Using Benthic Macroinvertebrate and Fish Communities as Bioindicators of the Tanshui River Basin Around the Greater Taipei Area — Multivariate Analysis of Spatial Variation Related to Levels of Water Pollution
Abstract
:1. Introduction
2. Experimental Section
2.1. Basic Environmental Variables
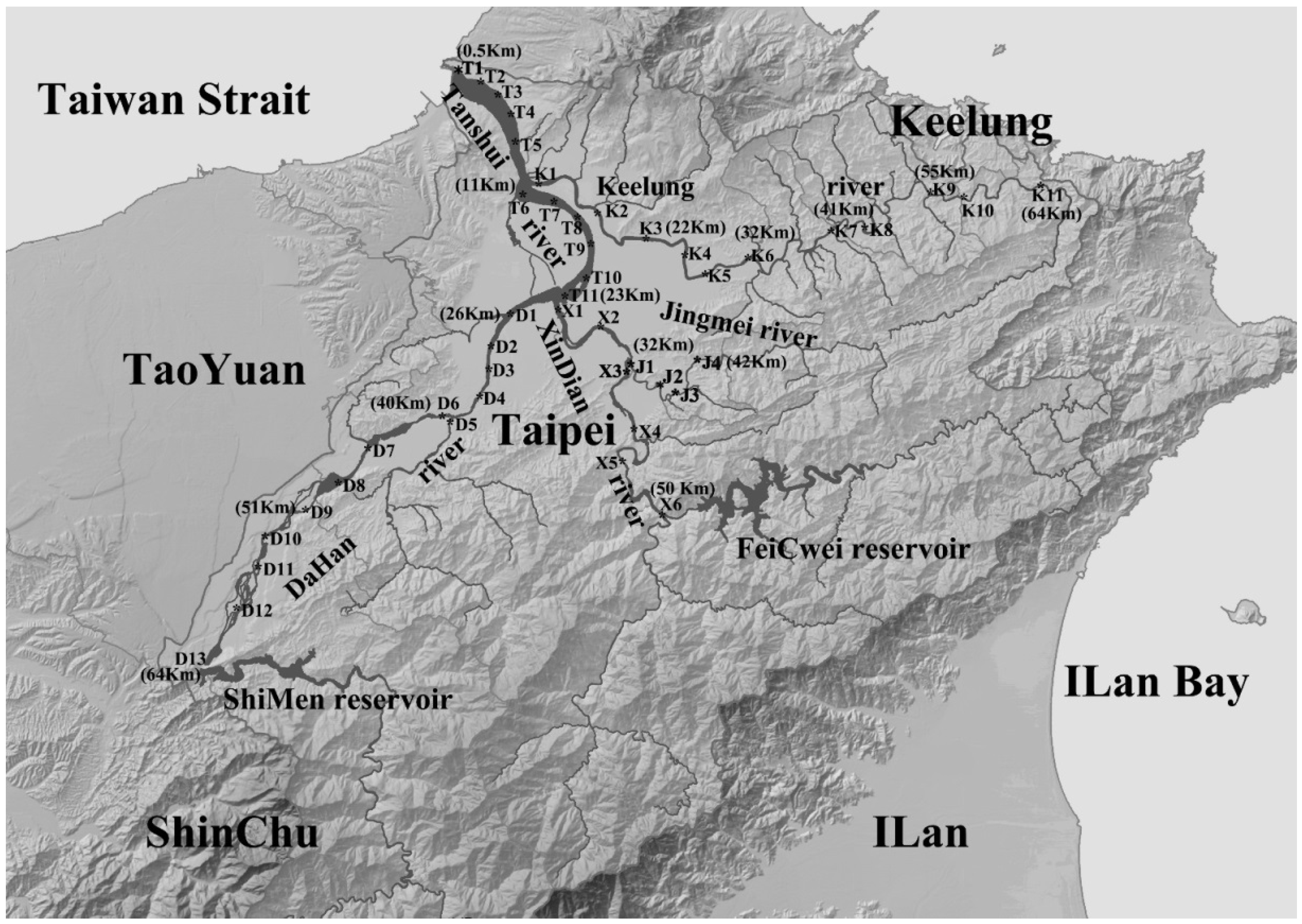
2.2. Biological Sampling
2.3. Community Structure Analysis and Biological Indexation
3. Results and Discussion
3.1. River Pollution Index
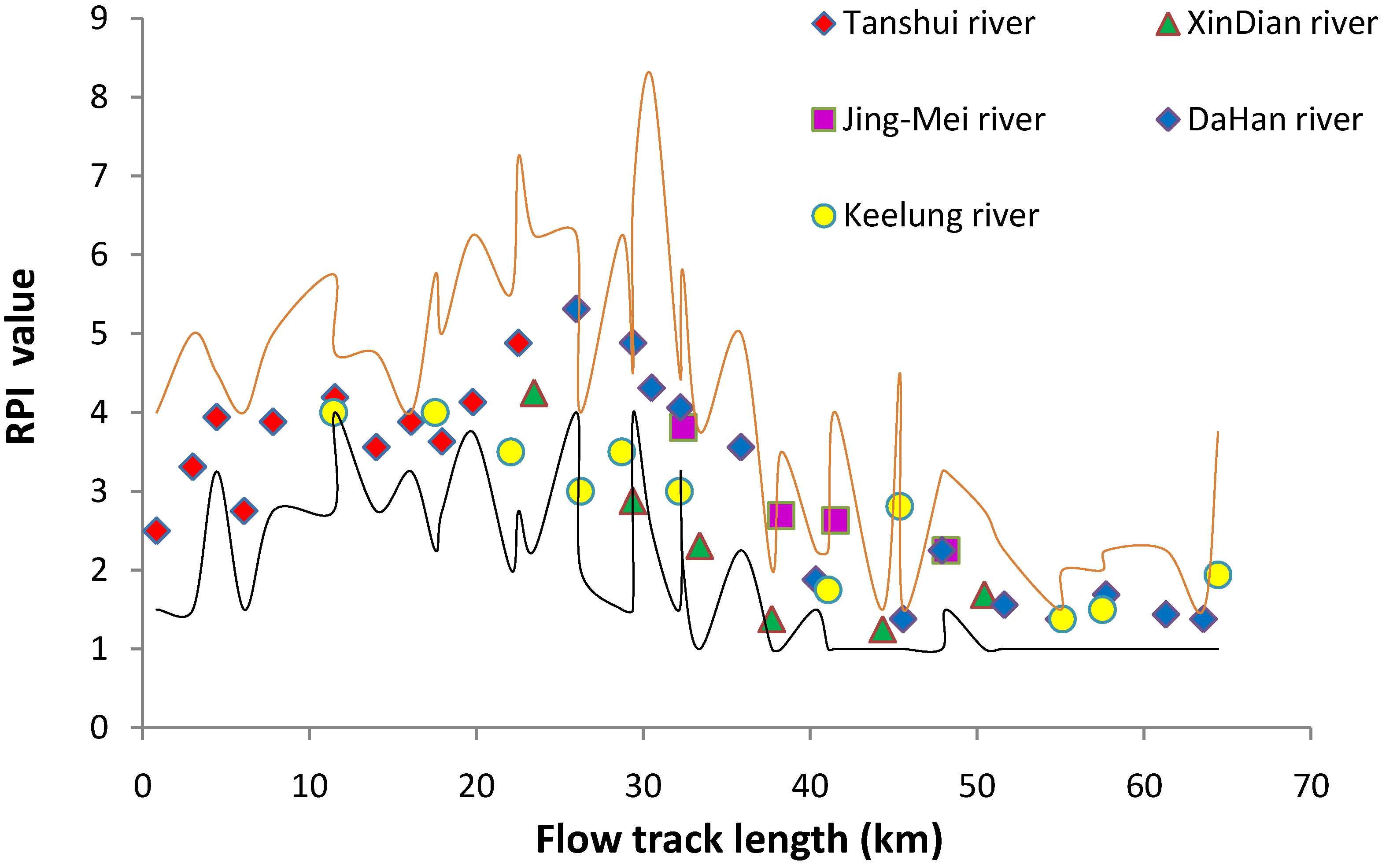
3.2. Species Richness and Distribution Pattern
3.3. Community Structure and Environmental Factor Correlations
| Axes | 1 | 2 | 3 | 4 | Total inertia |
|---|---|---|---|---|---|
| Eigenvalues | 0.719 | 0.367 | 0.212 | 0.182 | 10.375 |
| Taxa-environment correlations (r) | 0.887 | 0.682 | 0.739 | 0.592 | -- |
| Cumulative percentage variance | -- | -- | -- | -- | -- |
| Taxa | 6.9% | 10.5% | 12.5% | 14.3% | -- |
| Taxa-environment relation | 42.5% | 64.2% | 76.8% | 87.5% | -- |
| Sum of all eigenvalues | -- | -- | -- | -- | 10.375 |
| Sum of all canonical eigenvalues | -- | -- | -- | -- | 1.69 |

| Axes | 1 | 2 | 3 | 4 | Total Inertia |
|---|---|---|---|---|---|
| Upstream area without tidal effect | |||||
| Eigenvalues | 0.139 | 0.115 | 0.057 | 0.039 | 3.33 |
| Species-environment correlations | 0.661 | 0.63 | 0.622 | 0.578 | -- |
| Cumulative percentage variance | -- | -- | -- | -- | -- |
| Species | 4.2% | 7.6% | 9.3% | 10.5% | -- |
| Species-environment relation | 35.5% | 65% | 79.6% | 89.5% | -- |
| Sum of all eigenvalues | -- | -- | -- | -- | 3.33 |
| Sum of all canonical eigenvalues | -- | -- | -- | -- | 0.391 |
| Downstream area with tidal effect | |||||
| Eigenvalues | 0.505 | 0.208 | 0.098 | 0.087 | 5.656 |
| Species-environment correlations | 0.869 | 0.792 | 0.644 | 0.602 | -- |
| Cumulative percentage variance | -- | -- | -- | -- | -- |
| Species | 8.9% | 12.6% | 14.3% | 15.9% | -- |
| Species-environment relation | 50.3% | 71% | 80.7% | 89.4% | -- |
| Sum of all eigenvalues | -- | -- | -- | -- | 5.656 |
| Sum of all canonical eigenvalues | -- | -- | -- | -- | 1.004 |
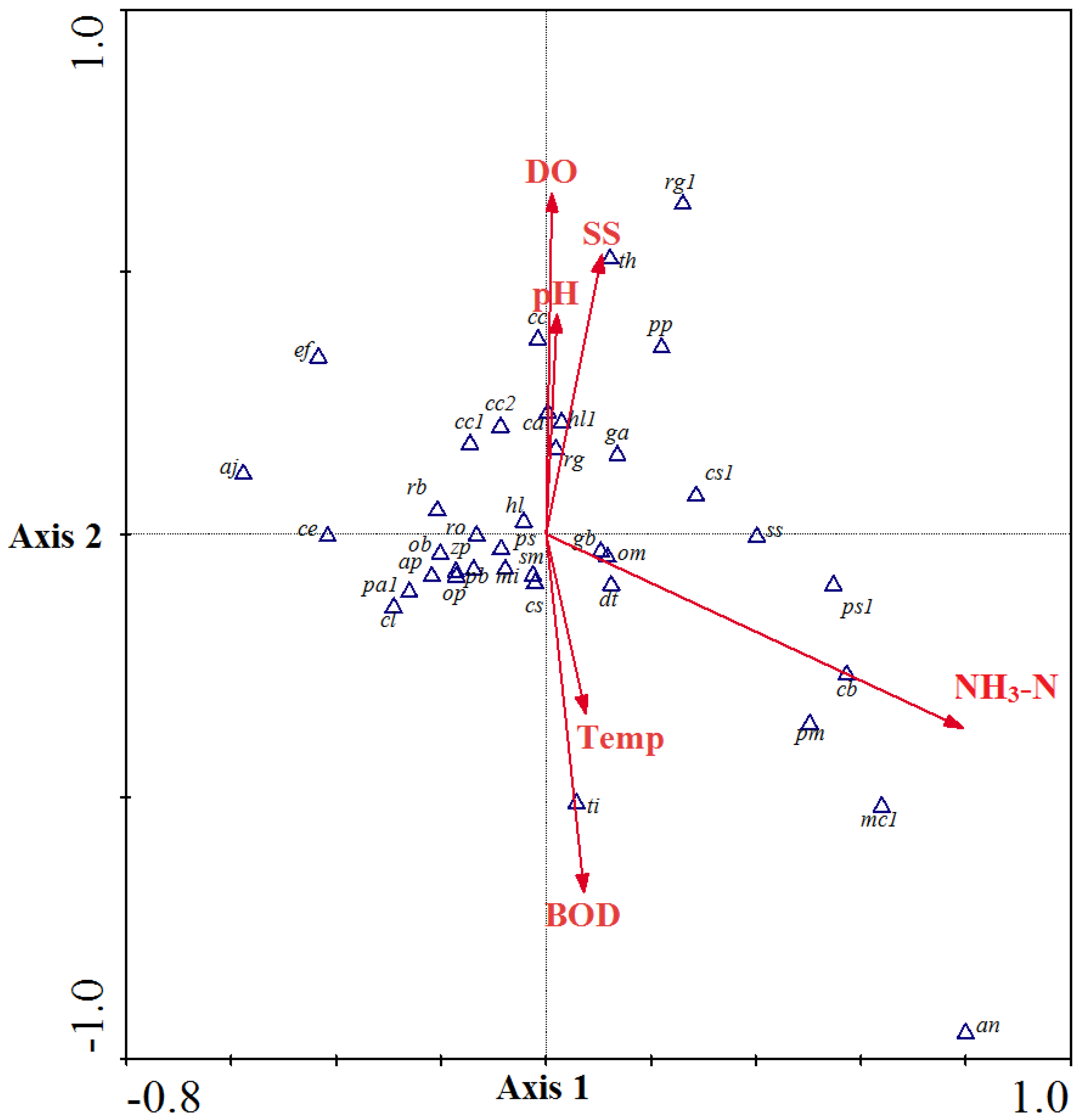
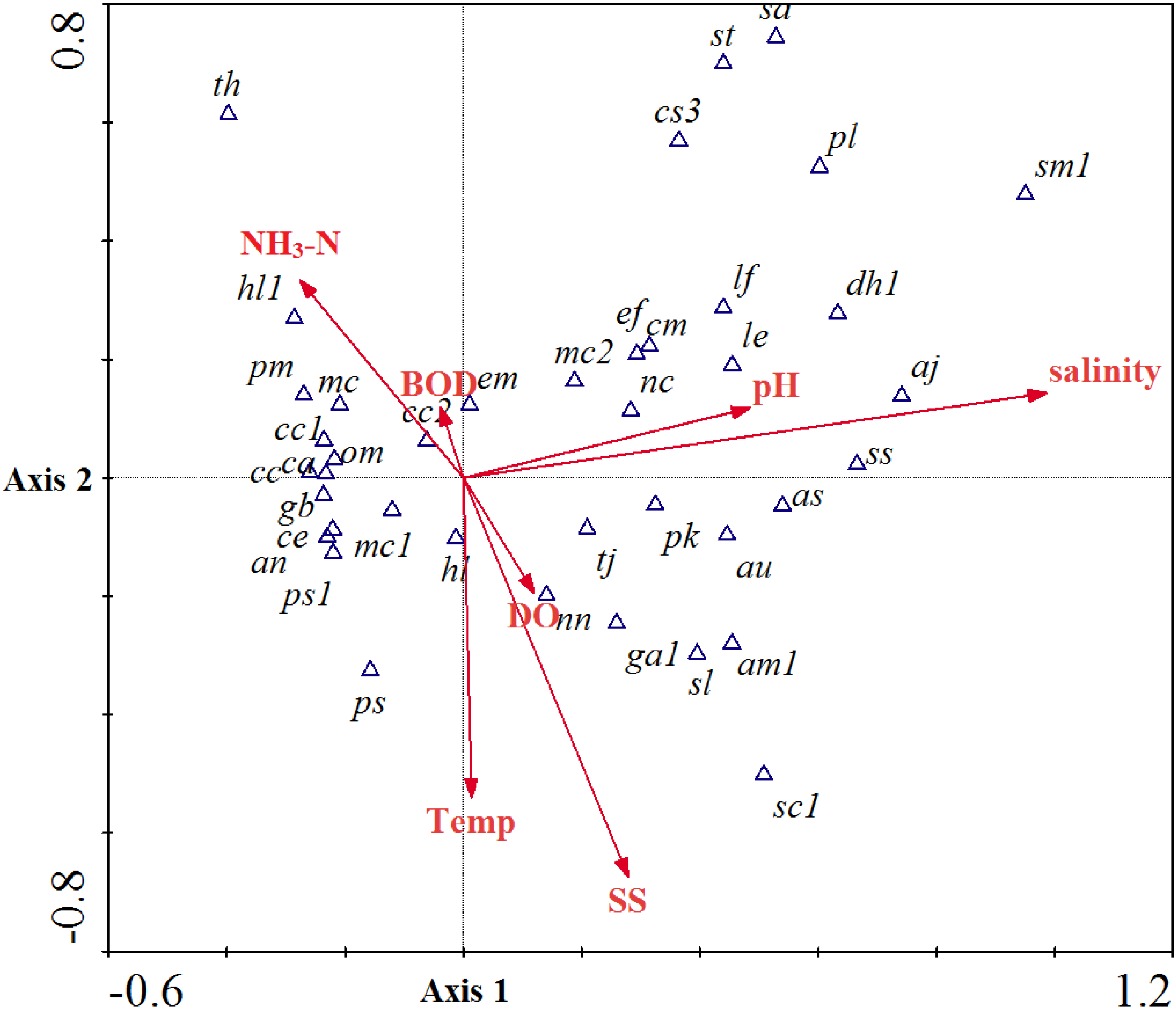
3.4. Biological Indexation


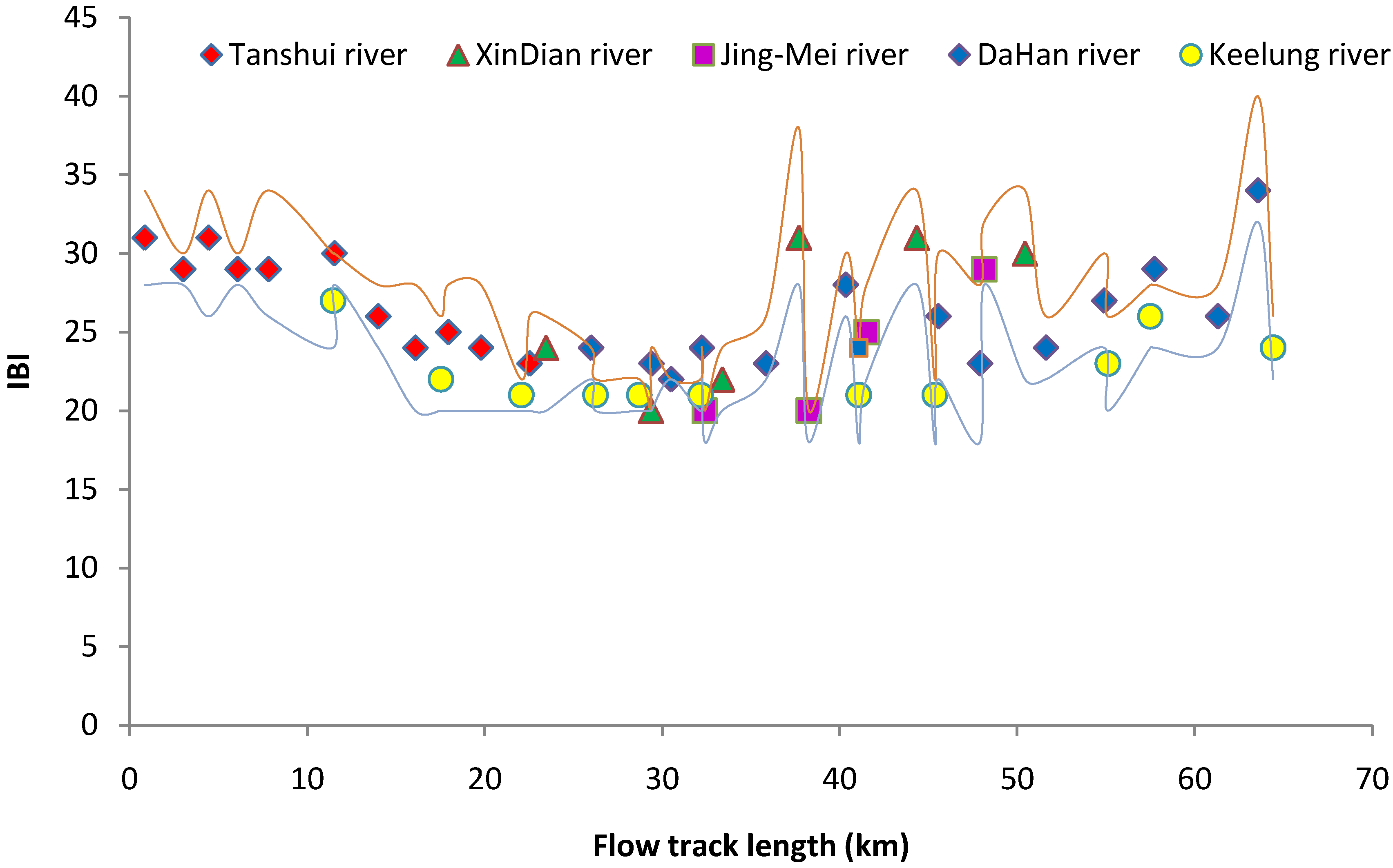
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hong, C.-C. The investigation of aquatic life of Tanshui River basin and assessment of its pollution degree. Biol. Bull. Natl. Taiwan Norm. Univ. 1979, 14, 23–31. (In Chinese) [Google Scholar]
- Hsu, T.-J.; Hsu, W.-H.; Wang, Z.-Z.; Hsu, F.-L.; Lai, S.-Ju.; Lai, S.-Je. Study of contamination in water sources of the Hsin-Tien River. J. Formos. Med. Assoc. 1972, 71, 467–478. (In Chinese) [Google Scholar]
- Wang, S.-B.; Gwo, J.-L.; Lee, J.-D. The study for causation of fish death in Xindian River. J. Chin. Taiwan Water Works Assoc. 1977, 14, 25–31. (In Chinese) [Google Scholar]
- Hong, C.-C.; Hsu, S.-J. The relationships between the pollution degree of the rivers and their biota- from the Hsin-Tien stream down to Tamsui River. Biol. Bull. Natl. Taiwan Norm. Univ. 1977, 14, 23–31. (In Chinese) [Google Scholar]
- Wong, K.-J.; Hau, D.-M.; Lin, W.-D.; Huang, H.-T.; Chang, C.-C. Ecological studies of contamination in water source of the Taipei City (Hsin-tein River). Taipei Med. Univ. Inst. Rep. 1973, 5, 39–48. (In Chinese) [Google Scholar]
- Construction and Planning Agency, Minister of Interior. Available online: http://www.cpami.gov.tw/chinese/index.php?option=com_content&view=article&id=9995&Itemid=50 (accessed on 17 February 2014).
- Environmental Protection Administration, R.O.C. (Taiwan), Research, Water quality. Available online: http://www.epa.gov.tw/ch/DocList.aspx?unit=14&clsone=490&clstwo=98&clsthree=1386&busin=235&path=17215 (accessed on 17 February 2014).
- Besley, C.H.; Chessman, B.C. Rapid biological assessment charts the recovery of stream macroinvertebrate assemblages after sewage discharges cease. Ecol. Indic. 2008, 8, 625–638. [Google Scholar] [CrossRef]
- Kelly, M.; Juggins, S.; Guthrie, R.; Pritchard, S.; Jamieson, J.; Ripper, B.; Hirst, H.; Yallop, M. Assessment of ecological status in UK rivers using diatoms. Freshwater Biol. 2008, 53, 403–422. [Google Scholar]
- Breine, J.B.; Quataert, P.; Stevens, M.; Ollevier, F.; Volckaert, F.A.M.; Bergh, E.V.D.; Maes, J. A zone-specific fish-based biotic index as a management tool for the Zeeschelde estuary (Belgium). Mar. Pollut. Bull. 2010, 60, 1099–1112. [Google Scholar] [CrossRef]
- Ceschin, S.; Zuccarello, V.; Caneva, G. Role of macrophyte communities as bioindicators of water quality: Application on the Tiber River basin (Italy). Plant Biosyst. 2010, 144, 528–536. [Google Scholar]
- Costa, P.F.; Schulz, UH. The fish community as an indicator of biotic integrity of the streams in the Sinos River basin, Brazil. Braz. J. Biol. 2010, 70, 1195–1205. [Google Scholar] [CrossRef]
- Dick, L.K.; Stelzer, E.A.; Bertke, E.E.; Fong, D.L.; Stoeckel, D.M. Relative decay of Bacteroidales microbial source tracking markers and cultivated Escherichia coli in freshwater microcosms. Appl. Environ. Microbiol. 2010, 76, 3255–3262. [Google Scholar] [CrossRef]
- Farnleitner, A.H.; Ryzinska-Paier, G.; Reischer, G.H.; Burtscher, M.M.; Knetsch, S.; Kirschner, A.K.T.; Dirnbo, T.; Kuschnig, G.; Mach, R.L.; Sommer, R. Escherichia coli and enterococci are sensitive and reliable indicators for human, livestock and wildlife faecal pollution in alpine mountainous water resources. J. Appl. Microbiol. 2010, 109, 1599–1608. [Google Scholar]
- Locka, K.; Asenovab, M.; Goethalsa, P.L.M. Benthic macroinvertebrates as indicators of the water quality in Bulgaria: A case-study in the Iskar River basin. Limnologica 2011, 41, 334–338. [Google Scholar] [CrossRef]
- Schmitter-Soto, J.J.; Lissie, L.E.; Ruiz-Cauich, E.; Herrera, R.L.; González-Solís, D. An index of biotic integrity for shallow streams of the Hondo River basin, Yucatan Peninsula. Sci. Total Environ. 2011, 409, 844–852. [Google Scholar]
- Ceschin, S.; Aleffi, M.; Bisceglie, S.; Savo, V.; Zuccarello, V. Aquatic bryophytes as ecological indicators of water quality in the Tiber basin, Italy. Ecol. Indicat. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Bhatt, J.P.; Pandit, M.K. A macro-invertebrate based new biotic index to monitor river water quality. Curr. Sci. 2010, 99, 196–203. [Google Scholar]
- Kireta, A.R.; Reavie, E.D.; Sgro, G.V.; Angradic, T.R.; Bolgrien, D.W.; Hill, B.H.; Jicha, T.M. Planktonic and periphytic diatoms as indicators of stress on great rivers of the United States: Testing water quality and disturbance models. Ecol. Indicat. 2012, 13, 222–231. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Grundsätze Für Die Biologische Beurteilung des Wassers Nach Seiner Flora und Fauna. In Mitt. aus d. Kgl. Prüfungsanstalt für Wasserversorgung u; Abwasserbeseitigung Berlin: Berlin, Germany, 1902. (In German) [Google Scholar]
- Kolkwitz, R.; Marsson, M. Ökologie der pflanzlichen Saprobien. Ber. Dt. Botan. Ges. 1908, 26, 505–519. [Google Scholar]
- Kolkwitz, R.; Marsson, M. Ökologie der tierischen Saprobien. Int. Revue ges. Hydrobiol. 1909, 2, 126–152. [Google Scholar]
- Policy on the Use of Biological Assessments and Criteria in the Water Quality Program. Available online: http://www.epa.gov/nscep/index.html (accessed on 10 July 2014).
- Lazorchak, J.M.; Hill, B.H.; Brown, B.S.; McCormick, F.H.; Engle, V.; Lattier, D.J.; Bagley, M.J.; Griffith, M.B.; Maciorowski, A.F.; Toth, G.P. Chapter 23 USEPA Biomonitoring and Bioindicator Concepts Needed to Evaluate the Biological Integrity of Aquatic Systems. In Bioindicators and Biomonitors; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Eselvier: Amsterdam, The Netherlands, 2002; pp. 831–873. [Google Scholar]
- Deflandre, A.; Jarvie, H. Nutrients and Eutrophication. In Indicators and Methods for the Ecological Status Assessment under the Water Framework Directive, linkages between Chemical and Biological Quality of Surface Waters; Solimini, A.G., Cardoso, A.C., Heiskanen, A.-S., Eds.; European Commission, Joint Research Center Press: Ispra, Italy, 2006; pp. 81–98. [Google Scholar]
- Pettine, M.; Ruggiero, A.; Fazi, S.; buffagni, A.; Andersen, J.M.; Roncak, P. ; Friberg, N. Organic Pollution in Rivers. In Indicators and Methods for the Ecological Status Assessment under the Water Framework Directive, Linkages between Chemical and Biological Quality of Surface Waters; Solimini, A.G., CardosoHeiskanen, A.C., Heiskanen, A.-S., Eds.; European Commission, Joint Research Center Press: Ispra, Italy, 2006; pp. 99–116. [Google Scholar]
- Wu, G. Phytoplankton as bioindicator for water quality in Taipei. Bot. Bull. Acad. Sinica 1986, 25, 205–214. [Google Scholar]
- Hilenhoff, W.L. Rapid field assessment of organic pollution in river Sutlej. Int. J. Environ. Sci. 1988, 7, 65–68. [Google Scholar]
- Suisei, K. Pollution Biology; Hokuryukan press: Tokyo, Japan, 1964; p. 193. (In Japanese) [Google Scholar]
- Karr, J.R. Assessment of biotic integrity using fish community. Fisheries 1981, 6, 21–27. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 283. [Google Scholar]
- Yang, P.S.; Xyu, C.B. Aquatic Insects as Bioindicators in River and Stream- Investigation methods. In Handbook for Investigation of Biota and Construction of Bioindicator for Tanshui River basin; EPA-88-G108-03-301; Environmental Protection Administration: Taipei, Taiwan, 1999; pp. 3-1–3-45. (In Chinese) [Google Scholar]
- Xie, H.-L.; Wang, Y.-H.; Liu, B.-R. Benthic invertebrate as bioindicator for tidal effect area of Tanshui River and Keelung River. In Handbook for Investigation of Biota and Construction of Bioindicator for Tanshui River basin; EPA-88-G108-03-301; Environmental Protection Administration: Taipei, Taiwan, 1999; pp. 4-1–4-44. (In Chinese) [Google Scholar]
- Soil and Water Conservation Society of Metro Halifax, Freshwater-Master Homepage. Available online: http://chebucto.ca/Science/SWCS/SWCS.html (accessed on 10 July 2014).
- Bressler, D.W.; Stribling, J.B.; Paul, M.J.; Hicks, M.B. Stressor tolerance values for benthic macroinvertebrates in Mississippi. Hydrobiologia 2006, 573, 155–172. [Google Scholar]
- Dwan, X.-H.; Wang, Z.-Z.; Xyu, M.-Z. Benthic Macroinvertebrate and Application in Assessment of Stream Ecology; QyingHwa University press: Peking, Chian, 2010; p. 120. [Google Scholar]
- Karr, J.R. Biological integrity: A long-neglected aspect of water resource management. Ecol. Appl. 1991, 1, 64–84. [Google Scholar]
- Southerland, M.T.; Rogers, G.M.; Kline, M.J.; Morgan, R.P.; Boward, D.M.; Stranko, S.A. Improving biological indicators to better assess the condition of streams. Ecol. Indicat. 2007, 7, 751–767. [Google Scholar]
- Zhang, M.S.; Wang, S.Z.; Shao, G.Z. The Investigation Methods for Bioindicator in Keelung River Basin-Fish. In Handbook for Investigation of Biota and Construction of Bioindicator for Tanshui River; EPA-88-G108-03-301; Environmental Protection Administration: Taipei, Taiwan, 1999; pp. 5-1–5-27. (In Chinese) [Google Scholar]
- The Study of Fishes as Biological Indicator of River Water Quality of Taiwan. Available online: http://www.niea.gov.tw/analysis/protect/Protect_srh.asp (accessed on 10 July 2014).
- Wang, H.Q. The Completed Records of River Ecology of Taiwan; ZhanLi Culture Press: Taipei, Taiwan, 2006; p. 174. (In Chinese) [Google Scholar]
- Cudmore, B.; Mandrak, N.E. Biological Synopsis of Grass Carp (Ctenopharyngodon idella); Canadian Manuscript Report of Fisheries and Aquatic Sciences; Fisheries and Oceans Canada, Great Lakes Laboratory for Fisheries and Aquatic Sciences: Burlington, Canada, 2004; p. 52. [Google Scholar]
- Chen, I.S.; Fang, L.S. The Freshwater and Estuarine Fishes of Taiwan; National Museum of Marine Biology and Aquarium: Pin-Tung, Taiwan, 1999; p. 287. (In Chinese) [Google Scholar]
- The Survey of Ecological Benefits for Tanshui River after Pollution Controls. Available online: http://epq.epa.gov.tw/mp.aspx. (accessed on 10 July 2014).
- Lin, H.-J.; Shao, K.-T.; Jan, R.-Q.; Hsieh, H.-L.; Chen, C.-P.; Hsieh, L.-Y.; Hsiao, Y.-T. A trophic model for the Danshuei River Estuary, a hypoxic estuary in northern Taiwan. Mar. Pollut. Bull. 2007, 54, 1789–1800. [Google Scholar]
- Lyang, Z.-S. The aquatic products industry of Taiwan before restoration. Q. J. Taiwan Bank 1951, 4, 74–109. (In Chinese) [Google Scholar]
- Tzeng, C.-H. The Freshwater Fishes of Taiwan; The Provincial Keelung Girl’s High School: Keelung, Taiwan, 1988; p. 195. [Google Scholar]
- Young, S.S. The Study of Biological and Ecological Indicators of Tanshui River (2/2).; EPA-98-1605-02-01; Environmental Protection Administration: Taipei, Taiwan, 2009; p. 400. (In Chinese) [Google Scholar]
- Physical Effects of Suspended Solids on Marine and Estuarine Fish and Shellfish, with Special Reference to Ocean Dumping: A Literature review. Available online: http://www.dfo-mpo.gc.ca/Library/114106.pdf (accessed on 10 July 2014).
- The Problem of Sediment in Water for Fish. Available online: http://www.mnr.gov.on.ca/stdprodconsume/groups/lr/@mnr/@nwsi/documents/document/mnr_e005389.pdf (accessed on 10 July 2014).
- Tyan, Q.Z.; Li, J.Z.; Siun, Y.M. The Survey on the Environmental Distribution of Toxic Substa nces from 2009 to 2010; EPA-98-J104-02-205; Environmental Protection Administration: Taipei, Taiwan, 2009; p. 239. (In Chinese) [Google Scholar]
- Chen, Y.-C.; Yeh, H.-C.; Wei, C. Estimation of river pollution index in a Tidal Stream using kriging analysis. Int. J. Environ. Res. Public Health 2012, 9, 3085–3100. [Google Scholar]
- Paavola, R.; Muotka, T.; Virtanen, R.; Heino, J.; Jackson, D.; Maki-Petays, A. Spatial scale affects community concordance among fish, benthic macroinvertebrates, and bryophytes in streams. Ecol. Appl. 2006, 16, 368–379. [Google Scholar]
- Infante, D.M.; Allan, J.D.; Linke, S.; Norris, R.H. Relationship of fish and macroinvertebrate assemblages to environmental factors: implications for community concordance. Hydrobiologia 2009, 623, 87–103. [Google Scholar] [CrossRef]
- Monaghan, K.A.; Soares, A.M.V.M. The bioassessment of fish and macroinvertebrates in a Mediterranean–Atlantic climate: Habitat assessment and concordance between contrasting ecological samples. Ecol. Indicat. 2010, 10, 184–191. [Google Scholar]
- Larsen, S.; Mancini, L.; Pace, G.; Scalici, M.; Tancioni, L. Weak concordance between fish and macroinvertebrates in Mediterranean streams. PLoS One 2012, 7. [Google Scholar] [CrossRef] [Green Version]
Appendix
| Item | Non-impaired | Slightly impaired | Moderately impaired | Severely impaired |
|---|---|---|---|---|
| (DO) mg/L | D ≥ 6.5 | 6.5 < D ≥ 4.6 | 4.5 ≥ D ≥ 2.0 | D < 2.0 |
| (BOD5) mg/L | B ≤ 3.0 | 3.0 < B ≤ 4.9 | 5.0 ≤ B ≤ 15.0 | B > 15.0 |
| (SS) mg/L | S ≤ 20.0 | 20.0 < S ≤ 49.9 | 50.0 ≤ S ≤ 100 | S > 100 |
| (NH3-N) mg/L | N ≤ 0.50 | 0.50 < N ≤ 0.99 | 1.00 ≤ N ≤ 3.00 | N > 3.00 |
| Score | 1 | 3 | 6 | 10 |
| RPI= (D + B + S + N)/4 | S ≤ 2.0 | 2.0 < S ≤ 3.0 | 3.1 ≤ S ≤ 6.0 | S > 6.0 |
| Taxa | Code | Tolerance value | ||
|---|---|---|---|---|
| Fish | ||||
| Anguillidae | ||||
| Anguilla japonica | aj | 4 | ||
| Anguilla marmorata | am | 4 | ||
| Cyprinidae | ||||
| Rhodeus ocellatus | ro | 5 | ||
| Tanakia himantegus | th | 5 | ||
| Microphysogobio brevirostris | mi | 3 | ||
| Acrossochelius paradoxus | ap | 3 | ||
| Aristichthys nobilis | an | 5 | ||
| Candidia barbata | cb | 2 | ||
| Carassius auratus | ca | 6 | ||
| Carassius cuvieri | cc | 6 | ||
| Ctenopharyngodon idella | ci | 6 | ||
| Chanodichthys erythropterus | ce | 5 | ||
| Cyprinus carpio carpio | cc1 | 6 | ||
| Distoechodon tumirostris | dt | 5 | ||
| Hemibarbus labeo | Hl | 6 | ||
| Hemiculter leucisculus | hl1 | 6 | ||
| Pseudorasbora parva | pp | 5 | ||
| Sinibrama macrops | sm | 4 | ||
| Onychostoma barbatulum | ob | 0 | ||
| Opsariichthys pachycephalus | op | 3 | ||
| Zacco platypus | zp | 3 | ||
| Mylopharyngodon piceus | mp | 5 | ||
| Crossostoma lacustre | cl | 2 | ||
| Hemimyzon formosanum | hf | 1 | ||
| Cobitididae | ||||
| Cobitis sinensis | cs | 2 | ||
| Misgurnus anguillicaudatus | ma | 6 | ||
| Bagridae | ||||
| Pseudobagrus adiposalis | pa | 2 | ||
| Pseudobagrus brevianalis | pb | 2 | ||
| Siluridae | ||||
| Silurus asotus | sa | 6 | ||
| Pangasius sutchi | ps | 8 | ||
| Siganidae | ||||
| Siganus canaliculatus | sc | 3 | ||
| Loricariidae | ||||
| Pterygoplichthys multiradiatus | pm | 9 | ||
| Hemichromis bimaculatus | hb | 8 | ||
| Oreochromis mossambicus | om | 8 | ||
| Tilapia sp. | ti | 8 | ||
| Paratheraps synspilum | ps1 | 8 | ||
| Labidochromis caeruleus | lc | 8 | ||
| Mugilidae | ||||
| Chelon macrolepis | cm | 8 | ||
| Mugil cephalus | mc1 | 7 | ||
| Moolgarda cunnesius | mc2 | 7 | ||
| Chelon subviridis | cs3 | 7 | ||
| Synbranchidae | ||||
| Monopterus albus | ma1 | 5 | ||
| Eleotridae | ||||
| Eleotris fusca | ef | 3 | ||
| Gobiidae | ||||
| Sicyopterus japonicus | sj | 3 | ||
| Rhinogobius brunneus | rb | 3 | ||
| Rhinogobius giurinus | rg | 4 | ||
| Rhinogobius gigas | rg1 | 3 | ||
| Moronidae | ||||
| Lateolabrax japonicus | lj | 3 | ||
| Channidae | ||||
| Channa striata | cs1 | 9 | ||
| Diodontidae | ||||
| Diodon holocanthus | dh1 | 3 | ||
| Sillaginidae | ||||
| Sillago sihama | ss | 3 | ||
| Sphyracenidae | ||||
| Sphyraena putnamae | sp | 3 | ||
| Lutjanidae | ||||
| Lutjanus fulviflamma | lf | 3 | ||
| Apogonidae | ||||
| Apogonidae sp. | ap1 | 3 | ||
| Haemulidae | ||||
| Pomadasys kaakan | pk | 3 | ||
| Serranidae | ||||
| Epinephelus coioides | ep | 3 | ||
| Epinephelus radiatus | er | 3 | ||
| Elopidae | ||||
| Elops machnata | em | 9 | ||
| Megalopidae | ||||
| Megalops cyprinoides | mc | 9 | ||
| Leiognathidae | ||||
| Nuchequula nuchalis | nn | 3 | ||
| Nuchequula mannusella | nm | 3 | ||
| Leiognathus equulus | le | 3 | ||
| Ambassidae | ||||
| Ambassis urotaenia | au | 3 | ||
| Gerreidae | ||||
| Gerres abbreviatus | ga1 | 3 | ||
| Scatophagidae | ||||
| Scatophagus argus | sa | 9 | ||
| Sparidae | ||||
| Rhabdosargus sarba | rs | 3 | ||
| Acanthopagrus latus | al | 3 | ||
| Acanthopagrus schlegelii | as | 3 | ||
| Ophichthidae | ||||
| Pisodonophis cancrivorus | pc | 3 | ||
| Sciaenidae | ||||
| Johnius dussumieri | jd | 3 | ||
| Clupeidae | ||||
| Sardinella melanura | sm1 | 3 | ||
| Sardinella lemuru | sl | 3 | ||
| Sardinella albella | sa1 | 3 | ||
| Nematalosa come | nc | 3 | ||
| Engraulidae | ||||
| Thryssa hamiltonii | th1 | 3 | ||
| Thryssa chefuensis | tc | 3 | ||
| Chanidae | ||||
| Chanos chanos | cc2 | 5 | ||
| Carangidae | ||||
| Scomberoides tol | st | 3 | ||
| Scomberoides commersonnianus | sc1 | 3 | ||
| Caranx sexfasciatus | cs2 | 3 | ||
| Carangoides malabaricus | cm1 | 3 | ||
| Macroinvertebrates | ||||
| Mollusca | ||||
| Thiaridae | ||||
| Stenomelania torulosa | st | 3 | ||
| Melanoides tubercufatus | mt | 3 | ||
| Tarebia granifera | tg | 3 | ||
| Thiara riqueti | tr | 3 | ||
| Pleuroceridae | ||||
| Semisulcospira libertina | sl | 3 | ||
| Viviparidae | ||||
| Bellamya quadrata | bq | 6 | ||
| Corbiculidae | ||||
| Corbicula fluminea | cf | 4 | ||
| Mytilidae | ||||
| Limnoperna fortunei | lf | 6 | ||
| Perna viridis | pv | 6 | ||
| Veneridae | ||||
| Meretrix petechialis | mp | |||
| Philinidae | 3 | |||
| Physa acuta | pa | 8 | ||
| Lymnaeidae | ||||
| Lymnaea pervia | lp | 6 | ||
| Radix auricularia | ra | 6 | ||
| Planorbidae | ||||
| Hippeutis umbilicalis | hu | 7 | ||
| Tellinidae | ||||
| Tellina virgata | tv | 3 | ||
| Unionidae | ||||
| Unio douglasiae | ud | 5 | ||
| Ampullariidae | ||||
| Pomacea canaliculata | pc | 8 | ||
| Nemertina | ne2 | 10 | ||
| Annelida | ||||
| Polychaeta | ||||
| Phyllodocimorpha | ||||
| Goniadidae | ||||
| Glycinde sp. | gl | 6 | ||
| Nereididae | ||||
| Neanthes sp. | ne | 6 | ||
| Neanthes glandicincta | ng | 6 | ||
| Perinereis aibuhitensis | pa1 | 6 | ||
| Sabellida | ||||
| Sabellidae | ||||
| Laonome albicingillum | la | 6 | ||
| Hesionidae | ||||
| Micropodarke sp. | mi | 6 | ||
| Hirudinea | ||||
| Rhynchobdellida | ||||
| Glossiophonidae | ||||
| Helobdella europaea | he | 9 | ||
| Alboglossiphonia lata | al | 9 | ||
| Barbronia weberi | bw | 9 | ||
| Erpobdellidae | ||||
| Bobronia sp. | bo | 9 | ||
| Arthropoda | ||||
| Malacostraca | ||||
| Amphipoda | ||||
| Melitidae | ||||
| Melitidae sp. | me | 6 | ||
| Corophiidae | ||||
| Corophium major | cm | 6 | ||
| Corophium sp. | co | 6 | ||
| Gammaropsis sp. | ga | 6 | ||
| Decapoda | ||||
| Palaemonidae | ||||
| Macrobrachium asperulum | ma | 6 | ||
| Macrobrachium nipponense | mn | 6 | ||
| Macrobrachium formosense | mf | 6 | ||
| Macrobrachium jaopnicum | mj | 6 | ||
| Macrobrachium australe | ma1 | 6 | ||
| Atyidae | ||||
| Neocaridina denticulata | nd | 6 | ||
| Caridina pseudodenticulata | cp | 6 | ||
| Penaeidae | ||||
| Metapenaeus monoceros | mm | 6 | ||
| Penaeus sp.1 | mm1 | 6 | ||
| Cambaridae | ||||
| Procambarus clarkii | pc1 | 6 | ||
| Potamidae | ||||
| Geothelphusa candidiensis | ga1 | 6 | ||
| Ocypodidae | ||||
| Ilyoplax fromosensis | if | 6 | ||
| Uca borealis | ub | 6 | ||
| Macrophthalmus banzai | mb | 6 | ||
| Grapsidae | ||||
| Eriocheir japonicus | ej | 6 | ||
| Helice wuana | hw | 6 | ||
| Portunidae | ||||
| Scylla serrata | ss | 6 | ||
| Portunus sanguinolentus | ps | 6 | ||
| Isopoda | is | 8 | ||
| Potamidae | ||||
| Insecta | ||||
| Ephemeroptera | ||||
| Baetidae | ||||
| Baetiella bispinosa | bb | 5 | ||
| Baetiella joponica | bj | 5 | ||
| Baetiella sp.1 | ba | 5 | ||
| Baetiella sp.2 | ba1 | 5 | ||
| Baetis sp. | ba2 | 5 | ||
| Caenidae | ||||
| Caenis bella | cb | 6 | ||
| Caenis sp. | ca | 6 | ||
| Ephemerellidae | ||||
| Ephemerella sp. | ep | 1 | ||
| Torleya japonixa | tj | 1 | ||
| Torleya glareosa | tg1 | 1 | ||
| Uracanthella sp. | ur | 1 | ||
| Ephemeridae | ||||
| Ephemera formosana | ef | 4 | ||
| Heptageniidae | ||||
| Ecdyonurus sp. | ec | 4 | ||
| Ecdyonurus viridis | ev | 4 | ||
| Ecdyonurus yoshidae | ey | 4 | ||
| Leptophlebiidae | ||||
| Choroterpes sp. | ch1 | 2 | ||
| Thraulis sp. | th | 2 | ||
| Paraleptophlebia sp. | pa2 | 2 | ||
| Odonata | ||||
| Euphaeidae | ||||
| Euphaea formosa | ef1 | 6 | ||
| Gomphidae | ||||
| Gomphus sp. | go | 1 | ||
| Lanthus fujiacus | lf1 | 1 | ||
| Ictinogomphus sp. | ic | 1 | ||
| Onychogomphus sp. | on | 1 | ||
| Plecoptera | ||||
| Perlodidae | ||||
| Neoperla sp. | ne1 | 1 | ||
| Trichoptera | ||||
| Hydropsychidae | ||||
| Hydropsyche orientalis | ho | 4 | ||
| Hydropsyche sp. | hy | 4 | ||
| Hydroptilidae | ||||
| Hydroptila sp. | hy1 | 4 | ||
| Philopotamidae | ||||
| Dolophilodes sp. | do | 3 | ||
| Wormaldia sp. | wo | 3 | ||
| Stenopsychidae | ||||
| Stenopsyche schmidi | ss1 | 4 | ||
| Stenopsyche sp. | st1 | 4 | ||
| Psychomyiidae | ||||
| Psychomyiella sp. | ps1 | 2 | ||
| Ecnomidae | ||||
| Ecnomus sp. | ec1 | 3 | ||
| Lepidoptera | le | 5 | ||
| Coleoptera | ||||
| Elminae | ||||
| Ordobrevia sp. | or | 4 | ||
| Stenelmis sp. | st2 | 4 | ||
| Psephenidae | ||||
| Eubrianax granicolis | eg | 4 | ||
| Eubrianax sp.1 | eu | 4 | ||
| Eubrianax sp.2 | eu1 | 4 | ||
| Psephenoidesjaponicus | pj | 4 | ||
| Ellipes sp. | el | 4 | ||
| Sialidae | ||||
| Corydalidae | ||||
| Protohermes grandis | pg | 4 | ||
| Diptera | ||||
| Chironomidae | ||||
| Chironomus sp. | ch | 7 | ||
| Tipulidae | ||||
| Antochasaxicola | as | 3 | ||
| Tipula sp. | ti | 3 | ||
| Formulation | Score and combination | Indication of water quality |
|---|---|---|
| FBI = Σ( ai × ni)/N | ai: tolerance value of i family ni: individuals of i family N: individuals in total | 0.00~3.75 Excellent 3.76~4.25 Very good 4.26~5.00 Good 5.01~5.75 Fair 5.76~6.50 Fairly poor 6.51~7.25 Poor 7.26~10.0 Very poor |
| BI = 2A + B + O | A: number of intolerant species B: number of tolerant species O: number of undefined species | ≥20: oligosaprobic zone (os) 11~19: β mesosaprobic zone (βms) 6~10: α mesosaprobic zone (αms) 0~5: polysaprobic zone (ps) |
| IBI = A + B + … + J | A: percentage of native species, 1 (≤ 33 %); 3 (33–66%); 5 (≥ 66%) B: percentage of benthic species, 1 (≤ 33 %); 3 (33–66%); 5 (≥ 66%) C: percentage of pelagic species, 1 (≤ 33 %); 3 (33–66%); 5 (≥ 66%) D: percentage of untolerant species, 1 (≤ 5 %); 3 (5–15 %); 5 (≥ 15%) E: percentage of tolerant species, 1 (≥15 %) ; 3 (5–15 %); 5 (≤ 5%) F: percentage of omnivorous species, 1 (≥40 %); 3 (20–40 %); 5 (≤ 20%) G: percentage of insectivorous species, 1 (≤ 5 %) ; 3 (5–20 %); 5 (≥20%) H: percentage of piscivorous species, 1 (≤ 3 %) ; 3 (3–10%); 5 (≥10%) I: catch per unit effort (CPUE) 1(≤ 100); 3(100–250); 5(≥250) J: percentage of exotic species, 1 (≥10 %) ; 3 (1–10 %); 5 (≤ 1%) | 45~50: Excellent 37~44: Good 28~36: Fair 16~27: Poor <16: Very poor *no fish |
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Young, S.-S.; Yang, H.-N.; Huang, D.-J.; Liu, S.-M.; Huang, Y.-H.; Chiang, C.-T.; Liu, J.-W. Using Benthic Macroinvertebrate and Fish Communities as Bioindicators of the Tanshui River Basin Around the Greater Taipei Area — Multivariate Analysis of Spatial Variation Related to Levels of Water Pollution. Int. J. Environ. Res. Public Health 2014, 11, 7116-7143. https://doi.org/10.3390/ijerph110707116
Young S-S, Yang H-N, Huang D-J, Liu S-M, Huang Y-H, Chiang C-T, Liu J-W. Using Benthic Macroinvertebrate and Fish Communities as Bioindicators of the Tanshui River Basin Around the Greater Taipei Area — Multivariate Analysis of Spatial Variation Related to Levels of Water Pollution. International Journal of Environmental Research and Public Health. 2014; 11(7):7116-7143. https://doi.org/10.3390/ijerph110707116
Chicago/Turabian StyleYoung, Shuh-Sen, Hsi-Nan Yang, Da-Ji Huang, Su-Miao Liu, Yueh-Han Huang, Chung-Ting Chiang, and Jin-Wei Liu. 2014. "Using Benthic Macroinvertebrate and Fish Communities as Bioindicators of the Tanshui River Basin Around the Greater Taipei Area — Multivariate Analysis of Spatial Variation Related to Levels of Water Pollution" International Journal of Environmental Research and Public Health 11, no. 7: 7116-7143. https://doi.org/10.3390/ijerph110707116




