Google Scholar]
- Kuate-Defo, B. Areal and socioeconomic differentials in infant and child mortality in Cameroon. Soc. Sci. Med. 1996, 42, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Welunmor, J.; Idris, O.; Adelakun, A.; Airhihenbuwa, C.O. Child malaria treatment decisions by mothers of children less than five years of age attending an outpatient clinic in south-west Nigeria: An application of the PEN-3 cultural model. Malar. J. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Sule, S.S. Childhood morbidity and treatment pattern at the multipurpose health centre, Ilesa, Nigeria. Niger J. Med. 2003, 12, 145–149. [Google Scholar] [PubMed]
- Feyisetan, B.J.; Asa, S.; Ebigbola, J.A. Mothers’management of childhood diseases in Yorubaland: The influence of cultural beliefs. Health Trans. Rev. 1997, 7, 221–234. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyekale, A.S. Assessment of Malawian Mothers’ Malaria Knowledge, Healthcare Preferences and Timeliness of Seeking Fever Treatments for Children Under Five. Int. J. Environ. Res. Public Health 2015, 12, 521-540. https://doi.org/10.3390/ijerph120100521
Oyekale AS. Assessment of Malawian Mothers’ Malaria Knowledge, Healthcare Preferences and Timeliness of Seeking Fever Treatments for Children Under Five. International Journal of Environmental Research and Public Health. 2015; 12(1):521-540. https://doi.org/10.3390/ijerph120100521
Chicago/Turabian StyleOyekale, Abayomi Samuel. 2015. "Assessment of Malawian Mothers’ Malaria Knowledge, Healthcare Preferences and Timeliness of Seeking Fever Treatments for Children Under Five" International Journal of Environmental Research and Public Health 12, no. 1: 521-540. https://doi.org/10.3390/ijerph120100521
APA StyleOyekale, A. S. (2015). Assessment of Malawian Mothers’ Malaria Knowledge, Healthcare Preferences and Timeliness of Seeking Fever Treatments for Children Under Five. International Journal of Environmental Research and Public Health, 12(1), 521-540. https://doi.org/10.3390/ijerph120100521





