Evaluating the Impact of Environmental Temperature on Global Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreaks in Domestic Poultry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Global HPAI H5N1 Outbreaks
2.2. Environmental Temperature
2.3. Global Polygon Maps
2.4. Statistical Analysis




3. Results
3.1. Environmental Temperature in Different Epidemic Waves
| EW | Sample size | Mean | std | CI95 | Median | Minimum | Maximum | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,540 | 20.832 | 5.615 | (8.777, 27.303) | 20.140 | −11.230 | 31.525 | 16.961 | 26.441 |
| 2 | 1,927 | 27.178 | 1.972 | (21.177, 29.374) | 27.641 | 6.344 | 30.679 | 26.726 | 28.392 |
| 3 | 2,338 | 17.578 | 9.158 | (−3.100, 31.180) | 17.949 | −11.360 | 34.214 | 14.538 | 22.483 |
| 4 | 657 | 19.742 | 8.512 | (1.563, 30.129) | 21.418 | −12.990 | 33.680 | 14.274 | 26.504 |
| 5 | 682 | 18.804 | 6.767 | (1.074, 28.864) | 19.279 | −7.417 | 33.632 | 14.279 | 24.622 |
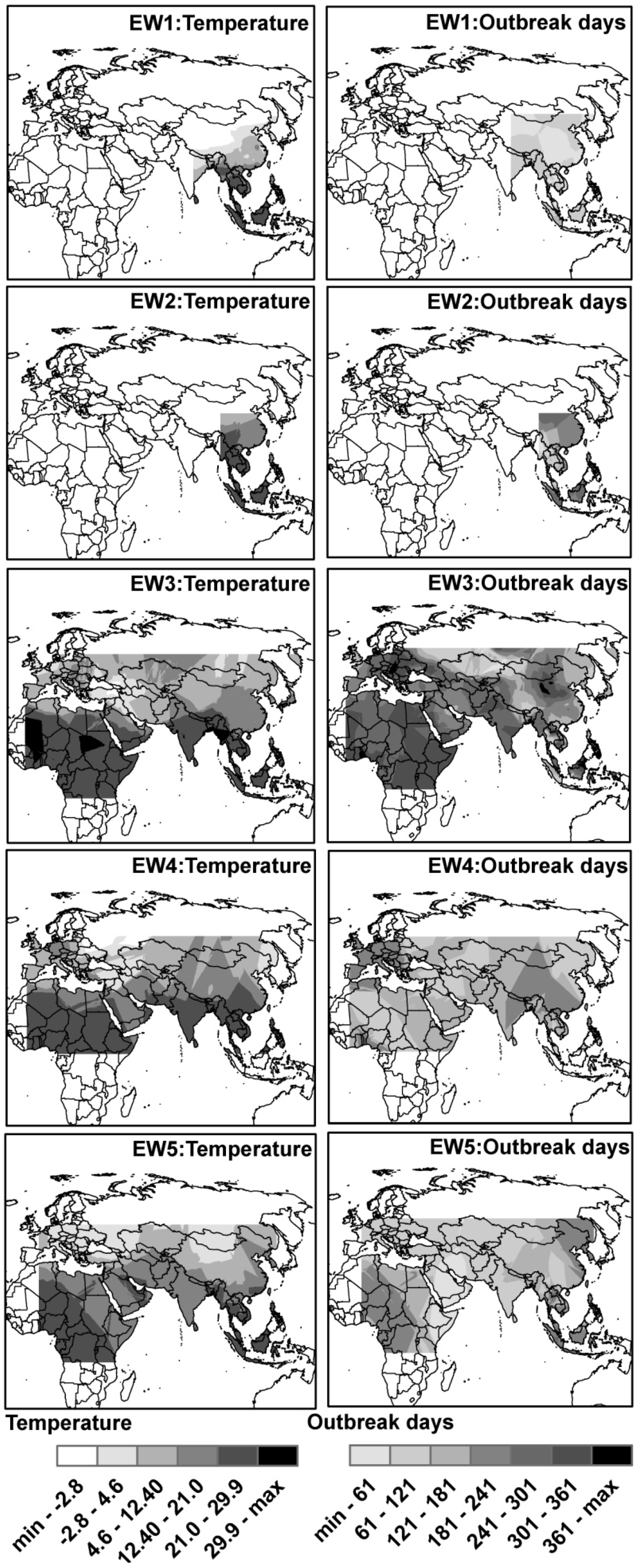
3.2. Spatial Distributions of Environmental Temperature and Outbreak Dates
3.3. Impact of Environmental Temperature on HPAI H5N1 Outbreaks
| EW | Node | Term | Mean | std | MC Error | 2.50% | Median | 97.50% |
|---|---|---|---|---|---|---|---|---|
| 1 | beta | temperature | −0.023 | 0.006 | 0.000 | −0.033 | −0.023 | −0.011 |
| sigma.u | std of u[] | 0.069 | 0.017 | 0.001 | 0.043 | 0.067 | 0.110 | |
| sigma.v | std of v[] | 0.086 | 0.021 | 0.002 | 0.051 | 0.083 | 0.134 | |
| 2 | beta | temperature | 0.232 | 0.019 | 0.002 | 0.198 | 0.230 | 0.275 |
| sigma.u | std of u[] | 0.258 | 0.056 | 0.004 | 0.166 | 0.252 | 0.375 | |
| sigma.v | std of v[] | 0.489 | 0.047 | 0.002 | 0.398 | 0.488 | 0.585 | |
| 3 | beta | temperature | −0.088 | 0.008 | 0.001 | −0.104 | −0.088 | −0.072 |
| sigma.u | std of u[] | 0.190 | 0.095 | 0.007 | 0.063 | 0.172 | 0.421 | |
| sigma.v | std of v[] | 1.634 | 0.085 | 0.004 | 1.472 | 1.633 | 1.806 | |
| 4 | beta | temperature | −0.185 | 0.015 | 0.001 | −0.217 | −0.184 | −0.156 |
| sigma.u | std of u[] | 0.359 | 0.271 | 0.010 | 0.077 | 0.279 | 1.070 | |
| sigma.v | std of v[] | 1.524 | 0.179 | 0.009 | 1.208 | 1.511 | 1.907 | |
| 5 | beta | temperature | −0.134 | 0.012 | 0.001 | −0.157 | −0.134 | −0.112 |
| sigma.u | std of u[] | 0.177 | 0.085 | 0.004 | 0.066 | 0.161 | 0.383 | |
| sigma.v | std of v[] | 0.777 | 0.090 | 0.004 | 0.614 | 0.773 | 0.967 |
4. Discussion and Conclusions
| Node | Term | Mean | std | MC error | 2.50% | Median | 97.50% |
|---|---|---|---|---|---|---|---|
| beta[0] | [20,25) | −1.634 | 1.451 | 0.122 | −3.783 | −1.843 | 1.286 |
| beta[1] | [min,20) | −1.461 | 0.406 | 0.007 | −2.285 | −1.450 | −0.689 |
| beta[2] | [25,max] | 0.841 | 0.107 | 0.004 | 0.628 | 0.843 | 1.047 |
| sigma.u | std of u[] | 0.253 | 0.053 | 0.003 | 0.155 | 0.250 | 0.361 |
| sigma.v | std of v[] | 0.484 | 0.046 | 0.002 | 0.396 | 0.484 | 0.577 |
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Subbarao, K.; Klimov, A.; Katz, J.; Regnery, H.; Lim, W.; Hall, H.; Perdue, M.; Swayne, D.; Bender, C.; Huang, J.; et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998, 279, 393–396. [Google Scholar]
- Shortridge, K.F.; Zhou, N.N.; Guan, Y.; Gao, P.; Ito, T.; Kawaoka, Y.; Kodihalli, S.; Krauss, S.; Markwell, D.; Murti, K.G.; et al. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 1998, 252, 331–342. [Google Scholar] [CrossRef]
- Claas, E.C.J.; Osterhaus, A.D.M.E.; van Beek, R.; de Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef]
- Alexander, D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis. 2007, 51, 161–166. [Google Scholar] [CrossRef]
- Ungchusak, K.; Auewarakul, P.; Dowell, S.F.; Kitphati, R.; Auwanit, W.; Puthavathana, P.; Uiprasertkul, M.; Boonnak, K.; Pittayawonganon, C.; Cox, N.J.; et al. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 2005, 352, 333–340. [Google Scholar] [CrossRef]
- Li, K.S.; Guan, Y.; Wang, J.; Smith, G.J.D.; Xu, K.M.; Duan, L.; Rahardjo, A.P.; Puthavathana, P.; Buranathai, C.; Nguyen, T.D.; et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 2004, 430, 209–213. [Google Scholar] [CrossRef]
- Chen, H.; Deng, G.; Li, Z.; Tian, G.; Li, Y.; Jiao, P.; Zhang, L.; Liu, Z.; Webster, R.G.; Yu, K. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 2004, 101, 10452–10457. [Google Scholar] [CrossRef]
- Farnsworth, M.L.; Ward, M.P. Identifying spatio-temporal patterns of transboundary disease spread: examples using avian influenza H5N1 outbreaks. Vet. Res. 2009, 40. [Google Scholar] [CrossRef]
- Jourdain, E.; Gauthier-Clerc, M.; Sabatier, P. Ecoregional dominance in spatial distribution of avian influenza (H5N1) outbreaks—In response. Emerg. Infect. Dis. 2007, 13, 1270–1271. [Google Scholar] [CrossRef]
- Gilbert, M.; Xiao, X.; Domenech, J.; Lubroth, J.; Martin, V.; Slingenbergh, J. Anatidae migration in the western Palearctic and spread of highly pathogenic avian influenza H5NI virus. Emerg. Infect. Dis. 2006, 12, 1650–1656. [Google Scholar] [CrossRef]
- Curseu, D.; Popa, M.; Sirbu, D.; Stoian, I. Potential impact of climate change on pandemic influenza risk. Green Energy Technol. 2010, 1, 643–657. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, D.M.; Liu, W.B.; Wang, L. Evaluating the Impact of Climate Change on Global HPAI H5N1 outbreaks. IEMSS 2010, Ottawa, Ontario, Canada; Available online: http://www.iemss.org/iemss2010/papers/S27/S.27.02 (accessed on 20 February 2014).
- Shahid, M.A.; Abubakar, M.; Hameed, S.; Hassan, S. Avian influenza virus (H5N1): Effects of physico-chemical factors on its survival. Virol. J. 2009, 6. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar]
- Chumpolbanchorn, K.; Suemanotham, N.; Siripara, N.; Puyati, B.; Chaichoune, K. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian J. Trop. Med. Public Health 2006, 37, 102–105. [Google Scholar]
- Tiensin, T.; Nielen, M.; Vernooij, H.; Songserm, T.; Kalpravidh, W.; Chotiprasatintara, S.; Chaisingh, A.; Wongkasemjit, S.; Chanachai, K.; Thanapongtham, W.; et al. Transmission of the highly pathogenic avian influenza virus H5N1 within flocks during the 2004 epidemic in Thailand. J. Infect. Dis. 2007, 196, 1679–1684. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar]
- Weber, T.P.; Stilianakis, N.I. Inactivation of influenza a viruses in the environment and modes of transmission: A critical review. J. Infect. 2008, 57, 361–373. [Google Scholar] [CrossRef]
- Si, Y.L.; Wang, T.J.; Skidmore, A.K.; de Boer, W.F.; Li, L.; Prins, H.H.T. Environmental factors influencing the spread of the highly pathogenic avian influenza H5N1 virus in wild birds in Europe. Ecol. Soc. 2010, 15, p. 26. Available online: http://www.ecologyandsociety.org/vol15/iss3/art26/ (accessed on 1 May 2014).
- Liu, C.M.; Lin, S.H.; Chen, Y.C.; Lin, K.C.M.; Wu, T.S.J.; King, C.C. Temperature drops and the onset of severe avian influenza a H5N1 virus outbreaks. PLoS One 2007, 2. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, D.M.; Chen, Y.; Liu, W.B.; Wang, L.; Zhao, F.; Yao, B.D. Spatio-temporal data comparisons for global highly pathogenic avian influenza (HPAI) H5N1 outbreaks. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, D.M.; Chen, Y.; Davies, T.M.; Vaillancourt, J.P.; Liu, W. Risk signals of an influenza pandemic caused by highly pathogenic avian influenza subtype H5N1: Spatio-temporal perspectives. Vet. J. 2012, 192, 417–421. [Google Scholar] [CrossRef]
- Clayton, D. Some approaches to the analysis of recurrent event data. Stat. Methods Med. Res. 1994, 3, 244–262. [Google Scholar] [CrossRef]
- Besag, J.; York, J.; Mollie, J. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Lawson, A.B.; Browne, W.J.; Rodeiro, C.L.V. Disease Mapping with WinBUGS and MLwiN; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Gilbert, M.; Slingenbergh, J.; Xiao, X. Climate change and avian influenza. Rev. Sci. Tech. 2008, 27, 459–466. [Google Scholar]
- Reperant, L.A.; Fuckar, N.S.; Osterhaus, A.D.M.E.; Dobson, A.P.; Kuiken, T. Spatial and temporal association of outbreaks of H5N1 influenza virus infection in wild birds with the 0 degrees C isotherm. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Tobler, W.R. Computer movie simulating urban growth in Detroit Region. Econ. Geogr. 1970, 46, 234–240. [Google Scholar] [CrossRef]
- Alonso, W.J.; Viboud, C.; Simonsen, L.; Hirano, E.W.; Daufenbach, L.Z.; Miller, M.A. Seasonality of influenza in Brazil: A traveling wave from the Amazon to the subtropics. Amer. J. Epidemiol. 2007, 165, 1434–1442. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Z.; Chen, D.; Chen, Y.; Wang, B.; Hu, Y.; Gao, J.; Sun, L.; Li, R.; Xiong, C. Evaluating the Impact of Environmental Temperature on Global Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreaks in Domestic Poultry. Int. J. Environ. Res. Public Health 2014, 11, 6388-6399. https://doi.org/10.3390/ijerph110606388
Zhang Z, Chen D, Chen Y, Wang B, Hu Y, Gao J, Sun L, Li R, Xiong C. Evaluating the Impact of Environmental Temperature on Global Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreaks in Domestic Poultry. International Journal of Environmental Research and Public Health. 2014; 11(6):6388-6399. https://doi.org/10.3390/ijerph110606388
Chicago/Turabian StyleZhang, Zhijie, Dongmei Chen, Yue Chen, Bo Wang, Yi Hu, Jie Gao, Liqian Sun, Rui Li, and Chenglong Xiong. 2014. "Evaluating the Impact of Environmental Temperature on Global Highly Pathogenic Avian Influenza (HPAI) H5N1 Outbreaks in Domestic Poultry" International Journal of Environmental Research and Public Health 11, no. 6: 6388-6399. https://doi.org/10.3390/ijerph110606388





